Endothelial Cell Protein Targeting by Myeloperoxidase-Derived 2-Chlorofatty Aldehyde
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Cell Culture
2.3. Metabolism of 2-ClHDyA in Cells
2.4. Visualization of Cell Protein on Gel Electrophoresis
2.5. Detection of 2-ClHDyA and 2-ClHyA in Cells
2.6. Confocal Microscopy
2.7. Click Reactions of 2-ClHDyA Modified Proteins with Biotin-Azide
2.8. Immunoprecipitation of Biotin-Azide Clicked Proteins with Streptavidin Beads
2.9. LC-MS/MS Analysis of Captured Proteins
2.10. Proteomic Analysis of Peptides of Captured Proteins
2.11. Gene Ontology Analysis
2.12. SDS-PAGE and Western Blotting
3. Results
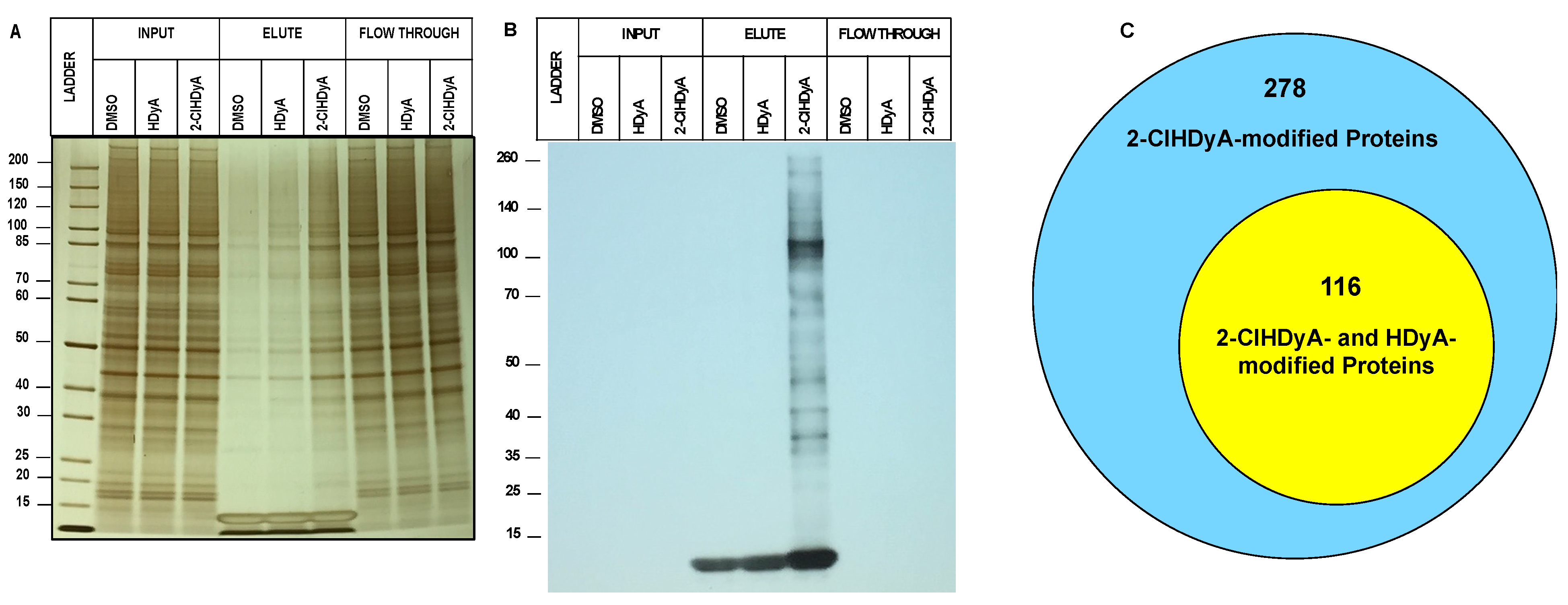
3.1. ClHDyA Metabolism and Protein Modification
3.2. Protein Targets of 2-ClHDyA in EA.hy926 Cells
3.3. Protein Targets of 2-ClHDyA in Human Lung Microvascular Endothelial Cells
3.4. Comparison of Protein Targets of 2-ClHDyA in EA.hy196 Cells and Human Lung Microvascular Endothelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Bardoel, B.W.; Kenny, E.F.; Sollberger, G.; Zychlinsky, A. The Balancing Act of Neutrophils. Cell Host Microbe 2014, 15, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Halade, G.V. Role of neutrophils in ischemic heart failure. Pharmacol. Ther. 2020, 205, 107424. [Google Scholar] [CrossRef]
- Döring, Y.; Drechsler, M.; Soehnlein, O.; Weber, C. Neutrophils in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 288–295. [Google Scholar] [CrossRef]
- Kovach, M.A.; Standiford, T.J. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 2012, 25, 321–327. [Google Scholar] [CrossRef]
- Anbukumar, D.S.; Shornick, L.P.; Albert, C.J.; Steward, M.M.; Zoeller, R.A.; Neumann, W.L.; Ford, D.A. Chlorinated lipid species in activated human neutrophils: Lipid metabolites of 2-chlorohexadecanal. J. Lipid Res. 2010, 51, 1085–1092. [Google Scholar] [CrossRef]
- Wildsmith, K.R.; Albert, C.J.; Anbukumar, D.S.; Ford, D.A. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J. Biol. Chem. 2006, 281, 16849–16860. [Google Scholar] [CrossRef]
- Thukkani, A.K.; Hsu, F.F.; Crowley, J.R.; Wysolmerski, R.B.; Albert, C.J.; Ford, D.A. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: Production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 2002, 277, 3842–3849. [Google Scholar] [CrossRef]
- Brahmbhatt, V.V.; Albert, C.J.; Anbukumar, D.S.; Cunningham, B.A.; Neumann, W.L.; Ford, D.A. ω-Oxidation of α-chlorinated fatty acids: Identification of α-chlorinated dicarboxylic acids. J. Biol. Chem. 2010, 285, 41255–41269. [Google Scholar] [CrossRef]
- Chilton, F.H.; Connell, T.R. 1-ether-linked phosphoglycerides. Major endogenous sources of arachidonate in the human neutrophil. J. Biol. Chem. 1988, 263, 5260–5265. [Google Scholar] [CrossRef]
- Ford, D.A.; Gross, R.W. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc. Natl. Acad. Sci. USA 1989, 86, 3479–3483. [Google Scholar] [CrossRef]
- Murphy, E.J.; Joseph, L.; Stephens, R.; Horrocks, L.A. Phospholipid composition of cultured human endothelial cells. Lipids 1992, 27, 150–153. [Google Scholar] [CrossRef]
- Thukkani, A.K.; Albert, C.J.; Wildsmith, K.R.; Messner, M.C.; Martinson, B.D.; Hsu, F.F.; Ford, D.A. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J. Biol. Chem. 2003, 278, 36365–36372. [Google Scholar] [CrossRef]
- Thukkani, A.K.; McHowat, J.; Hsu, F.F.; Brennan, M.L.; Hazen, S.L.; Ford, D.A. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation 2003, 108, 3128–3133. [Google Scholar] [CrossRef]
- Thukkani, A.K.; Martinson, B.D.; Albert, C.J.; Vogler, G.A.; Ford, D.A. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2955–H2964. [Google Scholar] [CrossRef]
- McHowat, J.; Shakya, S.; Ford, D.A. 2-Chlorofatty Aldehyde Elicits Endothelial Cell Activation. Front. Physiol. 2020, 11, 460. [Google Scholar] [CrossRef]
- Yu, H.; Wang, M.; Wang, D.; Kalogeris, T.J.; McHowat, J.; Ford, D.A.; Korthuis, R.J. Chlorinated lipids elicit inflammatory responses in vitro and in vivo. Shock 2019, 51, 114–122. [Google Scholar] [CrossRef]
- Nusshold, C.; Ullen, A.; Kogelnik, N.; Bernhart, E.; Reicher, H.; Plastira, I.; Glasnov, T.; Zangger, K.; Rechberger, G.; Kollroser, M.; et al. Assessment of electrophile damage in a human brain endothelial cell line utilizing a clickable alkyne analog of 2-chlorohexadecanal. Free Radic. Biol. Med. 2016, 90, 59–74. [Google Scholar] [CrossRef]
- Ullen, A.; Nusshold, C.; Glasnov, T.; Saf, R.; Cantillo, D.; Eibinger, G.; Reicher, H.; Fauler, G.; Bernhart, E.; Hallstrom, S.; et al. Covalent adduct formation between the plasmalogen-derived modification product 2-chlorohexadecanal and phloretin. Biochem. Pharm. 2015, 93, 470–481. [Google Scholar] [CrossRef]
- Amunugama, K.; Jellinek, M.J.; Kilroy, M.P.; Albert, C.J.; Rasi, V.; Hoft, D.F.; Shashaty, M.G.S.; Meyer, N.J.; Ford, D.A. Identification of novel neutrophil very long chain plasmalogen molecular species and their myeloperoxidase mediated oxidation products in human sepsis. Redox Biol. 2021, 48, 102208. [Google Scholar] [CrossRef]
- Wildsmith, K.R.; Albert, C.J.; Hsu, F.F.; Kao, J.L.; Ford, D.A. Myeloperoxidase-derived 2-chlorohexadecanal forms Schiff bases with primary amines of ethanolamine glycerophospholipids and lysine. Chem. Phys. Lipids 2006, 139, 157–170. [Google Scholar] [CrossRef]
- Duerr, M.A.; Aurora, R.; Ford, D.A. Identification of glutathione adducts of alpha-chlorofatty aldehydes produced in activated neutrophils. J. Lipid Res. 2015, 56, 1014–1024. [Google Scholar] [CrossRef]
- Duerr, M.A.; Palladino, E.N.D.; Hartman, C.L.; Lambert, J.A.; Franke, J.D.; Albert, C.J.; Matalon, S.; Patel, R.P.; Slungaard, A.; Ford, D.A. Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein. J. Lipid Res. 2018, 59, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.L.; Duerr, M.A.; Albert, C.J.; Neumann, W.L.; McHowat, J.; Ford, D.A. 2-Chlorofatty acids induce Weibel-Palade body mobilization. J. Lipid Res. 2018, 59, 113–122. [Google Scholar] [CrossRef]
- James, P.F.; Rizzo, W.B.; Lee, J.; Zoeller, R.A. Isolation and characterization of a Chinese hamster ovary cell line deficient in fatty alcohol:NAD+ oxidoreductase activity. Proc. Natl. Acad. Sci. USA 1990, 87, 6102–6106. [Google Scholar] [CrossRef]
- Rizzo, W.B.; Craft, D.A. Sjogren-Larsson syndrome. Deficient activity of the fatty aldehyde dehydrogenase component of fatty alcohol:NAD+ oxidoreductase in cultured fibroblasts. J. Clin. Investig. 1991, 88, 1643–1648. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Wang, W.Y.; Albert, C.J.; Ford, D.A. Approaches for the analysis of chlorinated lipids. Anal. Biochem. 2013, 443, 148–152. [Google Scholar] [CrossRef][Green Version]
- Wacker, B.K.; Albert, C.J.; Ford, B.A.; Ford, D.A. Strategies for the analysis of chlorinated lipids in biological systems. Free Radic. Biol. Med. 2013, 59, 92–99. [Google Scholar] [CrossRef][Green Version]
- Zhang, B.; Kirov, S.; Snoddy, J. WebGestalt: An integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005, 33, W741–W748. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duncan, D.; Shi, Z.; Zhang, B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013. Nucleic Acids Res. 2013, 41, W77–W83. [Google Scholar] [CrossRef] [PubMed]
- Kirov, S.; Ji, R.; Wang, J.; Zhang, B. Functional annotation of differentially regulated gene set using WebGestalt: A gene set predictive of response to ipilimumab in tumor biopsies. Methods Mol. Biol. 2014, 1101, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vasaikar, S.; Shi, Z.; Greer, M.; Zhang, B. WebGestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017, 45, W130–W137. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef]
- Prasch, J.; Bernhart, E.; Reicher, H.; Kollroser, M.; Rechberger, G.N.; Koyani, C.N.; Trummer, C.; Rech, L.; Rainer, P.P.; Hammer, A.; et al. Myeloperoxidase-Derived 2-Chlorohexadecanal Is Generated in Mouse Heart during Endotoxemia and Induces Modification of Distinct Cardiomyocyte Protein Subsets In Vitro. Int. J. Mol. Sci. 2020, 21, 9235. [Google Scholar] [CrossRef]
- Ullen, A.; Fauler, G.; Bernhart, E.; Nusshold, C.; Reicher, H.; Leis, H.J.; Malle, E.; Sattler, W. Phloretin ameliorates 2-chlorohexadecanal-mediated brain microvascular endothelial cell dysfunction in vitro. Free Radic. Biol. Med. 2012, 53, 1770–1781. [Google Scholar] [CrossRef]
- Davda, D.; El Azzouny, M.A.; Tom, C.T.; Hernandez, J.L.; Majmudar, J.D.; Kennedy, R.T.; Martin, B.R. Profiling targets of the irreversible palmitoylation inhibitor 2-bromopalmitate. ACS Chem. Biol. 2013, 8, 1912–1917. [Google Scholar] [CrossRef]
- Turriziani, B.; Garcia-Munoz, A.; Pilkington, R.; Raso, C.; Kolch, W.; von Kriegsheim, A. On-beads digestion in conjunction with data-dependent mass spectrometry: A shortcut to quantitative and dynamic interaction proteomics. Biology 2014, 3, 320–332. [Google Scholar] [CrossRef]
- Ma, X.; Sickmann, A.; Pietsch, J.; Wildgruber, R.; Weber, G.; Infanger, M.; Bauer, J.; Grimm, D. Proteomic differences between microvascular endothelial cells and the EA.hy926 cell line forming three-dimensional structures. Proteomics 2014, 14, 689–698. [Google Scholar] [CrossRef]
- Ford, D.A.; Honavar, J.; Albert, C.J.; Duerr, M.A.; Oh, J.Y.; Doran, S.; Matalon, S.; Patel, R.P. Formation of chlorinated lipids post-chlorine gas exposure. J. Lipid Res. 2016, 57, 1529–1540. [Google Scholar] [CrossRef]
- Pike, D.P.; Vogel, M.J.; McHowat, J.; Mikuzis, P.A.; Schulte, K.A.; Ford, D.A. 2-Chlorofatty acids are biomarkers of sepsis mortality and mediators of barrier dysfunction in rats. J. Lipid Res. 2020, 61, 1115–1127. [Google Scholar] [CrossRef]
- Meyer, N.J.; Reilly, J.P.; Feng, R.; Christie, J.D.; Hazen, S.L.; Albert, C.J.; Franke, J.D.; Hartman, C.L.; McHowat, J.; Ford, D.A. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI Insight 2017, 2, e96432. [Google Scholar] [CrossRef]
- Edgell, C.J.; Haizlip, J.E.; Bagnell, C.R.; Packenham, J.P.; Harrison, P.; Wilbourn, B.; Madden, V.J. Endothelium specific Weibel-Palade bodies in a continuous human cell line, EA.hy926. In Vitro Cell. Dev. Biol. 1990, 26, 1167–1172. [Google Scholar] [CrossRef]
- Cerutti, C.; Ridley, A.J. Endothelial cell-cell adhesion and signaling. Exp. Cell Res. 2017, 358, 31–38. [Google Scholar] [CrossRef]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997, 16, 3797–3804. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakya, S.; Herr, R.A.; Carlson, H.L.; Zoeller, R.A.; Albert, C.J.; Ford, D.A. Endothelial Cell Protein Targeting by Myeloperoxidase-Derived 2-Chlorofatty Aldehyde. Antioxidants 2022, 11, 940. https://doi.org/10.3390/antiox11050940
Shakya S, Herr RA, Carlson HL, Zoeller RA, Albert CJ, Ford DA. Endothelial Cell Protein Targeting by Myeloperoxidase-Derived 2-Chlorofatty Aldehyde. Antioxidants. 2022; 11(5):940. https://doi.org/10.3390/antiox11050940
Chicago/Turabian StyleShakya, Shubha, Roger A. Herr, Haley L. Carlson, Raphael A. Zoeller, Carolyn J. Albert, and David A. Ford. 2022. "Endothelial Cell Protein Targeting by Myeloperoxidase-Derived 2-Chlorofatty Aldehyde" Antioxidants 11, no. 5: 940. https://doi.org/10.3390/antiox11050940
APA StyleShakya, S., Herr, R. A., Carlson, H. L., Zoeller, R. A., Albert, C. J., & Ford, D. A. (2022). Endothelial Cell Protein Targeting by Myeloperoxidase-Derived 2-Chlorofatty Aldehyde. Antioxidants, 11(5), 940. https://doi.org/10.3390/antiox11050940






