Zeolitic Imidazolate Framework-8 Nanoparticles Exhibit More Severe Toxicity to the Embryo/Larvae of Zebrafish (Danio rerio) When Co-Exposed with Cetylpyridinium Chloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Test Organisms
2.3. Exposure Experiment
2.4. Light-Dark Locomotion Test of Newly Hatched Larval
2.5. Biochemical Assays
2.6. Statistical Analysis
3. Results
3.1. Effects on Mortality and Hatching
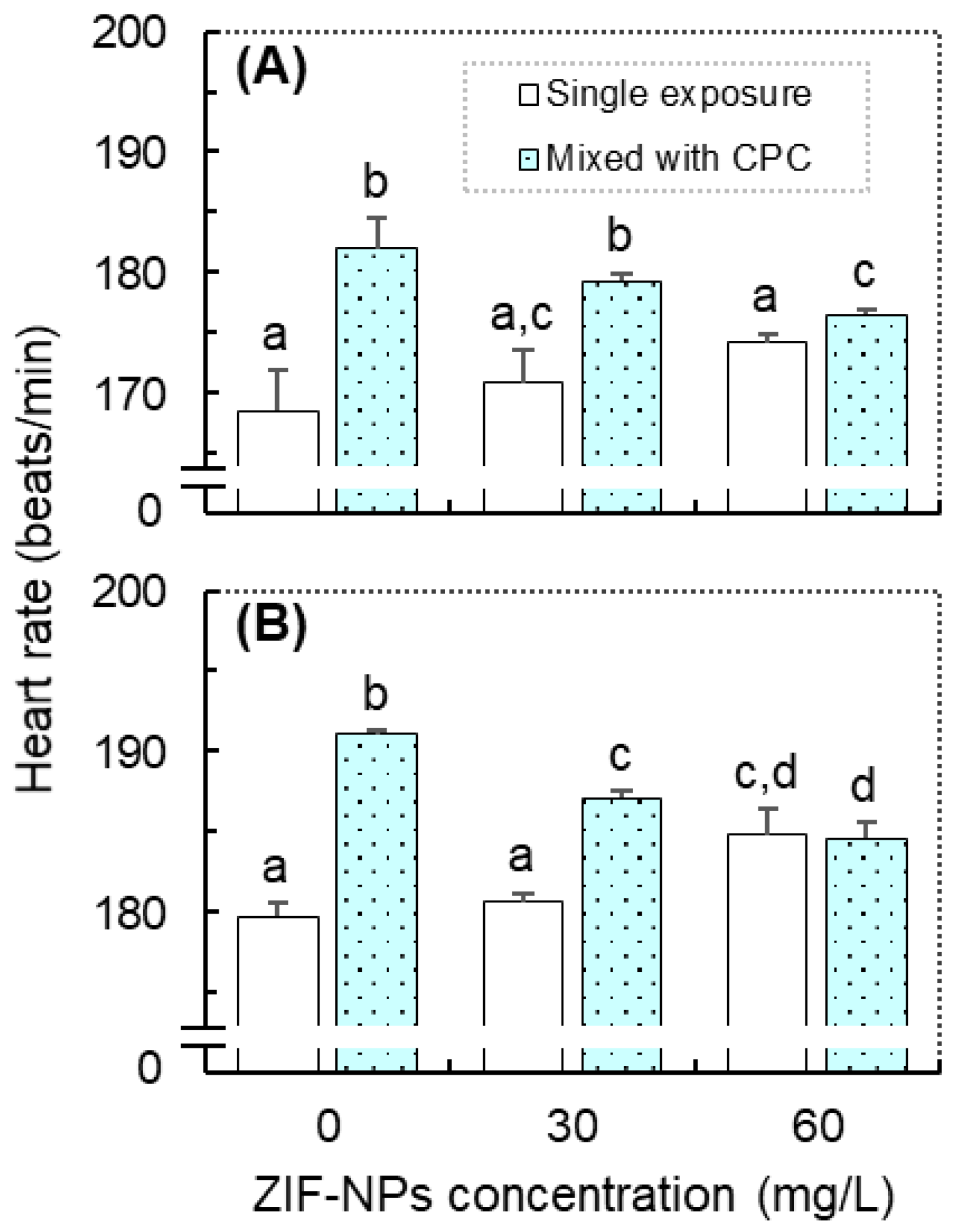
3.2. Effects on the Heart Rate
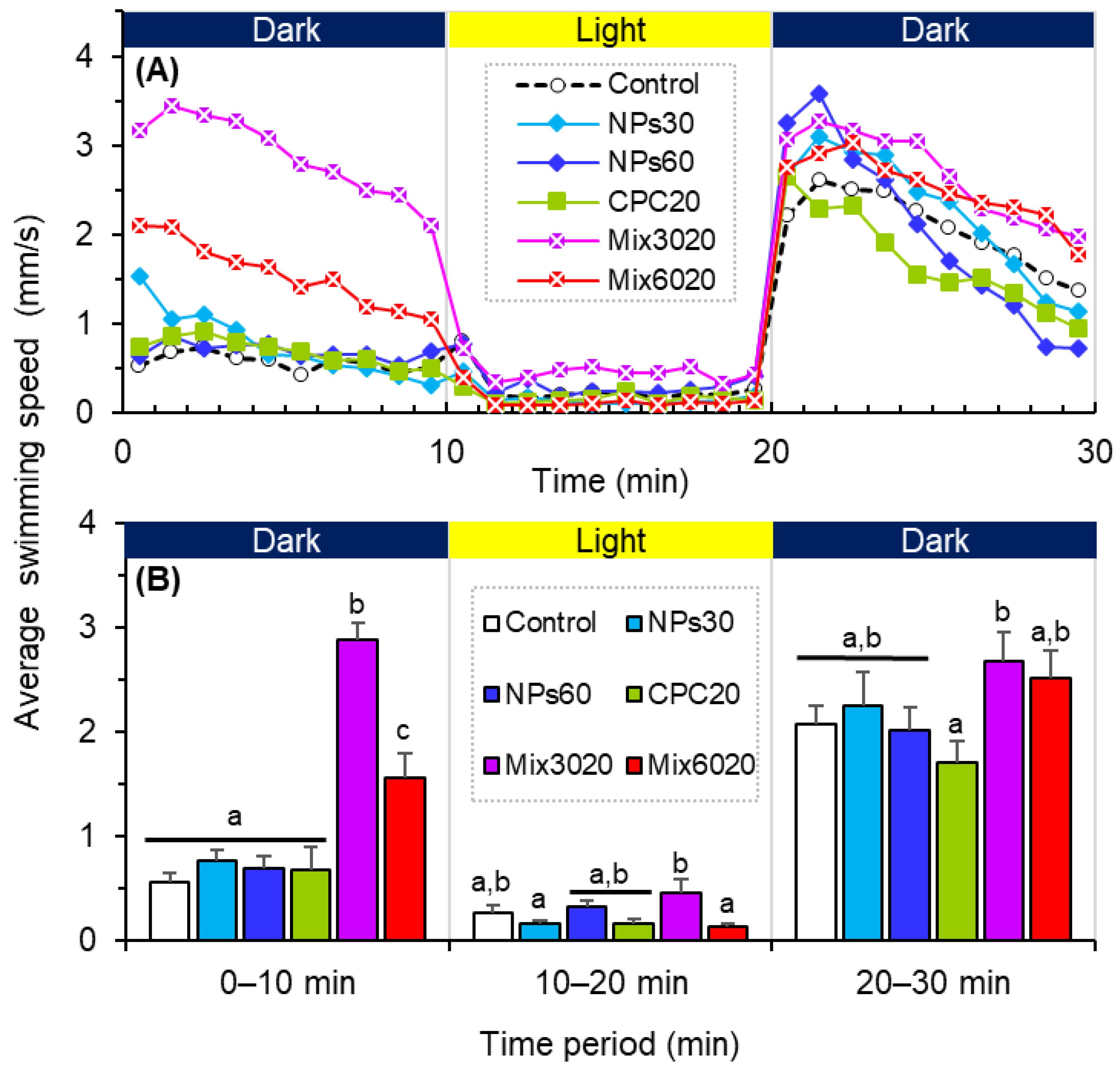
3.3. Effects on the Behavioral Traits
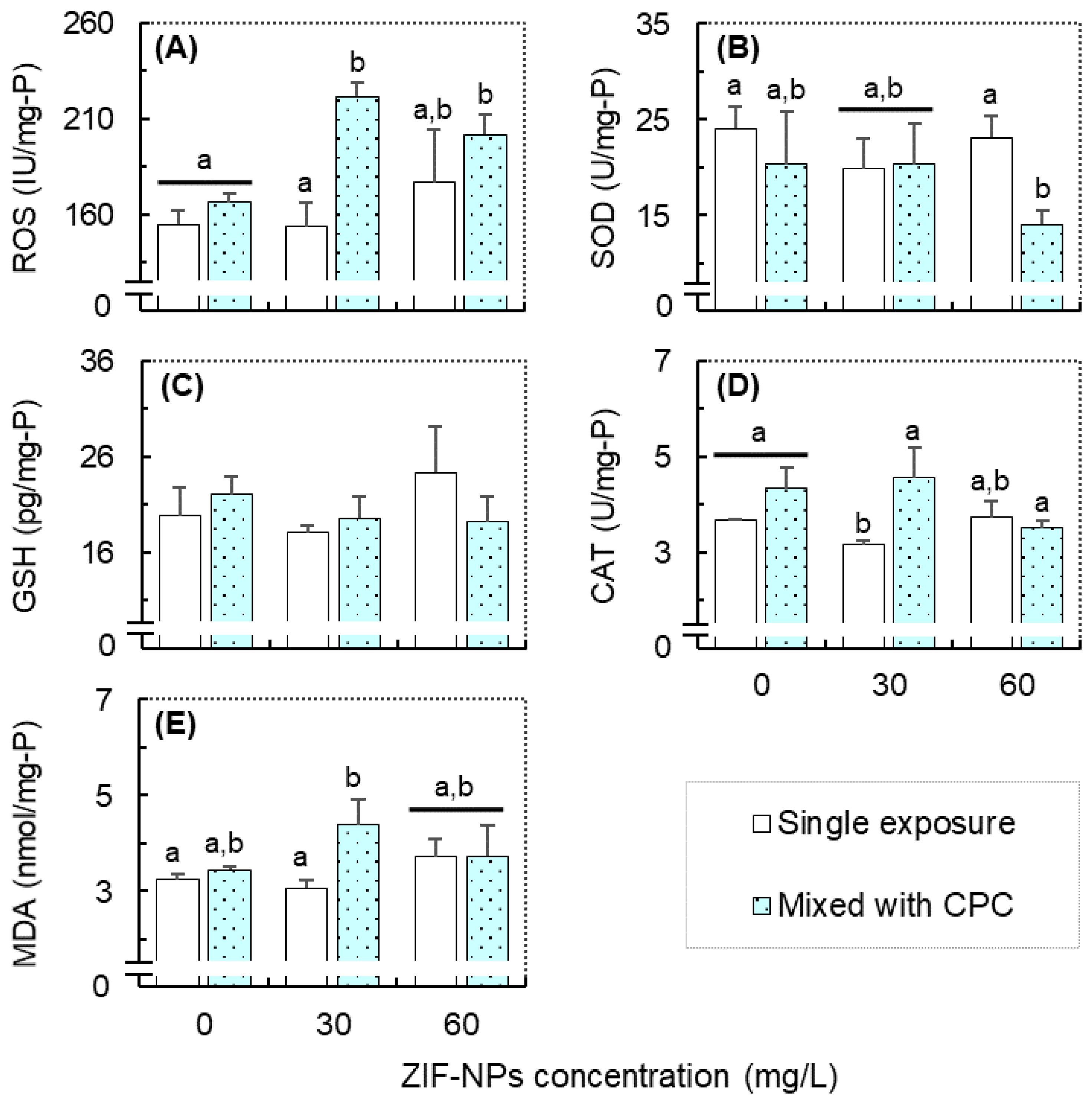
3.4. Responses in the Oxidative Stress-Related Biomarkers and Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A critical review of the use of surfactant-coated nanoparticles in nanomedicine and food nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, A.L.; Truong, L.; Tanguay, R.L.; Hutchison, J.E. Synergistic toxicity produced by mixtures of biocompatible gold nanoparticles and widely used surfactants. ACS Nano 2018, 12, 5312–5322. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, X.; Tian, F.; Liu, X.; Yuan, J.; Huang, B. Review on nanoparticle-surfactant nanofluids: Formula fabrication and applications in enhanced oil recovery. J. Dispers. Sci. Technol. 2020, 43, 745–759. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, J.; Wang, J.; Xue, Q.; Li, S.; Yu, W.; Li, Z. The Impact of Ionic Liquid and Nanoparticles on Stabilizing Foam for Enhanced Oil Recovery. ChemistrySelect 2018, 3, 12461–12468. [Google Scholar] [CrossRef]
- Modi, A.; Jiang, Z.; Kasher, R. Hydrostable ZIF-8 layer on polyacrylonitrile membrane for efficient treatment of oilfield produced water. Chem. Eng. J. 2021, 434, 133513. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.S.; Bu, X.; Feng, P. Metal-organic frameworks for separation. Adv. Mater. 2018, 30, e1705189. [Google Scholar] [CrossRef]
- Wan, Y.; Xu, W.; Ren, X.; Wang, Y.; Dong, B.; Wang, L. Microporous frameworks as promising platforms for antibacterial strategies against oral diseases. Front. Bioeng. Biotechnol. 2020, 8, 628. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Li, C.; Dong, B.; Wang, J.; Weir, M.D.; Imazato, S.; Du, L.; Lynch, C.D.; Xu, L.; et al. Novel nanoparticles of cerium-doped zeolitic imidazolate frameworks with dual benefits of antibacterial and anti-inflammatory functions against periodontitis. J. Mater. Chem. B 2019, 7, 6955–6971. [Google Scholar] [CrossRef]
- Fromm-Dornieden, C.; Rembe, J.D.; Schäfer, N.; Böhm, J.; Stuermer, E.K. Cetylpyridinium chloride and miramistin as antiseptic substances in chronic wound management—prospects and limitations. J. Med. Microbiol. 2015, 64, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Johari, S.A.; Sarkheil, M.; Veisi, S. Cytotoxicity, oxidative stress, and apoptosis in human embryonic kidney (HEK293) and colon cancer (SW480) cell lines exposed to nanoscale zeolitic imidazolate framework 8 (ZIF-8). Environ. Sci. Pollut. Res. 2021, 28, 56772–56781. [Google Scholar] [CrossRef]
- Chen, P.; He, M.; Chen, B.; Hu, B. Size- and dose-dependent cytotoxicity of ZIF-8 based on single cell analysis. Ecotoxicol. Environ. Saf. 2020, 205, 111110. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kita, F.; Ikesugi, S.; Abe, M.; Sogabe, S.; Nishimura-Danjobara, Y.; Miura, H.; Oyama, Y. Cetylpyridinium chloride at sublethal levels increases the susceptibility of rat thymic lymphocytes to oxidative stress. Chemosphere 2017, 170, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Chatterjee, A.; Chatterjee, S.; Saha, N.C. Acute toxicity and impact of sublethal exposure to commonly used surfactants sodium dodecyl sulphate, cetylpyridinium chloride and sodium laureth sulphate on oxidative stress enzymes in oligochaete worm Branchiura sowerbyi (Beddard, 1892). Aquac. Res. 2021, 52, 6367–6379. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Chatterjee, A.; Chatterjee, S.; Saha, N.C. Oxidative stress in benthic oligochaete worm, Tubifex tubifex induced by sublethal exposure to a cationic surfactant cetylpyridinium chloride and an anionic surfactant sodium dodecyl sulfate. Comp. Biochem. Physiol. Part-C Toxicol. 2021, 240, 108906. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Chatterjee, A.; Chatterjee, S.; Saha, N.C. Commonly used surfactants sodium dodecyl sulphate, cetylpyridinium chloride and sodium laureth sulphate and their effects on antioxidant defence system and oxidative stress indices in Cyprinus carpio L.: An integrated in silico and in vivo approach. Environ. Sci. Pollut. Res. 2022, 29, 30622–30637. [Google Scholar] [CrossRef]
- Qiu, X.; Tengbe, M.S.; Xia, X.; Dong, K.; Chen, C.; Shi, Y.; Li, M.; Xu, H.; Wu, X.; Chen, K. Impacts of cetylpyridinium chloride on the survival, development, behavior, and oxidative stress of early-life-stage zebrafish (Danio rerio). Antioxidants 2022, 11, 676. [Google Scholar] [CrossRef]
- Chen, K.; Wu, M.; Chen, C.; Xu, H.; Wu, X.; Qiu, X. Impacts of chronic exposure to sublethal diazepam on behavioral traits of female and male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 208, 111747. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, C.; Shi, Y.; Chen, K.; Li, M.; Xu, H.; Wu, X.; Takai, Y.; Shimasaki, Y.; Oshima, Y. Persistent impact of amitriptyline on the behavior, brain neurotransmitter, and transcriptional profile of zebrafish (Danio rerio). Aquat. Toxicol. 2022, 245, 106129. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar] [CrossRef]
- Ganesan, S.; Anaimalai Thirumurthi, N.; Raghunath, A.; Vijayakumar, S.; Perumal, E. Acute and sub-lethal exposure to copper oxide nanoparticles causes oxidative stress and teratogenicity in zebrafish embryos. J. Appl. Toxicol. 2016, 36, 554–567. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wu, Y.; You, H.; Lv, L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat. Toxicol. 2013, 136–137, 49–59. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Q.; Chu, T.; Mo, Y.; Cai, S.; Chen, M.; Zhu, G. High-dose acute exposure of paraquat induces injuries of swim bladder, gastrointestinal tract and liver via neutrophil-mediated ROS in zebrafish and their relevance for human health risk assessment. Chemosphere 2018, 205, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Bernardo, I.D.; Lowe, A.; Nisbet, D.R.; Tsuzuki, T. Green full conversion of ZnO nanopowders to well-dispersed zeolitic imidazolate framework-8 (ZIF-8) nanopowders via a stoichiometric mechanochemical reaction for fast dye adsorption. Cryst. Growth Des. 2020, 20, 2761–2773. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Han, L.; He, Q.; Hou, H.; Han, J.; Wang, X.; Li, C.; Cen, J.; Liu, K. Oxidative stress-mediated developmental toxicity induced by isoniazide in zebrafish embryos and larvae. J. Appl. Toxicol. 2017, 37, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Rong, X.S.; Chen, C.; Wu, M.; Takai, Y.; Qiu, X.C.; Wang, C.C.; Shimasaki, Y.; Oshima, Y. Effects of ZIF-8 nanoparticles on the survival, development, and locomotor activity of early-life-stages of zebrafish (Danio rerio). J. Fac. Agric. Kyushu Univ. 2021, 66, 211–216. [Google Scholar]
- Deng, J.; Yu, L.; Liu, C.; Yu, K.; Shi, X.; Yeung, L.W.; Lam, P.K.; Wu, R.S.; Zhou, B. Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat. Toxicol. 2009, 93, 29–36. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, X.; Xie, Y.; Song, M.; Zhu, K.; Ding, S. Macrolides induce severe cardiotoxicity and developmental toxicity in zebrafish embryos. Sci. Total Environ. 2019, 649, 1414–1421. [Google Scholar] [CrossRef]
- Garbinato, C.; Schneider, S.E.; Sachett, A.; Decui, L.; Conterato, G.M.; Muller, L.G.; Siebel, A.M. Exposure to ractopamine hydrochloride induces changes in heart rate and behavior in zebrafish embryos and larvae. Environ. Sci. Pollut. Res. 2020, 27, 21468–21475. [Google Scholar] [CrossRef]
- Chen, K.; Iwasaki, N.; Qiu, X.; Xu, H.; Takai, Y.; Tashiro, K.; Shimasaki, Y.; Oshima, Y. Obesogenic and developmental effects of TBT on the gene expression of juvenile Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2021, 237, 105907. [Google Scholar] [CrossRef]
- Chen, C.; Li, L.; Li, M.; Wu, M.; Liang, W.; Takai, Y.; Qiu, X.; Shimasaki, Y.; Oshima, Y. Impacts of diazepam on the survival, development, and response to light stimulation in early-life stages of zebrafish (Danio rerio). J. Fac. Agric. Kyushu Univ. 2021, 66, 205–210. [Google Scholar]
- Du, J.; Wang, S.; You, H.; Jiang, R.; Zhuang, C.; Zhang, X. Developmental toxicity and DNA damage to zebrafish induced by perfluorooctane sulfonate in the presence of ZnO nanoparticles. Environ. Toxicol. 2016, 31, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Saputra, F.; Uapipatanakul, B.; Lee, J.-S.; Hung, S.-M.; Huang, J.-C.; Pang, Y.-C.; Muñoz, J.E.R.; Macabeo, A.P.G.; Chen, K.H.-C.; Hsiao, C.-D. Co-treatment of copper oxide nanoparticle and carbofuran enhances cardiotoxicity in zebrafish embryos. Int. J. Mol. Sci. 2021, 22, 8259. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lin, B.; Hu, C.; Zhang, H.; Lin, Z.; Xi, Z. The combined toxicological effects of titanium dioxide nanoparticles and bisphenol A on zebrafish embryos. Nanoscale Res. Lett. 2014, 9, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellou, J. Behavioural ecotoxicology, an “early warning” signal to assess environmental quality. Environ. Sci. Pollut. Res. 2011, 18, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colwill, R.M.; Creton, R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav. Process. 2011, 86, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drapeau, P.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Brustein, E. Development of the locomotor network in zebrafish. Prog. Neurobiol. 2002, 68, 85–111. [Google Scholar] [CrossRef]
- Wu, M.; Qiu, X.; Chen, C.; Chen, K.; Li, M.; Xu, H.; Wu, X.; Shimasaki, Y.; Oshima, Y. Short-term and persistent impacts of sublethal exposure to diazepam on behavioral traits and brain GABA levels in juvenile zebrafish (Danio rerio). Sci. Total Environ. 2020, 740, 140392. [Google Scholar] [CrossRef]
- Svanbäck, R.; Zha, Y.; Brönmark, C.; Johansson, F. The interaction between predation risk and food ration on behavior and morphology of Eurasian perch. Ecol. Evol. 2017, 7, 8567–8577. [Google Scholar] [CrossRef] [Green Version]
- Vieira, E.A.; Duarte, L.F.L.; Dias, G.M. How the timing of predation affects composition and diversity of species in a marine sessile community? J. Exp. Mar. Biol. Ecol. 2012, 412, 126–133. [Google Scholar] [CrossRef]
- Hong, X.; Zha, J. Fish behavior: A promising model for aquatic toxicology research. Sci. Total Environ. 2019, 686, 311–321. [Google Scholar] [CrossRef]
- Stoliar, O.B.; Lushchak, V.I. Environmental pollution and oxidative stress in fish. In Oxidative Stress-Environmental Induction and Dietary Antioxidants; Lushcha, V., Ed.; Sasa Leporic: Rijeka, Croatia, 2012; pp. 131–166. [Google Scholar]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Wen, J.; Xue, Z.; Yin, X.; Li, Y.; Yuan, L. The accumulation and toxicity of ZIF-8 nanoparticles in Corbicula fluminea. J. Environ. Sci. 2022. In Press. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Wu, H.; Mu, L. Persistence and Recovery of ZIF-8 and ZIF-67 Phytotoxicity. Environ. Sci. Technol. 2021, 55, 15301–15312. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kim, S.; Kang, I.J.; Hano, T.; Shimasaki, Y.; Oshima, Y. Combined toxicities of tributyltin and polychlorinated biphenyls on the development and hatching of Japanese medaka (Oryzias latipes) embryos via in ovo nanoinjection. Chemosphere 2019, 225, 927–934. [Google Scholar] [CrossRef]
- Qiu, X.; Tanoue, W.; Kawaguchi, A.; Yanagawa, T.; Seki, M.; Shimasaki, Y.; Honjo, T.; Oshima, Y. Interaction patterns and toxicities of binary and ternary pesticide mixtures to Daphnia magna estimated by an accelerated failure time model. Sci. Total Environ. 2017, 607–608, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cai, J.; Wang, S.; You, H. Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZnO nanoparticles. Int. J. Occup. Med. Environ. Health 2017, 30, 213–229. [Google Scholar] [CrossRef] [Green Version]
- Ogunsuyi, O.I.; Fadoju, O.M.; Akanni, O.O.; Alabi, O.A.; Alimba, C.G.; Cambier, S.; Eswara, S.; Gutleb, A.C.; Adaramoye, O.A.; Bakare, A.A. Genetic and systemic toxicity induced by silver and copper oxide nanoparticles, and their mixture in Clarias gariepinus (Burchell, 1822). Environ. Sci. Pollut. Res. 2019, 26, 27470–27481. [Google Scholar] [CrossRef]
- Olguín-Albuerne, M.; Morán, J. ROS produced by NOX2 control in vitro development of cerebellar granule neurons development. ASN Neuro 2015, 7, 1759091415578712. [Google Scholar] [CrossRef]
- Daugaard, M.; Nitsch, R.; Razaghi, B.; McDonald, L.; Jarrar, A.; Torrino, S.; Castillo-Lluva, S.; Rotblat, B.; Li, L.; Malliri, A.; et al. Hace1 controls ROS generation of vertebrate Rac1-dependent NADPH oxidase complexes. Nat. Commun. 2013, 4, 2180. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2015, 42, 711–747. [Google Scholar] [CrossRef]
- Border, S.E.; DeOliveira, G.M.; Janeski, H.M.; Piefke, T.J.; Brown, T.J.; Dijkstra, P.D. Social rank, color morph, and social network metrics predict oxidative stress in a cichlid fish. Behav. Ecol. 2019, 30, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Sarasamma, S.; Audira, G.; Samikannu, P.; Juniardi, S.; Siregar, P.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Behavioral impairments and oxidative stress in the brain, muscle, and gill caused by chronic exposure of C(70) nanoparticles on adult zebrafish. Int. J. Mol. Sci. 2019, 20, 5795. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Biological Parameters | Average Swimming Velocity | Oxidative Stress-Related Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOR | HAT | HB48 | HB72 | 0–10 min | 10–20 min | 20–30 min | ROS | T-SOD | GSH | CAT | MDA | |
| CPC (df = 1) | 0.03 | 4.68 * | 34.6 ** | 92.1 ** | 65.4 ** | 0.00 | 0.91 | 13.8 ** | 3.24 | 0.06 | 7.12 ** | 4.15 * |
| ZIF-NPs (df = 2) | 0.64 | 1.09 | 0.06 | 11.8 ** | 58.9 ** | 2.07 | 5.75 | 17.7 ** | 2.06 | 2.40 | 2.64 | 4.13 |
| CPC × ZIF (df = 2) | 1.34 | 2.17 | 15.9 ** | 56.9 ** | 41.6 ** | 12.4 ** | 3.84 | 16.2 ** | 4.36 | 2.13 | 8.69 * | 6.06 * |
| Heart Rate | ASV Under the Light (L) and Dark (D) Shifts | ||||
|---|---|---|---|---|---|
| 48 hpf | 72 hpf | 0–10 min (D) | 10–20 min (L) | 20–30 min (D) | |
| ROS | 0.494 * | 0.540 * | 0.507 * | −0.063 | 0.513 * |
| T-SOD | −0.282 | −0.126 | −0.453 | 0.193 | 0.001 |
| GSH | 0.361 | 0.562 * | −0.119 | −0.092 | −0.007 |
| CAT | 0.441 | 0.663 ** | 0.073 | 0.108 | 0.127 |
| MDA | 0.485 * | 0.558 * | 0.411 | 0.417 | 0.485 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Liu, L.; Xu, W.; Chen, C.; Li, M.; Shi, Y.; Wu, X.; Chen, K.; Wang, C. Zeolitic Imidazolate Framework-8 Nanoparticles Exhibit More Severe Toxicity to the Embryo/Larvae of Zebrafish (Danio rerio) When Co-Exposed with Cetylpyridinium Chloride. Antioxidants 2022, 11, 945. https://doi.org/10.3390/antiox11050945
Qiu X, Liu L, Xu W, Chen C, Li M, Shi Y, Wu X, Chen K, Wang C. Zeolitic Imidazolate Framework-8 Nanoparticles Exhibit More Severe Toxicity to the Embryo/Larvae of Zebrafish (Danio rerio) When Co-Exposed with Cetylpyridinium Chloride. Antioxidants. 2022; 11(5):945. https://doi.org/10.3390/antiox11050945
Chicago/Turabian StyleQiu, Xuchun, Lei Liu, Wei Xu, Chen Chen, Ming Li, Yanhong Shi, Xiangyang Wu, Kun Chen, and Chongchen Wang. 2022. "Zeolitic Imidazolate Framework-8 Nanoparticles Exhibit More Severe Toxicity to the Embryo/Larvae of Zebrafish (Danio rerio) When Co-Exposed with Cetylpyridinium Chloride" Antioxidants 11, no. 5: 945. https://doi.org/10.3390/antiox11050945






