Salicylic Acid Enhances Heat Stress Resistance of Pleurotus ostreatus (Jacq.) P. Kumm through Metabolic Rearrangement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material and Culture Conditions
2.2. Temperature Stress and SA Treatments
2.3. Enzyme Activity Assays
2.4. Detection of H2O2 and MDA Production
2.5. Detection of GSH and Proline Production
2.6. Determination of the Recovery Growth Rate of Hyphae
2.7. Metabolome Analysis
2.8. Transcriptome Analysis and qRT-PCR Verification
2.9. Statistical Analysis
3. Results
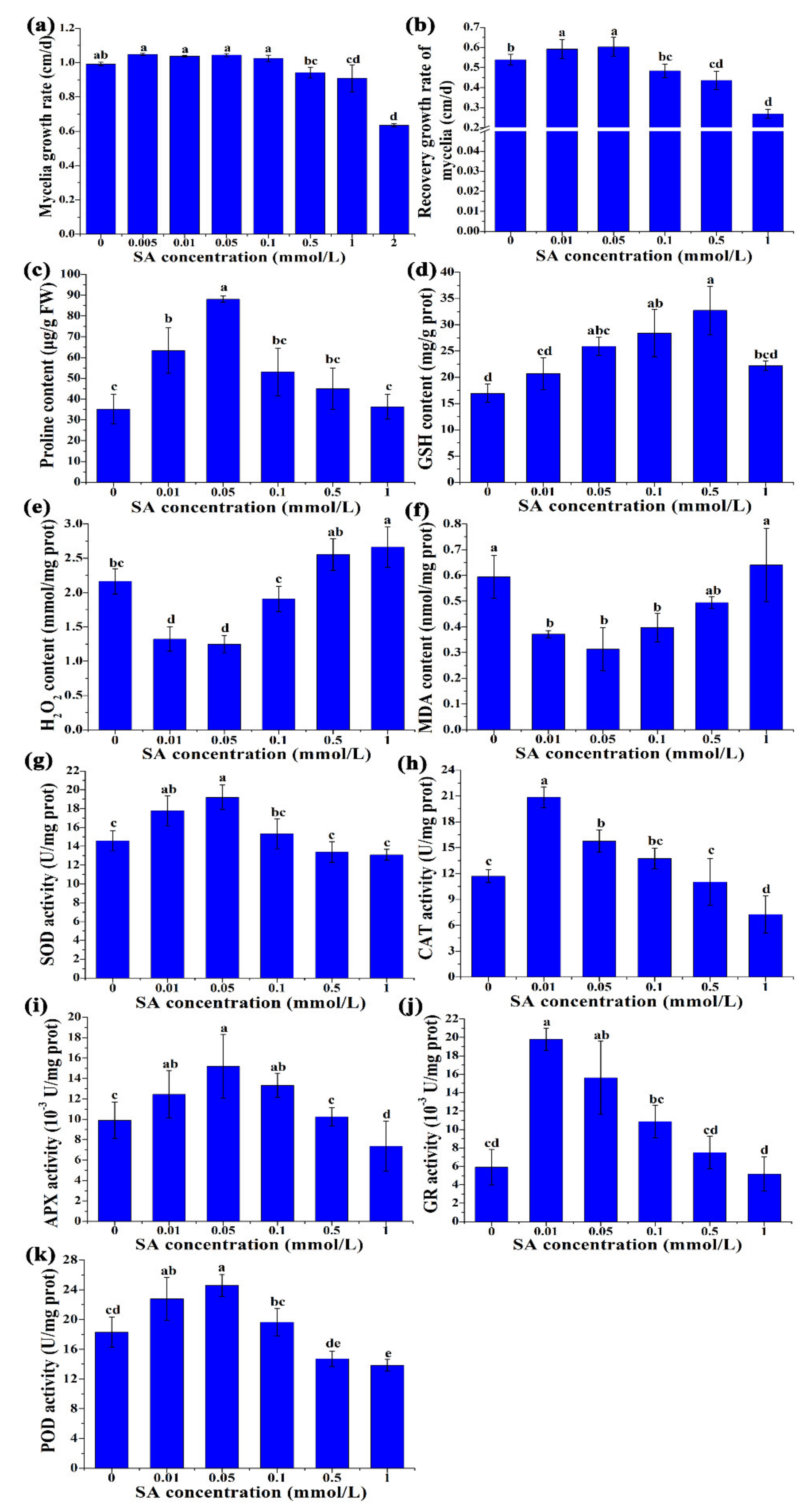
3.1. Physiological and Biochemical Changes
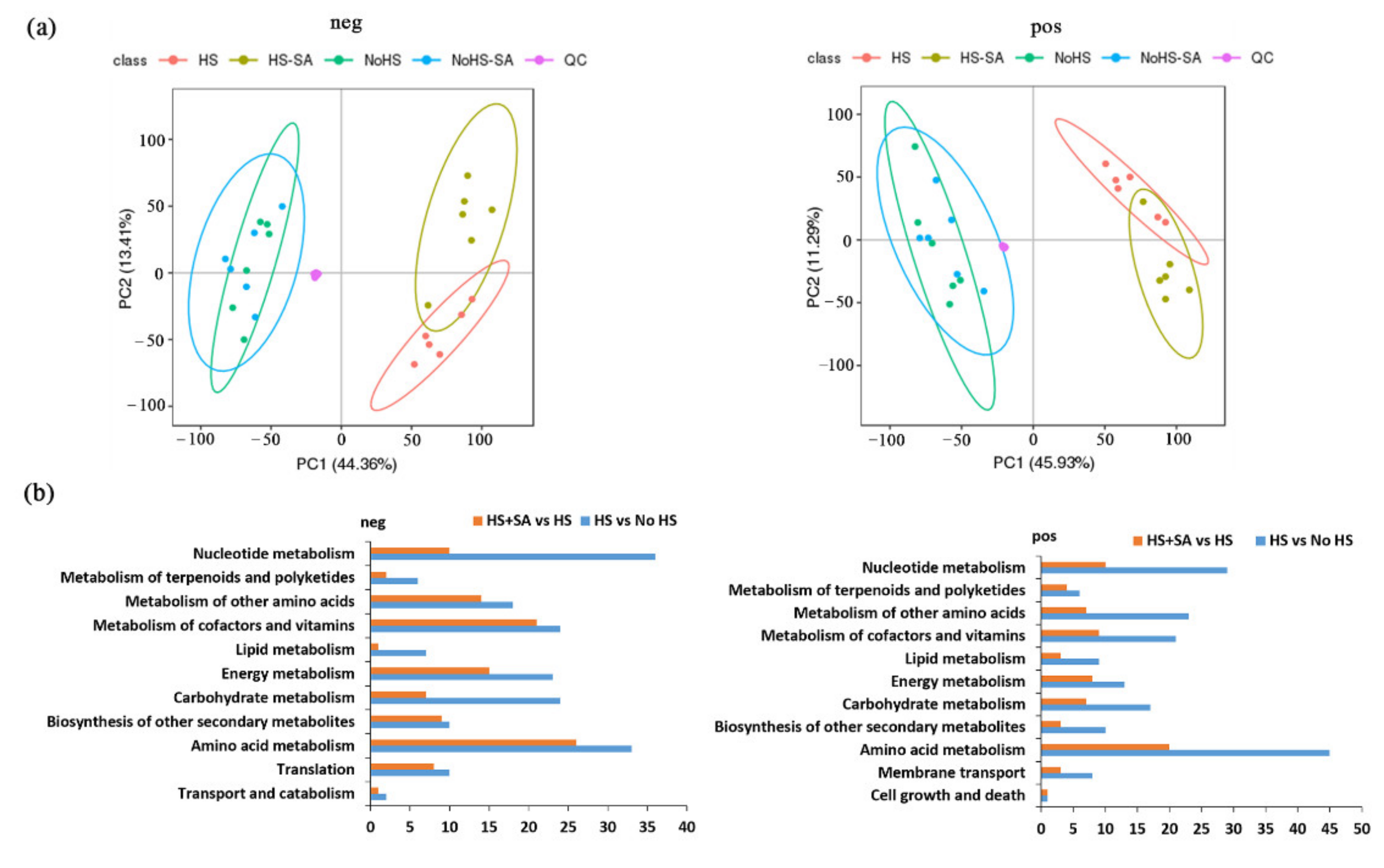
3.2. Metabolite Changes
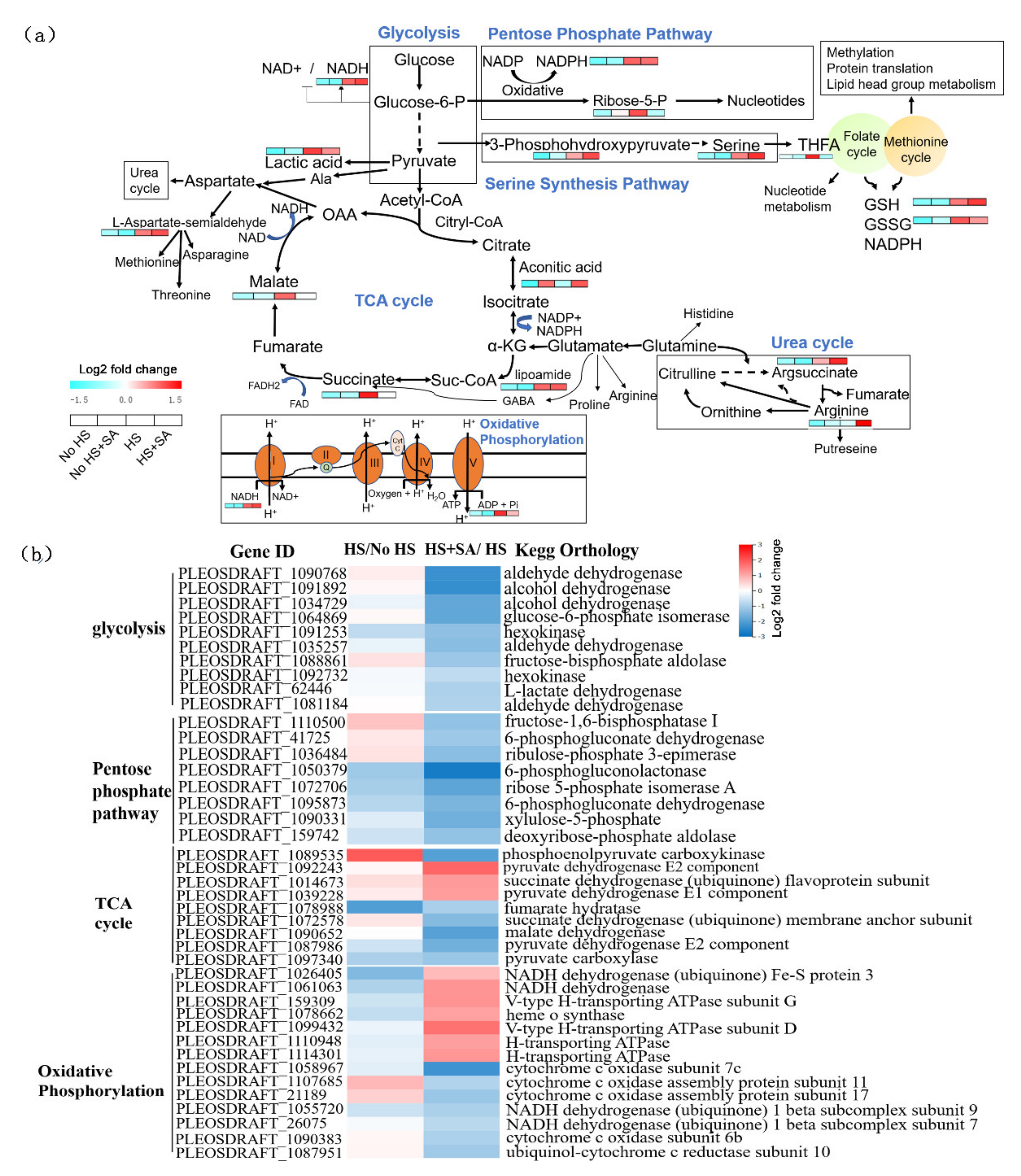
3.3. Energy Metabolism and Central Carbon Metabolism Analysis
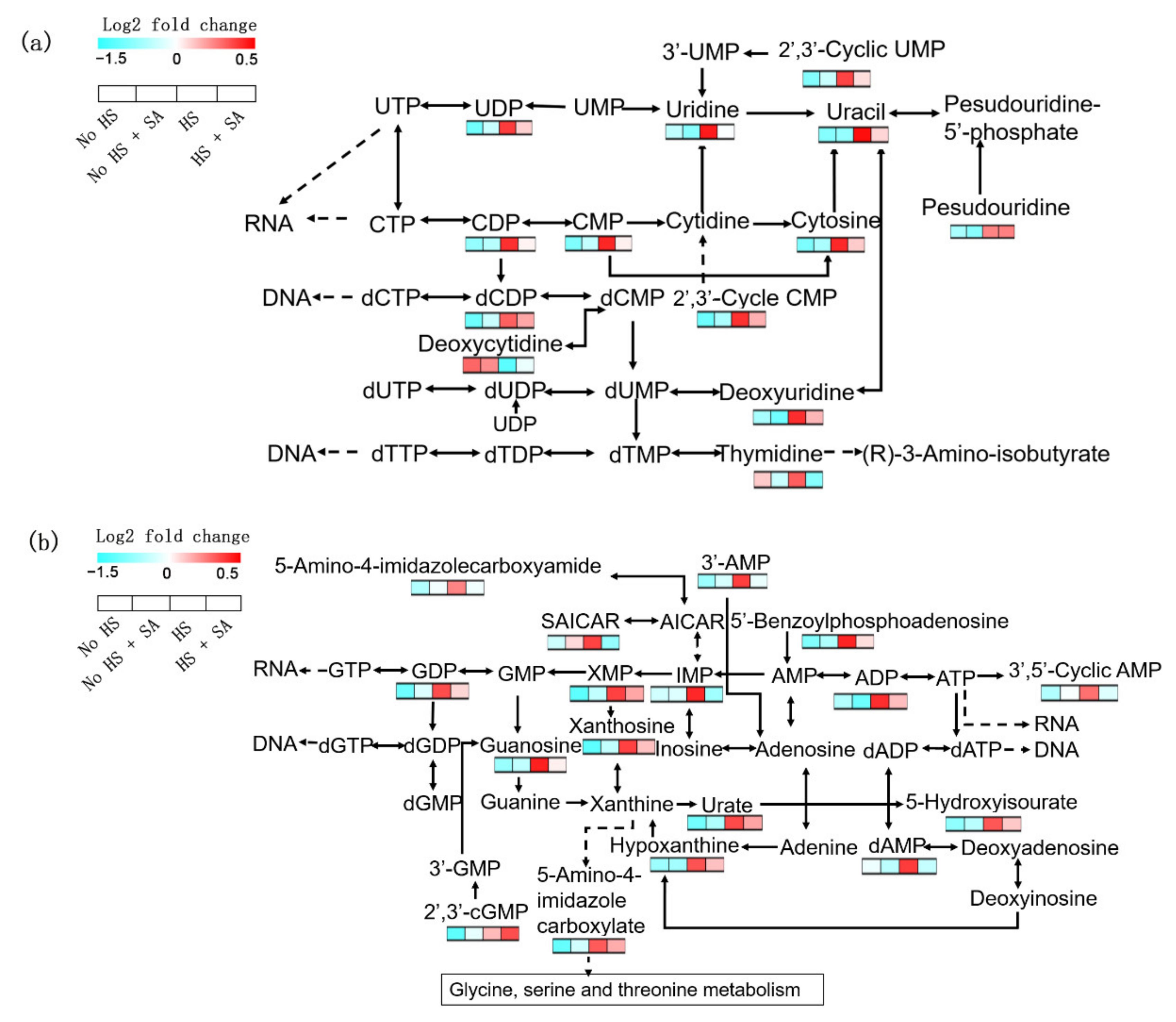
3.4. Nucleotide Metabolism Analysis
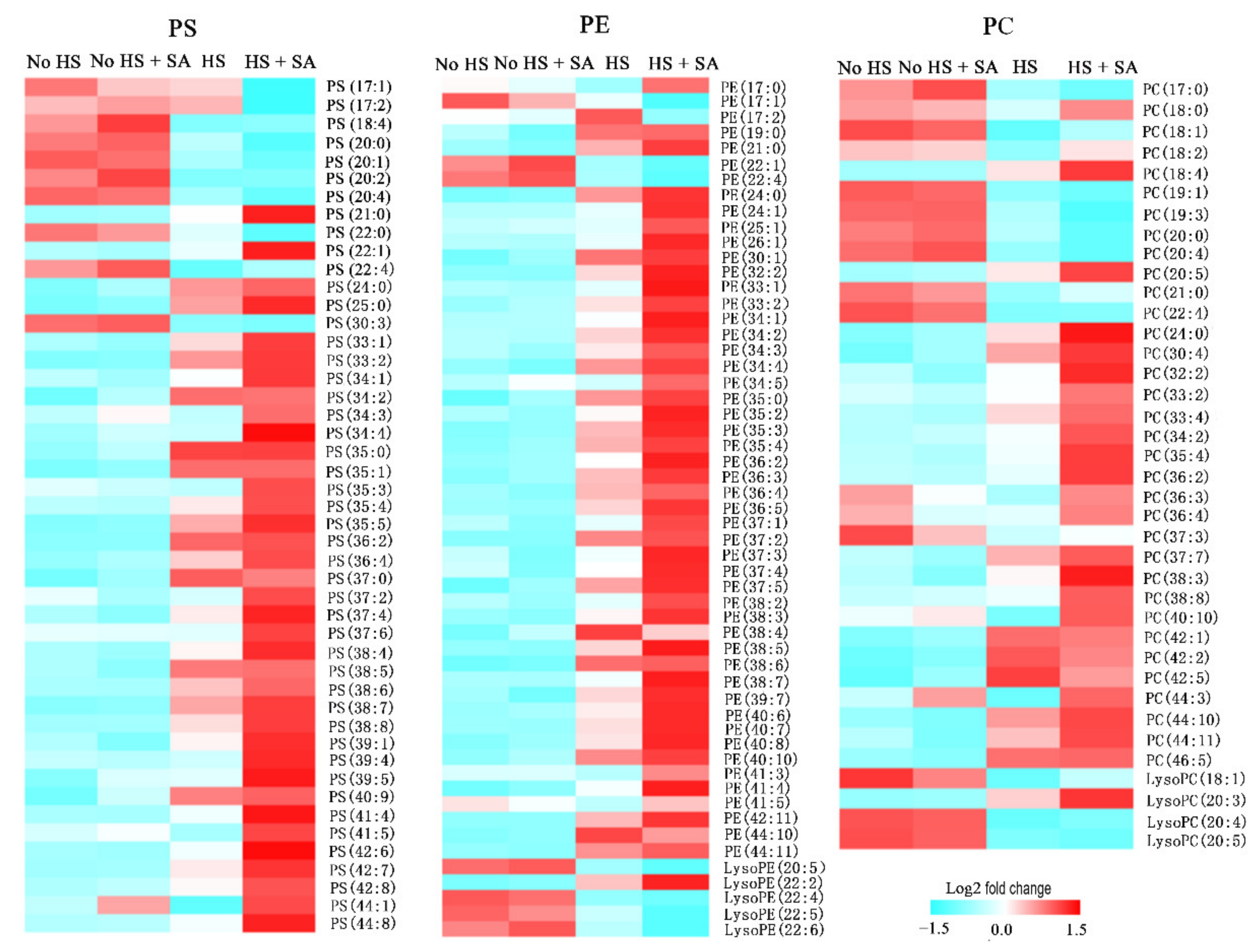
3.5. Membrane Lipids Remodeling
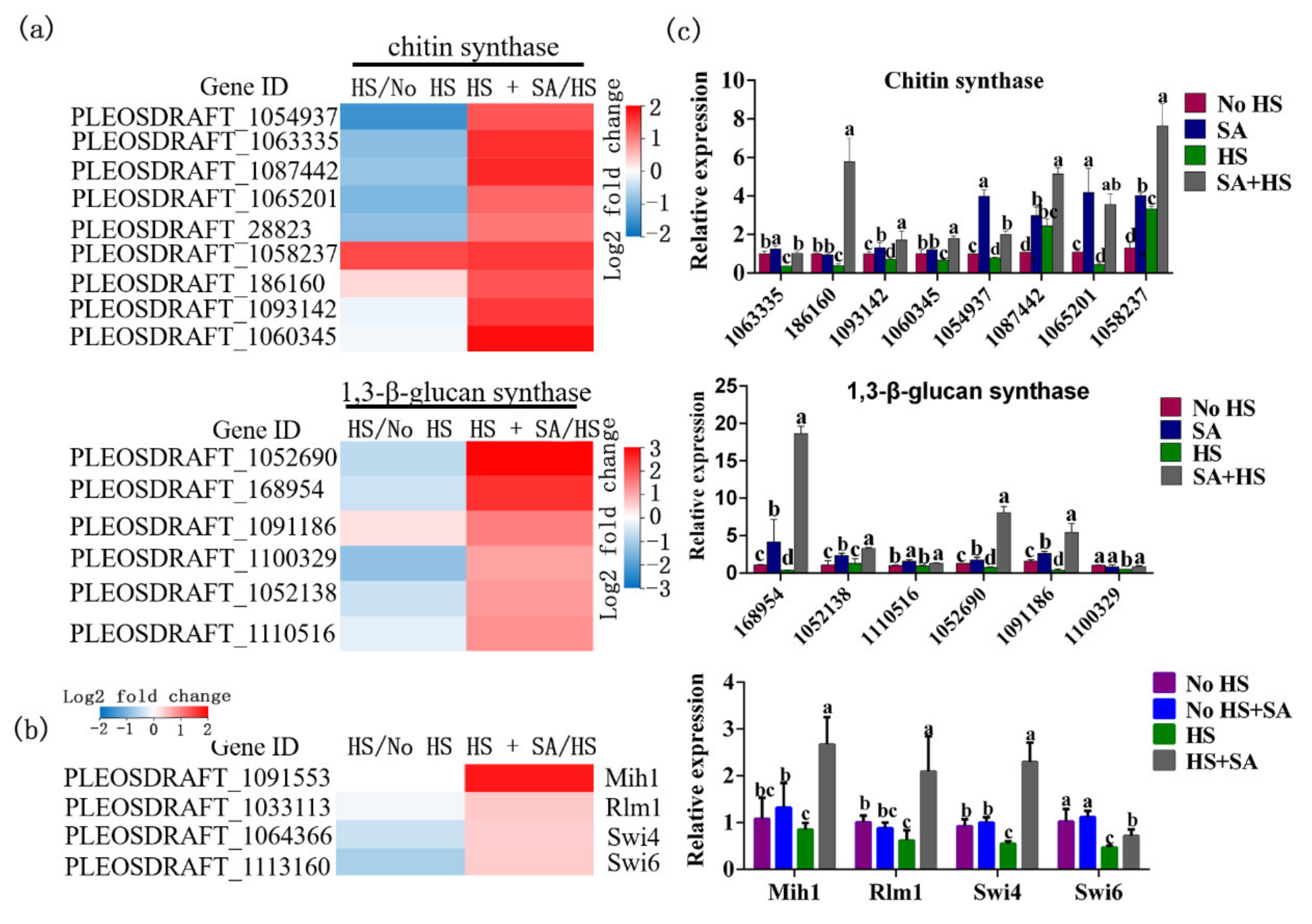
3.6. Transcriptome Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Arora, K.; Goswami, S.; Sakhare, A.; Singh, B.; Chinnusamy, V.; Praveen, S. MAPK Enzymes: A ROS activated signaling sensors involved in modulating heat stress response, tolerance and grain stability of wheat under heat stress. 3 Biotech 2020, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Vilaprinyo, E.; Belli, G.; Herrero, E.; Salvado, B.; Sorribas, A.; Altés, G.; Alves, R. Quantitative operating principles of yeast metabolism during adaptation to heat stress. Cell Rep. 2018, 22, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and Biological Properties of Satureja cuneifolia and Thymus spinulosus Ten.: Two Wild Officinal Species of Conservation Concern in Apulia (Italy). A Preliminary Survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Fan, L.; Loescher, W.; Duan, W.; Liu, G.J.; Cheng, J.S.; Luo, H.B.; Li, S.H. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; Xu, S.H.; Ding, P.T.; Wang, D.M.; Cheng, Y.T.; He, J.; Gao, M.H.; Xu, F.; Li, Y.; Zhu, Z.H.; et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.N.; Qi, M.; Mei, C.S. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004, 40, 909–919. [Google Scholar] [CrossRef]
- Liu, R.; Cao, P.F.; Ren, A.; Wang, S.L.; Yang, T.; Zhu, T.; Shi, L.; Zhu, J.; Jiang, A.L.; Zhao, M.W. SA inhibits complex III activity to generate reactive oxygen species and thereby induces GA overproduction in Ganoderma lucidum. Redox Biol. 2018, 16, 388–400. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bumm, E.; Dixon, K. Acetylsalicylic (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Sánchez, C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl. Microbiol. Biotechnol. 2000, 85, 1321–1337. [Google Scholar] [CrossRef]
- Reisa, F.S.; Lillian Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Z.H.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. High-temperature induced cell wall integrity and structure disruption of Pleurotus ostreatus mycelia. Appl. Microbiol. Biotechnol. 2018, 102, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.D.; Zhao, M.R.; Huang, C.Y.; Wu, X.L.; Zhang, J.X. Nitric oxide improves the tolerance of Pleurotus ostreatus to heat stress by inhibiting mitochondrial aconitase. Appl. Environ. Microbiol. 2020, 86, e02303-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.Y.; Zhao, M.R.; Huang, C.Y.; Zhang, L.J.; Zhang, J.X. Trehalose alleviates high-temperature stress in Pleurotus ostreatus by affecting central carbon metabolism. Microb. Cell Fact. 2021, 20, 82. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, M.; Wu, X.; Zhang, J. Metabolic response of Pleurotus ostreatus to continuous heat stress. Front. Microbiol. 2020, 10, 3148. [Google Scholar] [CrossRef]
- Ren, A.; Liu, R.; Miao, Z.G.; Zhang, X.; Cao, P.F.; Chen, T.X.; Li, C.Y.; Shi, L.; Jiang, A.L.; Zhao, M.W. Hydrogen-rich water regulates effects of ROS balance on morphology, growth and secondary metabolism via glutathione peroxidase in Ganoderma lucidum. Environ. Microbiol. 2017, 19, 566–583. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Krishnan, N.; Dickman, M.B.; Becker, D.F. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic. Biol. Med. 2007, 144, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Klein, D.A.; Paschke, M.W. Filamentous fungi: The indeterminate lifestyle and microbial ecology. Microbial Ecol. 2004, 47, 224–235. [Google Scholar] [CrossRef]
- Hu, Y.R.; Xu, W.Z.; Hu, S.S.; Lian, L.D.; Zhu, J.; Shi, L.; Zhao, M.W. Glsnf1-mediated metabolic rearrangement participates in coping with heat stress and influencing secondary metabolism in Ganoderma lucidum. Free Radic. Biol. Med. 2020, 147, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Planchais, S.; Djafi, N.; Martinec, J.; Burketova, L.; Valentova, O.; Zachowski, A.; Ruelland, E. Phosphoglycerolipids are master players in plant hormone signal transduction. Plant Cell Rep. 2013, 32, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Chen, H.; Zhang, M.; Wen, Q.; Qiu, L.Y.; Shen, J.W. Identification and expression analysis of Pofst3 suggests a role during Pleurotus ostreatus primordia formation. Fungal Biol. 2019, 123, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Song, C.; Chen, Q.; Wu, X.L.; Zhang, J.X.; Huang, C.Y. Heat stress induces apoptotic-like cell death in two Pleurotus species. Curr. Microbiol. 2014, 69, 611–616. [Google Scholar] [CrossRef]
- Mirshekari, M.; Einali, A.; Jafar Valizadeh, J. Metabolic changes and activity pattern of antioxidant enzymes induced by salicylic acid treatment in green microalga Dunaliella salina under nitrogen deficiency. J. Appl. Phycol. 2019, 31, 1709–1719. [Google Scholar] [CrossRef]
- Cui, W.T.; Li, L.; Gao, Z.Z.; Wu, H.H.; Xie, Y.J.; Shen, W.B. Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J. Exp. Bot. 2012, 63, 5521–5534. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential physiological, transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, H.; Franchina, D.G.; Guerra, L.; Bonetti, L.; Soriano-Baguet, L.; Grusdat, M. Glutathione restricts serine metabolism to preserve regulatory T cell function. Cell Metab. 2020, 31, 920–936. [Google Scholar] [CrossRef]
- Xu, H.; Ma, S.; Liu, Q.; Huang, L.; Wu, P.; Liu, X.; Huang, Y.; Wang, X.L.; Xu, H.; Lou, K.Y.; et al. A naphthalimide-aminal-based pH-sensitive fluorescent donor for lysosome-targeted formaldehyde release and fluorescence turn-on readout. Chem. Commun. 2019, 55, 7053–7056. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.; Lane, A.N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Zhang, H.X.; et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Lastres, D.; Fontanesi, F.; García-Consuegra, I.; Martín, M.A.; Arenas, J.; Barrientos, A.; Ugaide, C. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 2012, 15, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.S.; Tang, K.; Lu, Y.J.; Tian, Z.; Huang, Z.L.; Wang, M.J.; Zhao, J.P.; Xie, J.G. Revealing the role of glycerophospholipid metabolism in asthma through plasma lipidomics. Clin. Chim. Acta 2021, 513, 34–42. [Google Scholar] [CrossRef]
- Hofbauer, H.F.; Schopf, F.H.; Schleifer, H.; Knittelfelder, O.L.; Pieber, B.; Rechberger, G.N.; Wolinski, H.; Gaspar, M.L.; Oliver Kappe, C.; Stadlmann, J.; et al. Regulation of gene expression through a transcriptional repressor that senses acyl-chain length in membrane phospholipids. Dev. Cell 2014, 29, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Khamari, R.; Trinh, A.; Gabert, P.E.; Corazao-Rozas, P.; Riveros-Cruz, S.; Balayssac, S.; Malet-Martino, M.; Dekiouk, S.; Curt, M.J.C.; Maboudou, P.; et al. Glucose metabolism and NRF2 coordinate the antioxidant response in melanoma resistant to MAPK inhibitors. Cell Death Dis. 2018, 9, 325. [Google Scholar] [CrossRef] [Green Version]
- Levin, D.E. Cell Wall Integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef] [Green Version]
- Maus, M.; Cuk, M.; Patel, B.; Lian, J.; Ouimet, M.; Kaufmann, U.; Yang, J.; Horvath, R.; Horing-Do, H.T.; Chrzanowska-Lightowlers, Z.M.; et al. Store-operated Ca2+ entry controls induction of lipolysis and the transcriptional reprogramming to lipid metabolism. Cell Metab. 2017, 25, 698–712. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Liu, Z.; Yu, X.; Ye, P.; Liu, H.; Xue, X.; Yang, L.X.; Li, Z.T.; Wu, Y.; Fang, C.; et al. Direct gating of the TRPM2 channel by cADPR via specific interactions with the ADPR binding pocket. Cell Rep. 2019, 27, 3684–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Han, W.; Qin, A.; Wang, Z.; Xu, J.; Qian, Y. The emerging role of Hippo signaling pathway in regulating osteoclast formation. J. Cell. Physiol. 2018, 233, 4606–4617. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Feder, M.E.; Kang, L. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Mol. Ecol. 2018, 27, 3040–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.-R.; Wang, Y.; Chen, Y.-J.; Chai, Q.-Q.; Dong, H.-Z.; Shen, J.-W.; Qi, Y.-C.; Wang, F.-Q.; Wen, Q. Salicylic Acid Enhances Heat Stress Resistance of Pleurotus ostreatus (Jacq.) P. Kumm through Metabolic Rearrangement. Antioxidants 2022, 11, 968. https://doi.org/10.3390/antiox11050968
Hu Y-R, Wang Y, Chen Y-J, Chai Q-Q, Dong H-Z, Shen J-W, Qi Y-C, Wang F-Q, Wen Q. Salicylic Acid Enhances Heat Stress Resistance of Pleurotus ostreatus (Jacq.) P. Kumm through Metabolic Rearrangement. Antioxidants. 2022; 11(5):968. https://doi.org/10.3390/antiox11050968
Chicago/Turabian StyleHu, Yan-Ru, Yue Wang, Yu-Jie Chen, Qian-Qian Chai, Hao-Zhe Dong, Jin-Wen Shen, Yuan-Cheng Qi, Feng-Qin Wang, and Qing Wen. 2022. "Salicylic Acid Enhances Heat Stress Resistance of Pleurotus ostreatus (Jacq.) P. Kumm through Metabolic Rearrangement" Antioxidants 11, no. 5: 968. https://doi.org/10.3390/antiox11050968
APA StyleHu, Y.-R., Wang, Y., Chen, Y.-J., Chai, Q.-Q., Dong, H.-Z., Shen, J.-W., Qi, Y.-C., Wang, F.-Q., & Wen, Q. (2022). Salicylic Acid Enhances Heat Stress Resistance of Pleurotus ostreatus (Jacq.) P. Kumm through Metabolic Rearrangement. Antioxidants, 11(5), 968. https://doi.org/10.3390/antiox11050968







