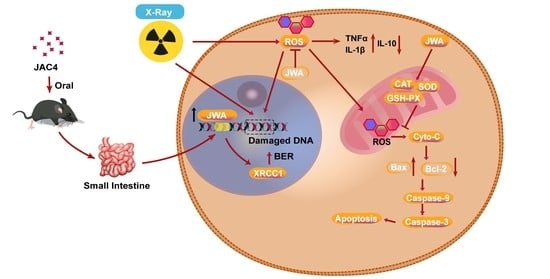
JAC4 Protects from X-ray Radiation-Induced Intestinal Injury by JWA-Mediated Anti-Oxidation/Inflammation Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. JAC4 Synthesis and Preparation
2.2. Animals and Irradiation
2.3. Cell Culture and Irradiation
2.4. Histopathological Score of the Small Intestine
2.5. Intestinal Permeability Assay
2.6. Western Blotting Analysis
2.7. Intracellular ROS Assay
2.8. Flow Cytometric Assay
2.9. Biochemical Analysis
2.10. Hoechst Staining
2.11. Measurement of Mitochondrial Membrane Potential
2.12. TNF-α, IL-1β, and IL-10 Assay
2.13. Immunofluorescence Assay
2.14. The Cytochrome C Release Assay
2.15. Statistics Analysis
3. Results
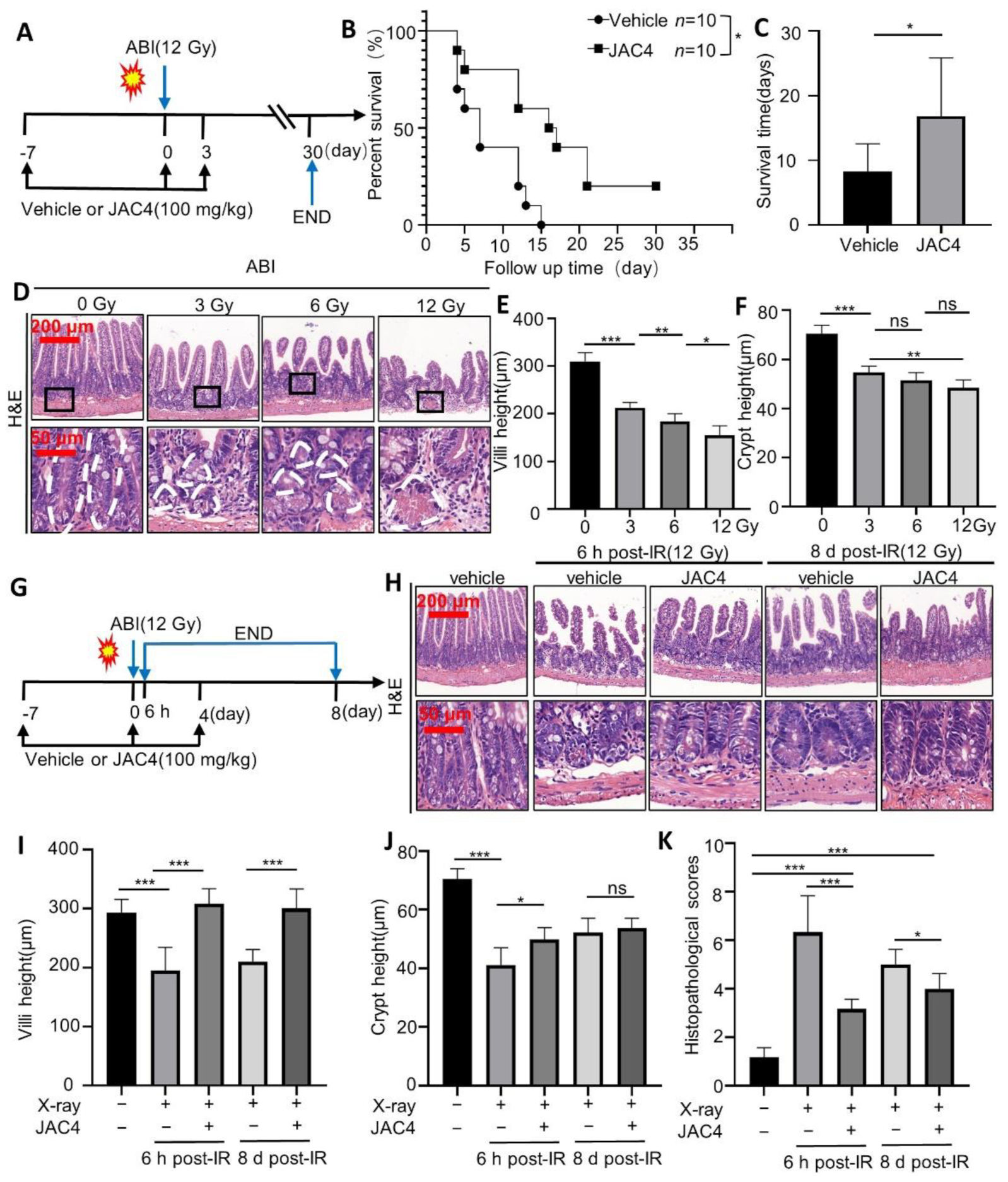
3.1. JAC4 Improves the Survival Rate and Reduces the Damages of Intestinal Epithelium after ABI in Mice
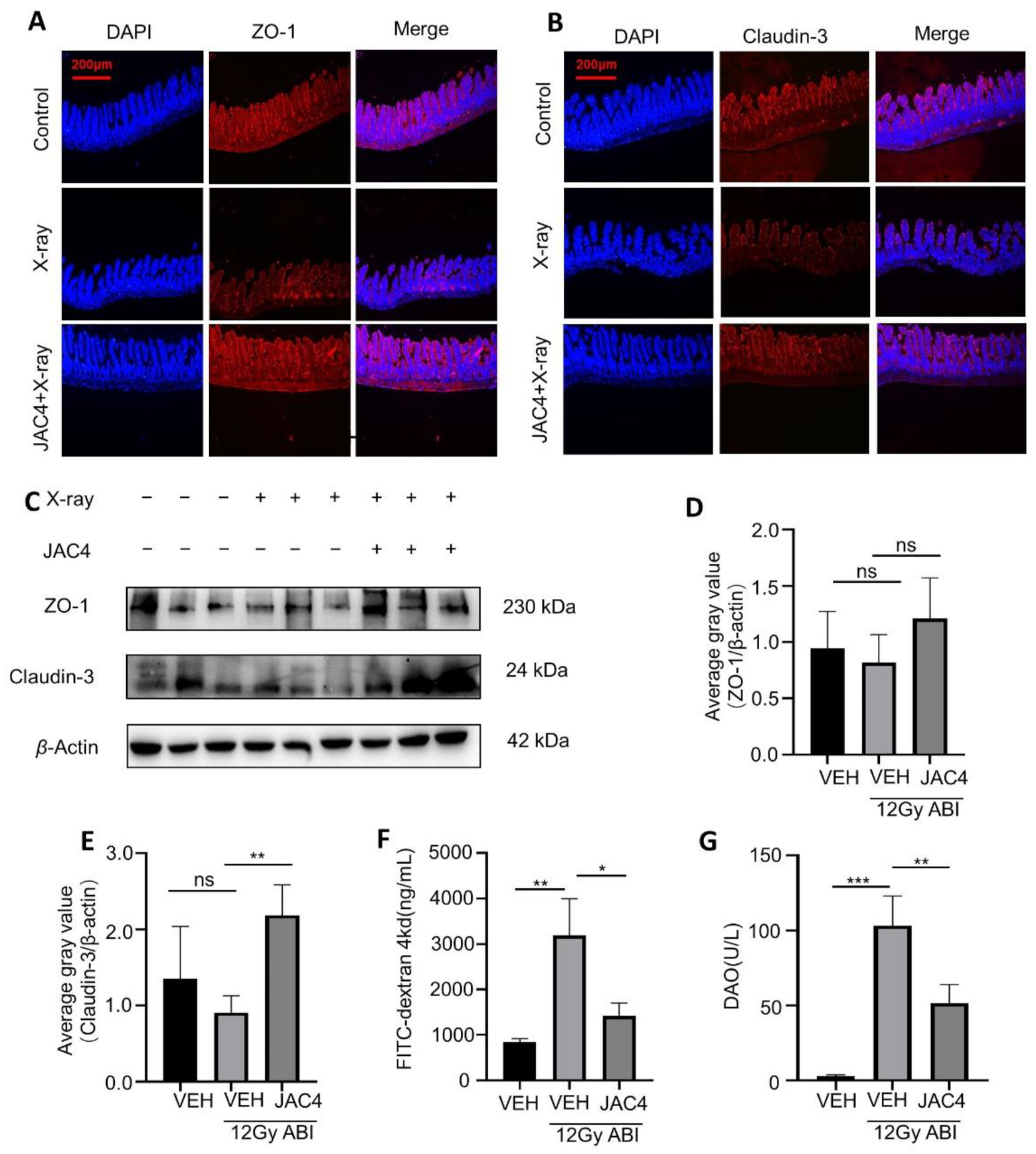
3.2. JAC4 Improves the Intestinal Mucosal Barrier from Radiation Damage in Mice
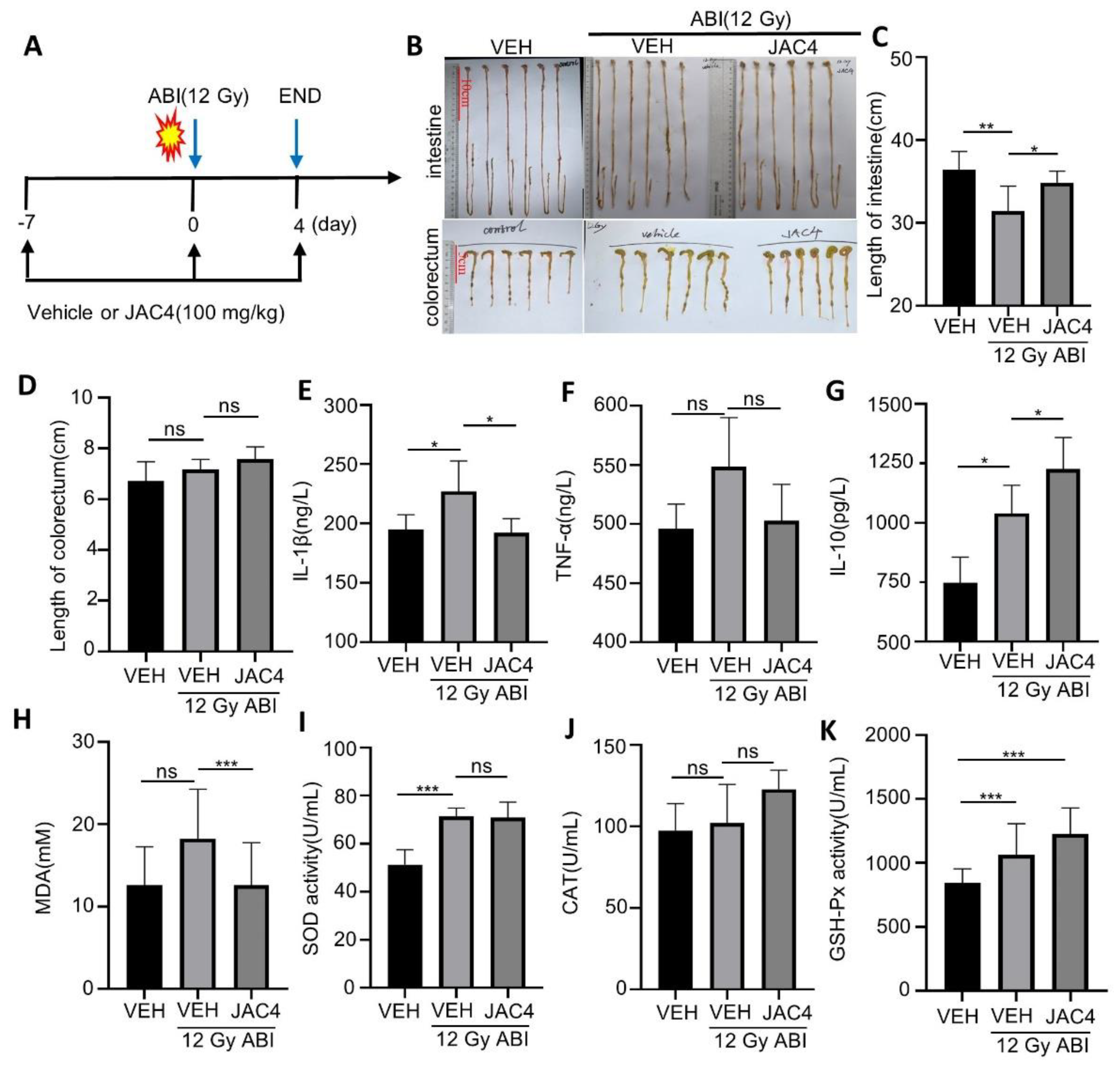
3.3. JAC4 Inhibits X-ray-Triggered Inflammation and Oxidative Stress
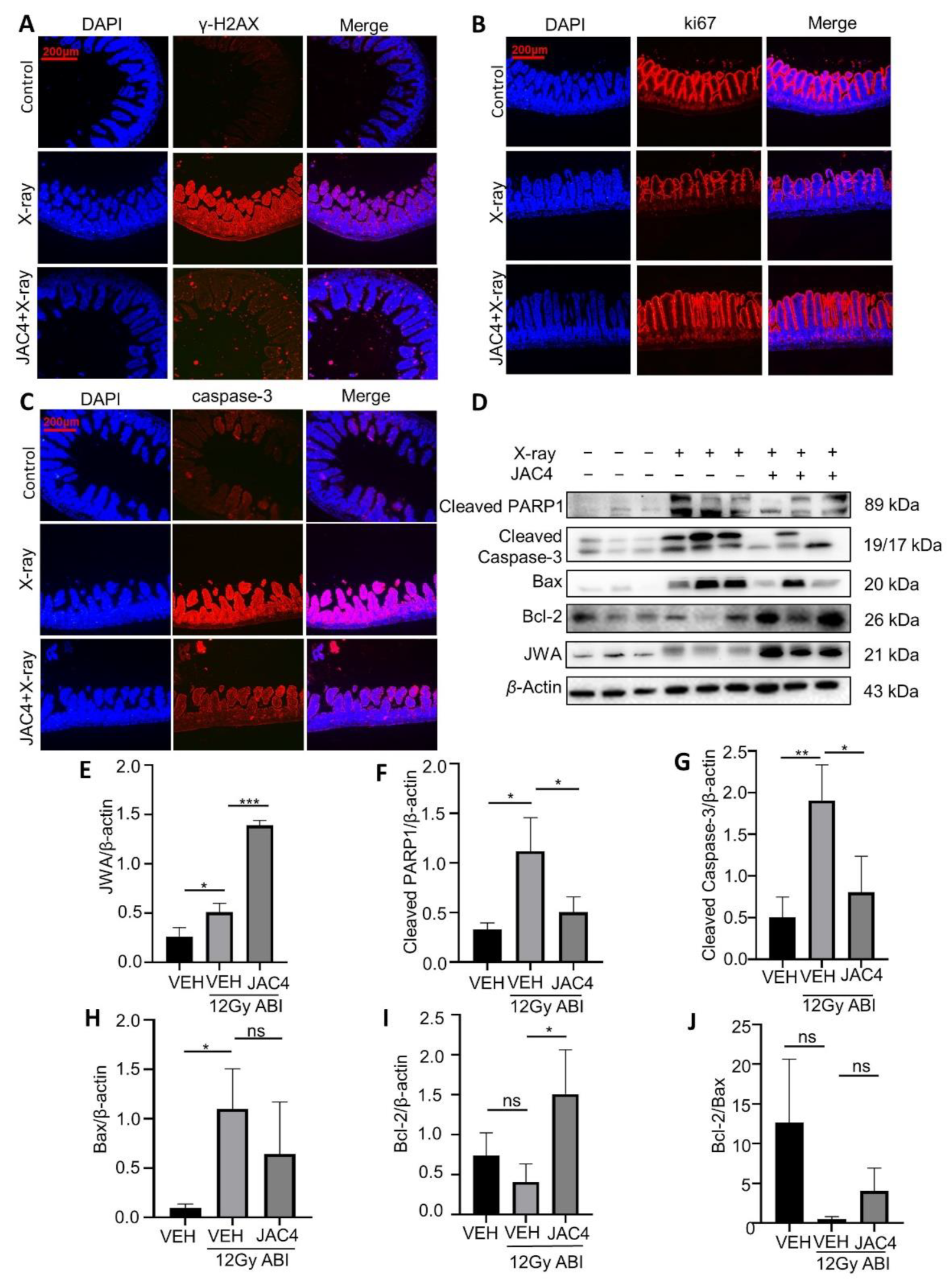
3.4. JAC4 Attenuates X-ray-Triggered DNA Damage and Apoptosis of the Small Intestinal Epithelial Cells in Mice
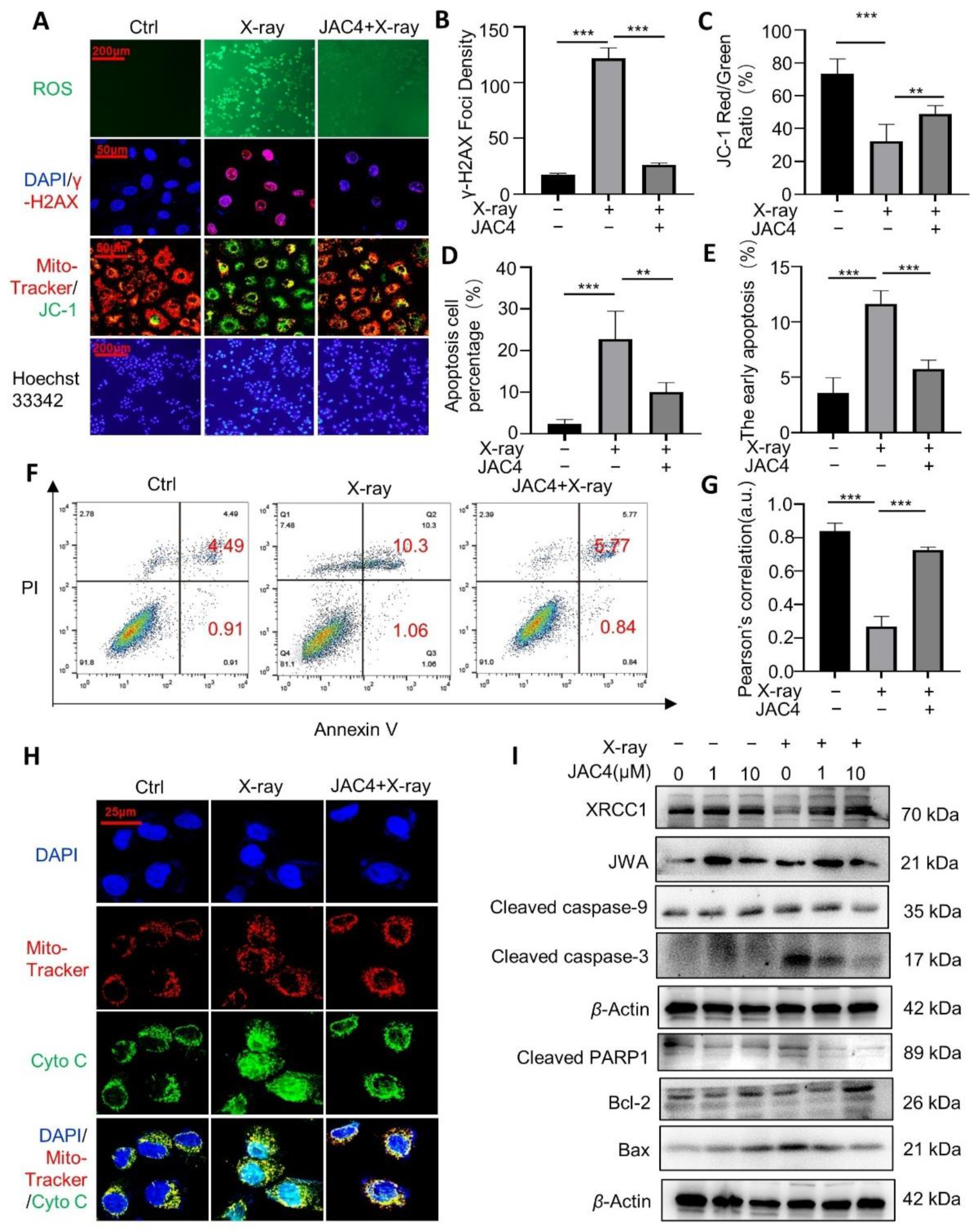
3.5. JAC4 Protects ICE-6 Cells from X-ray-Triggered DNA Damage/Apoptosis through Mitochondria Signaling
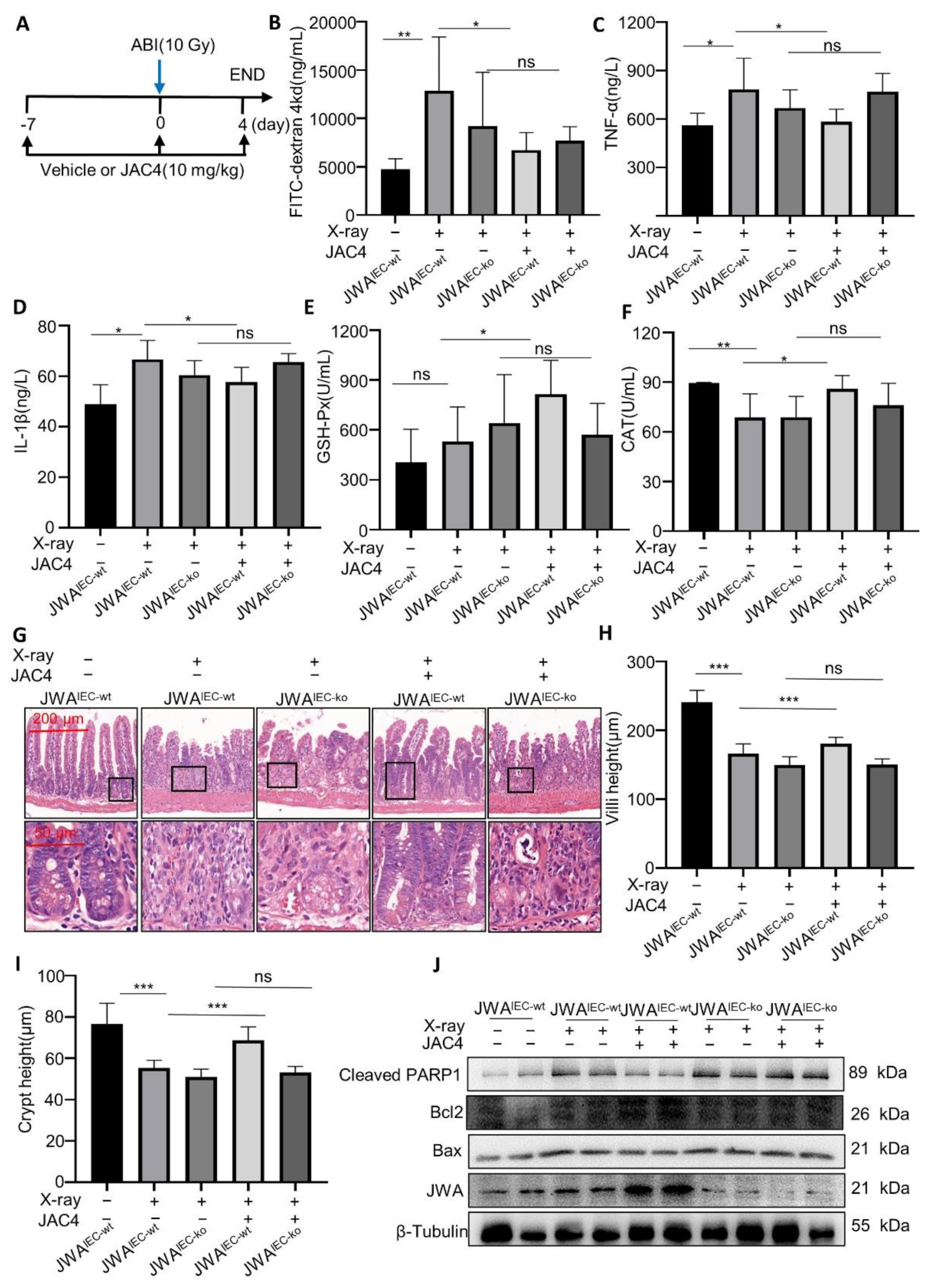
3.6. JAC4 Protects Mice from X-ray-Induced Intestinal Barrier Damage Dependent upon Intestinal Epithelium JWA Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Dong, K.; Guan, J.; He, J. Hydrogen attenuates radiation-induced intestinal damage by reducing oxidative stress and inflammatory response. Int. Immunopharmacol. 2020, 84, 106517. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pérez, A.; Yilmaz, M.; Perna, C.; de la Rosa, S.; Djouder, N. URI is required to maintain intestinal architecture during ionizing radiation. Science 2019, 364, eaaq1165. [Google Scholar] [CrossRef]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Rezapoor, S.; Shabeeb, D.; Musa, A.E.; Najafi, M.; et al. Reduction-oxidation (redox) system in radiation-induced normal tissue injury: Molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018, 20, 975–988. [Google Scholar] [CrossRef]
- Garg, S.; Sadhukhan, R.; Banerjee, S.; Savenka, A.V.; Basnakian, A.G.; McHargue, V.; Wang, J.; Pawar, S.A.; Ghosh, S.P.; Ware, J.; et al. Gamma-Tocotrienol Protects the Intestine from Radiation Potentially by Accelerating Mesenchymal Immune Cell Recovery. Antioxid. Basel 2019, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Shen, Q.; Mao, W.G.; Li, A.P.; Ye, J.; Liu, Q.Z.; Zou, C.P.; Zhou, J.W. JWA, a novel signaling molecule, involved in the induction of differentiation of human myeloid leukemia cells. Biochem. Biophys. Res. Commun. 2006, 341, 440–450. [Google Scholar] [CrossRef]
- Chen, R.; Qiu, W.; Liu, Z.; Cao, X.; Zhu, T.; Li, A.; Wei, Q.; Zhou, J. Identification of JWA as a novel functional gene responsive to environmental oxidative stress induced by benzo[a]pyrene and hydrogen peroxide. Free Radic. Biol. Med. 2007, 42, 1704–1714. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, X.; Xu, J.; Wen, Y.; Li, A.; Lu, M.; Zhou, J. Astrocytic JWA deletion exacerbates dopaminergic neurodegeneration by decreasing glutamate transporters in mice. Cell Death Dis. 2018, 9, 352. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Che, Z.; Shu, C.; Chen, D.; Ding, K.; Li, A.; Zhou, J. JP3 enhances the toxicity of cisplatin on drug-resistant gastric cancer cells while reducing the damage to normal cells. J. Cancer 2021, 12, 1894–1906. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, Y.; Bryant, S.H. Investigating the correlations among the chemical structures, bioactivity profiles and molecular targets of small molecules. Bioinform. Oxf. Engl. 2010, 26, 2881–2888. [Google Scholar] [CrossRef]
- Johnke, R.M.; Sattler, J.A.; Allison, R.R. Radioprotective agents for radiation therapy: Future trends. Future Oncol. 2014, 10, 2345–2357. [Google Scholar] [CrossRef]
- Gong, Z.; Shi, Y.; Zhu, Z.; Li, X.; Ye, Y.; Zhang, J.; Li, A.; Li, G.; Zhou, J. JWA deficiency suppresses dimethylbenz[a]anthracene-phorbol ester induced skin papillomas via inactivation of MAPK pathway in mice. PLoS ONE 2012, 7, e34154. [Google Scholar] [CrossRef]
- Chiu, C.J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef]
- Chen, J.; Chen, R.; Huang, S.; Zu, B.; Zhang, S. Atezolizumab alleviates the immunosuppression induced by PD-L1-positive neutrophils and improves the survival of mice during sepsis. Mol. Med. Rep. 2021, 23, 144. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Dong, X.; Mei, L.; Zhao, M.; Leng, Z.; Hu, H.; Li, L.; Gu, Z.; Zhao, Y. Clinically Approved Carbon Nanoparticles with Oral Administration for Intestinal Radioprotection via Protecting the Small Intestinal Crypt Stem Cells and Maintaining the Balance of Intestinal Flora. Small 2020, 16, e1906915. [Google Scholar] [CrossRef]
- Menon, S.S.; Uppal, M.; Randhawa, S.; Cheema, M.S.; Aghdam, N.; Usala, R.L.; Ghosh, S.P.; Cheema, A.K.; Dritschilo, A. Radiation Metabolomics: Current Status and Future Directions. Front. Oncol. 2016, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Seed, T.M.; Olabisi, A.O. Drug discovery strategies for acute radiation syndrome. Expert Opin. Drug Discov. 2019, 14, 701–715. [Google Scholar] [CrossRef] [Green Version]
- Merritt, A.J.; Potten, C.S.; Kemp, C.J.; Hickman, J.A.; Balmain, A.; Lane, D.P.; Hall, P.A. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994, 54, 614–617. [Google Scholar]
- Segers, C.; Verslegers, M.; Baatout, S.; Leys, N.; Lebeer, S.; Mastroleo, F. Food Supplements to Mitigate Detrimental Effects of Pelvic Radiotherapy. Microorganisms 2019, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. CR 2018, 37, 266. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Zhou, X.; Wang, H.; Yang, Y.; Zhang, J.; Wang, H. The protective effects of Resveratrol against radiation-induced intestinal injury. BMC Complement. Altern. Med. 2017, 17, 410. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Dong, Y.; Hou, Q.; Wu, J.; Zhang, W.; Tian, H.; Li, D. The protective effects of XH-105 against radiation-induced intestinal injury. J. Cell. Mol. Med. 2019, 23, 2238–2247. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Bian, C.; Wang, X.; Liu, X.; Ahmad Kassab, M.; Yu, Y.; Yu, X. ADP-ribosylation of histone variant H2AX promotes base excision repair. EMBO J. 2021, 40, e104542. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Chen, L.; Su, Q.; Wang, Y.; Guo, Z.; Lu, Y.; Liu, B.; Qin, S. Radiation-induced intestinal damage: Latest molecular and clinical developments. Future Oncol. 2019, 15, 4105–4118. [Google Scholar] [CrossRef]
- Al-Qadami, G.; Van Sebille, Y.; Le, H.; Bowen, J. Gut microbiota: Implications for radiotherapy response and radiotherapy-induced mucositis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 485–496. [Google Scholar] [CrossRef]
- Hoffmann, M.; Waller, K.; Last, A.; Westhuyzen, J. A critical literature review on the use of bellyboard devices to control small bowel dose for pelvic radiotherapy. Rep. Pract. Oncol. Radiother. J. 2020, 25, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, K.; Wang, Z.; Li, A.; Chen, D.; Zhou, J. JWA regulates energy metabolism reprogramming and inhibits migration of pancreatic cancer cell. J. Nanjing Med. Univ. Nat. Sci. 2019, 39, 1728–1736. [Google Scholar]
- Xu, W.; Chen, Q.; Wang, Q.; Sun, Y.; Wang, S.; Li, A.; Xu, S.; Røe, O.D.; Wang, M.; Zhang, R.; et al. JWA reverses cisplatin resistance via the CK2-XRCC1 pathway in human gastric cancer cells. Cell Death Dis. 2014, 5, e1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wu, X.; Chen, Y.; Zhang, J.; Ding, J.; Zhou, Y.; He, S.; Tan, Y.; Qiang, F.; Bai, J.; et al. Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2987–2996. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shen, X.; Wang, X.; Li, A.; Wang, P.; Jiang, P.; Zhou, J.; Feng, Q. EGCG regulates the cross-talk between JWA and topoisomerase IIα in non-small-cell lung cancer (NSCLC) cells. Sci. Rep. 2015, 5, 11009. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Qian, C.; Xie, Y.; Huang, X.; Chen, J.; Ren, Y.; Fu, Z.; Li, Y.; Zeng, T.; Yang, F.; et al. JWA suppresses proliferation in trastuzumab-resistant breast cancer by downregulating CDK12. Cell Death Discov. 2021, 7, 306. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, D.; Zhai, Z.; Chen, J.; Li, A.; Liang, Y.; Zhou, J. JAC1 suppresses proliferation of breast cancer through the JWA/p38/SMURF1/HER2 signaling. Cell Death Discov. 2021, 7, 85. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Huang, Y.; Xia, X.; Zhang, J.; Zhou, Y.; Tan, Y.; He, S.; Qiang, F.; Li, A.; et al. JWA suppresses tumor angiogenesis via Sp1-activated matrix metalloproteinase-2 and its prognostic significance in human gastric cancer. Carcinogenesis 2014, 35, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Zhang, J.; Wu, J.; Shen, L.; Zeng, J.; Ding, J.; Wu, Y.; Gong, Z.; Li, A.; Xu, S.; et al. JWA regulates melanoma metastasis by integrin alphaVbeta3 signaling. Oncogene 2010, 29, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Cheng, W.; Qu, W.; Shao, G.; Liu, S. Antibiotic Alleviates Radiation-Induced Intestinal Injury by Remodeling Microbiota, Reducing Inflammation, and Inhibiting Fibrosis. ACS Omega 2020, 5, 2967–2977. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, H.; Li, Y.; Zhou, L.; Zhao, S.; Luo, D.; Zheng, Q.; Dong, J.; Zhao, Y.; Zhang, X.; et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 2017, 9, 448–461. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Liu, J.; Li, X.; Wang, L.; Hu, L.; Li, A.; Zhou, J. JAC4 Protects from X-ray Radiation-Induced Intestinal Injury by JWA-Mediated Anti-Oxidation/Inflammation Signaling. Antioxidants 2022, 11, 1067. https://doi.org/10.3390/antiox11061067
Zhou Y, Liu J, Li X, Wang L, Hu L, Li A, Zhou J. JAC4 Protects from X-ray Radiation-Induced Intestinal Injury by JWA-Mediated Anti-Oxidation/Inflammation Signaling. Antioxidants. 2022; 11(6):1067. https://doi.org/10.3390/antiox11061067
Chicago/Turabian StyleZhou, Yan, Jingwen Liu, Xiong Li, Luman Wang, Lirong Hu, Aiping Li, and Jianwei Zhou. 2022. "JAC4 Protects from X-ray Radiation-Induced Intestinal Injury by JWA-Mediated Anti-Oxidation/Inflammation Signaling" Antioxidants 11, no. 6: 1067. https://doi.org/10.3390/antiox11061067
APA StyleZhou, Y., Liu, J., Li, X., Wang, L., Hu, L., Li, A., & Zhou, J. (2022). JAC4 Protects from X-ray Radiation-Induced Intestinal Injury by JWA-Mediated Anti-Oxidation/Inflammation Signaling. Antioxidants, 11(6), 1067. https://doi.org/10.3390/antiox11061067