Antioxidant Effect of Chrysanthemum morifolium (Chuju) Extract on H2O2-Treated L-O2 Cells as Revealed by LC/MS-Based Metabolic Profiling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
3. Test Method
3.1. Cell Viability Assay
3.2. Measurement of MDA Content and SOD, GSH Px, CAT Activities
3.3. Cell Cycle Analysis
3.4. Mitochondrial Membrane Potential Analysis
3.5. Apoptosis Assay
3.6. Reactive Oxygen (ROS) Detection
3.7. UHPLC-QE-MS Untargeted Metabolomics
3.8. Statistics
4. Results and Analysis
4.1. The Effect of H2O2 and Chrysanthemum Extract on Cell Viability
4.2. Protective Effect of Chrysanthemum Extract against Oxidative Damage of Cells
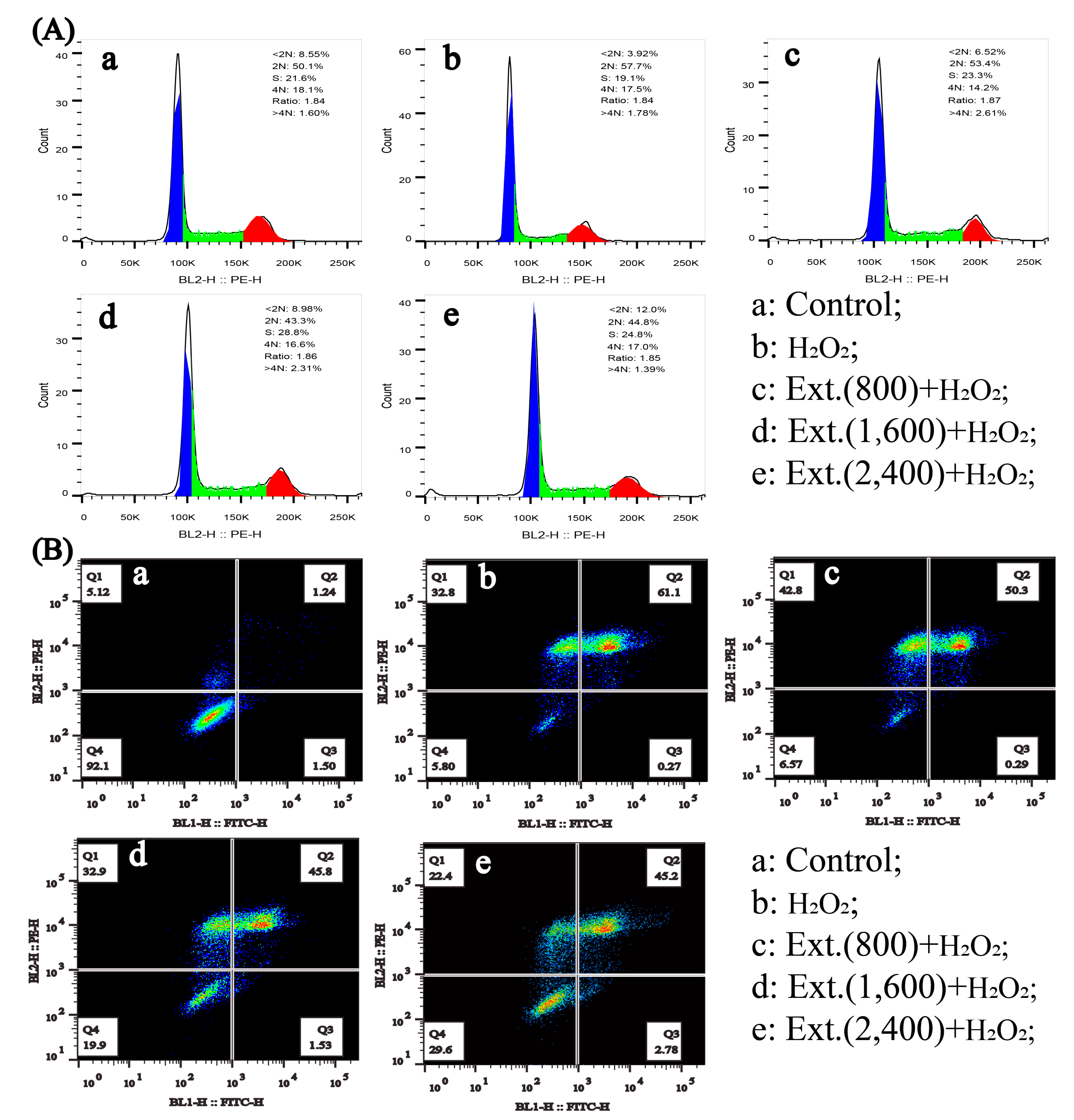
4.2.1. Cell Cycle and Apoptosis
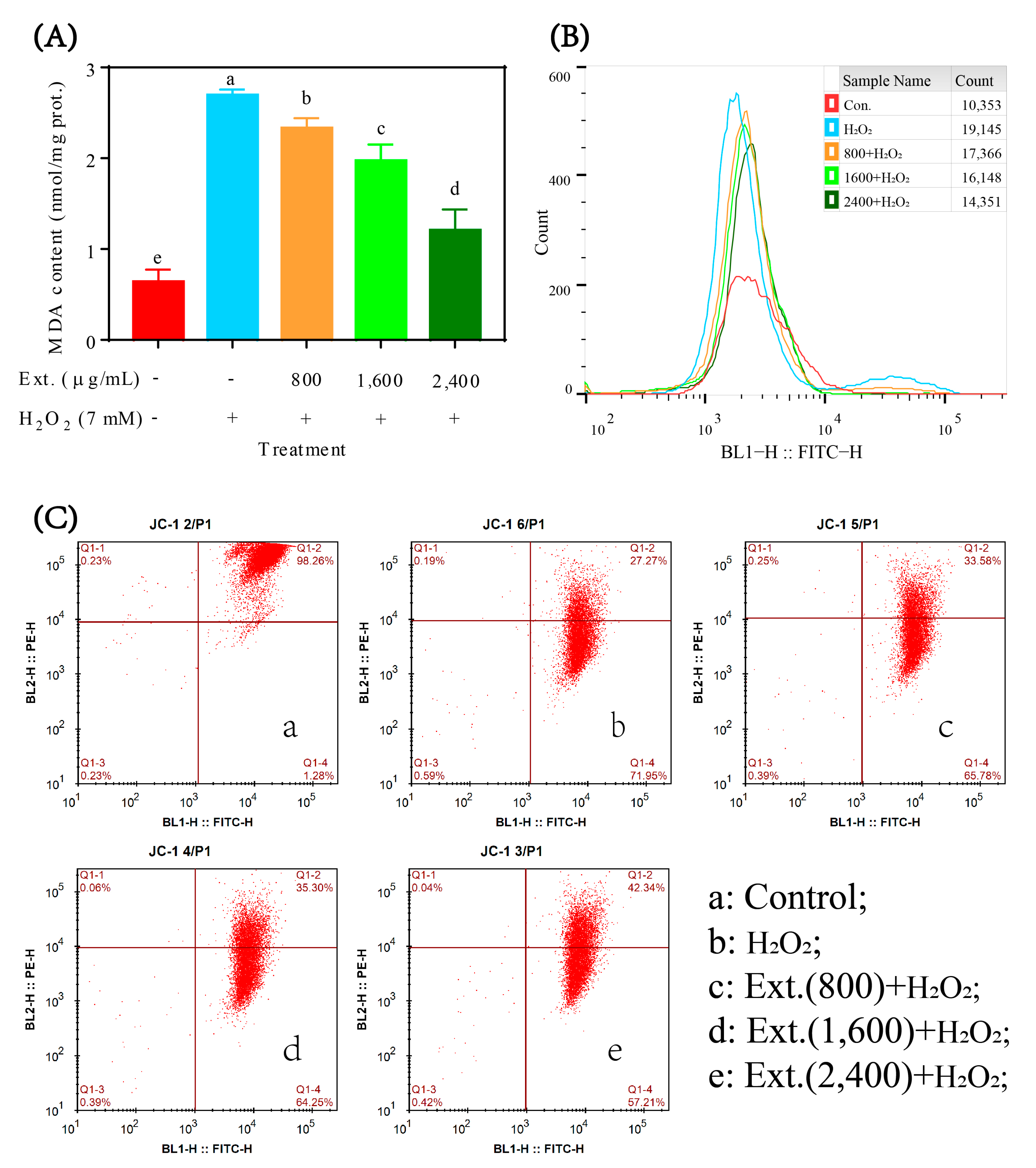
4.2.2. MDA, ROS Content and Mitochondrial Membrane Potential
4.3. Antioxidant Enzyme Activities
4.4. Untargeted Metabolomics Analysis
4.4.1. Principal Component Analysis
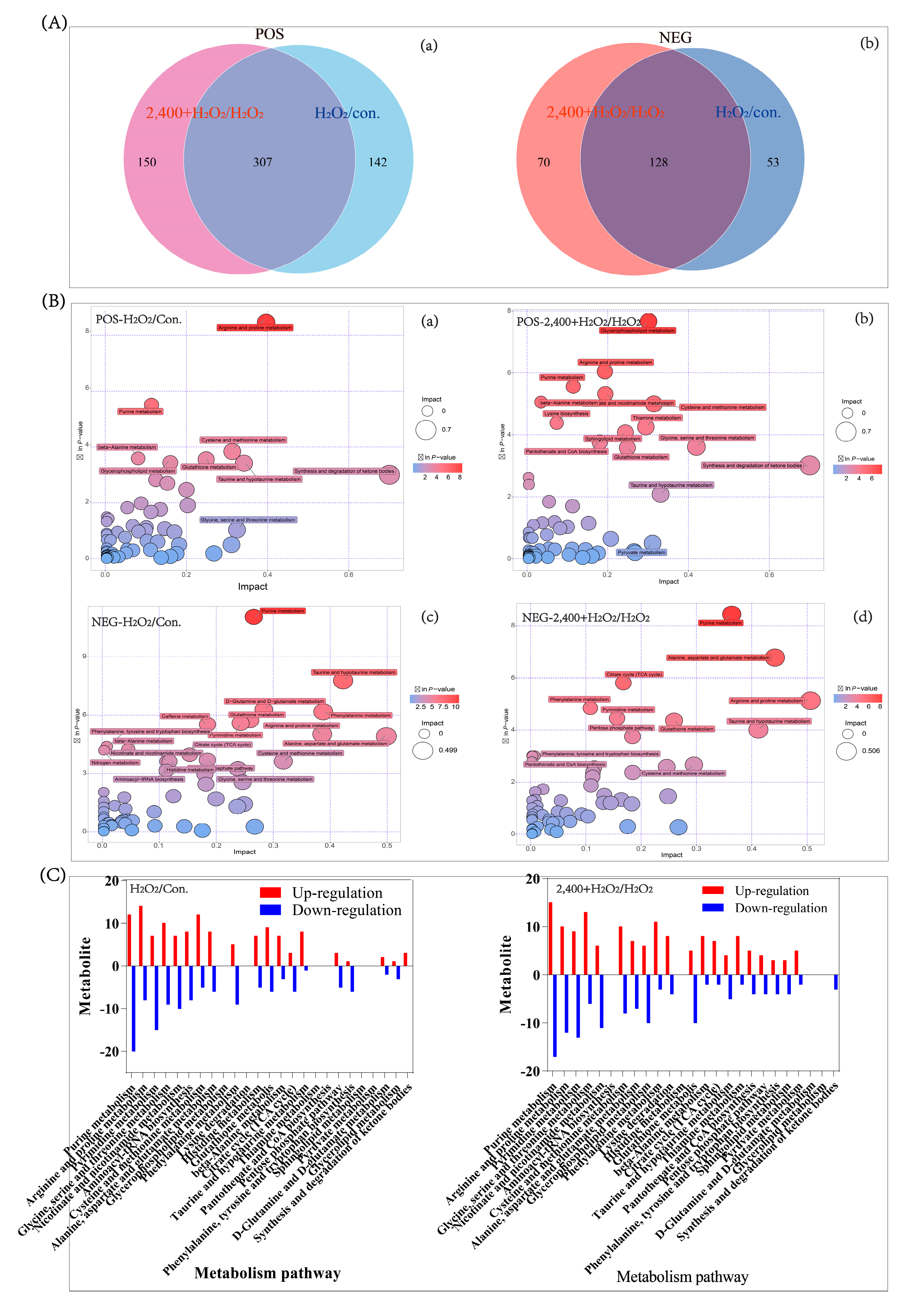
4.4.2. Pathway Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, H.; Jiang, S.; Liu, Y.; Daniyal, M.; Jian, Y.; Peng, C.; Shen, J.; Liu, S.; Wang, W. The flower head of Chrysanthemum morifolium Ramat. (Juhua): A paradigm of flowers serving as Chinese dietary herbal medicine. J. Ethnopharmacol. 2020, 261, 113043. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Kim, S.M.; Jeong, Y.T.; Song, C.H. Hepatoprotective effect of water extract from Chrysanthemum indicum L. flower. Chin. Med. 2013, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Zheng, Q.; Pan, K.; Xu, X. Protective effect of Chrysanthemum morifolium Ramat. ethanol extract on lipopolysaccharide induced acute lung injury in mice. BMC Complement. Med. Ther. 2020, 20, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, C.; Kou, J.; Wang, M.; Rong, X.; Pu, X.; Xie, X.; Han, G.; Pang, X. Chrysanthemi Flos extract alleviated acetaminophen-induced rat liver injury via inhibiting oxidative stress and apoptosis based on network pharmacology analysis. Pharm. Biol. 2021, 59, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kong, S.; Song, F.; Li, L.; Jiang, H. Pharmacokinetic study of luteolin, apigenin, chrysoeriol and diosmetin after oral administration of Flos Chrysanthemi extract in rats. Fitoterapia 2012, 83, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Eid, S.Y.; Alshammari, E.; Ashour, M.L.; Wink, M.; El-Readi, M.Z. Chrysanthemum indicum and Chrysanthemum morifolium: Chemical composition of their essential oils and their potential use as natural preservatives with antimicrobial and antioxidant activities. Foods 2020, 9, 1460. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Cicala, C.; Musciacco, G.; De Feo, V.; Amat, A.G.; Ialenti, A.; Mascolo, N. Phenols, alkaloids and terpenes from medicinal plants with antihypertensive and vasorelaxant activities. A review of natural products as leads to potential therapeutic agents. Nat. Prod. Commun. 2013, 8, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, L.; Luo, J.; Zhang, J. Protection against acute renal injury by naturally occurring medicines which act through Nrf2 signaling pathway. J. Food Biochem. 2021, 45, e13556. [Google Scholar] [CrossRef]
- Jarco, S.; Pilawa, B.; Ramos, P. Free radical scavenging activity of infusions of different medicinal plants for use in obstetrics. Plants 2021, 10, 2016. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Butterweck, V.; Lieflander-Wulf, U.; Winterhoff, H.; Nahrstedt, A. Plasma levels of hypericin in presence of procyanidin B2 and hyperoside: A pharmacokinetic study in rats. Planta Med. 2003, 69, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.M.; Pereira, N.R.; Costa, C.A.; Mann, G.E.; Cordeiro, V.S.; de Moura, R.S.; Brunini, T.M.; Mendes-Ribeiro, A.C.; Resende, A.C. L-arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsia. Hypertens. Res. 2013, 36, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pervin, M.; Unno, K.; Konishi, T.; Nakamura, Y. L-arginine exerts excellent anti-stress effects on stress-induced shortened lifespan, cognitive decline and depression. Int. J. Mol. Sci. 2021, 22, 508. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.S.; Rojas, D.B.; Oliveira, L.; Nunes, M.L.; de Castro, F.L.; Garcia, C.; Gemelli, T.; de Andrade, R.B.; Wannmacher, C.M. Creatine and pyruvate prevent behavioral and oxidative stress alterations caused by hypertryptophanemia in rats. Mol. Cell. Biochem. 2012, 362, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, L.M.; Fernandes de Lima, L.; Ferraz-Bannitz, R.; Takaara, D.; Coimbra Romano, B.; Braga Costa, T.M.; Foss de Freitas, M.C.; Bueno, A.C.; Barbosa Junior, F.; Marliere Navarro, A. Association between creatine kinase activity, oxidative stress and selenoproteins mRNA expression changes after Brazil nut consumption of patients using statins. Clin. Nutr. 2020, 39, 3175–3181. [Google Scholar] [CrossRef]
- Evangelista-Vargas, S.; Santiani, A. Detection of intracellular reactive oxygen species (superoxide anion and hydrogen peroxide) and lipid peroxidation during cryopreservation of alpaca spermatozoa. Reprod. Domest. Anim. 2017, 52, 819–824. [Google Scholar] [CrossRef]
- Rancourt, R.C.; Hayes, D.D.; Chess, P.R.; Keng, P.C.; O’Reilly, M.A. Growth arrest in G1 protects against oxygen-induced DNA damage and cell death. J. Cell. Physiol. 2002, 193, 26–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.S.; Zhao, S.; Yang, Z.; Zhong, C.Q.; Chen, X.; Cai, Q.; Yang, Z.H.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8, 14329. [Google Scholar] [CrossRef] [Green Version]
- Csordas, A.; Wick, G.; Bernhard, D. Hydrogen peroxide-mediated necrosis induction in HUVECs is associated with an atypical pattern of caspase-3 cleavage. Exp. Cell Res. 2006, 312, 1753–1764. [Google Scholar] [CrossRef]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, Z.; Li, X.; Jia, H.; Sun, L.; Tian, C.; Jia, L.; Liu, J. alpha-Tocopherol is an effective Phase II enzyme inducer: Protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Yekta, R.; Dehghan, G.; Rashtbari, S.; Sheibani, N.; Moosavi-Movahedi, A.A. Activation of catalase by pioglitazone: Multiple spectroscopic methods combined with molecular docking studies. J. Mol. Recognit. 2017, 30, e2648. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zhang, J.; Zheng, Z.; Wang, Z. Antioxidant apigenin relieves age-related muscle atrophy by inhibiting oxidative stress and hyperactive mitophagy and Apoptosis in skeletal muscle of mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B. The potential protective effects of phenolic compounds against low-density lipoprotein oxidation. Curr. Pharm. Des. 2017, 23, 2754–2766. [Google Scholar] [CrossRef] [PubMed]
- Neukirch, H.; D’Ambrosio, M.; Sosa, S.; Altinier, G.; Della Loggia, R.; Guerriero, A. Improved anti-inflammatory activity of three new terpenoids derived, by systematic chemical modifications, from the abundant triterpenes of the flowery plant Calendula officinalis. Chem. Biodivers. 2005, 2, 657–671. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef]
- Cavalloni, G.; Peraldo-Neia, C.; Massa, A.; Bergamini, C.; Trentini, A.; De Rosa, G.; Daniele, L.; Ciccosanti, F.; Cervellati, C.; Leone, F.; et al. Proteomic analysis identifies deregulated metabolic and oxidative-associated proteins in Italian intrahepatic cholangiocarcinoma patients. BMC Cancer 2021, 21, 865. [Google Scholar] [CrossRef]
- Popova, N.R.; Gudkov, S.V.; Bruskov, V.I. Natural purine compounds as radioprotective agents. Radiatsionnaya Biol. Radioekol. 2014, 54, 38–49. [Google Scholar]
- Srinivasan, S.; Torres, A.G.; Ribas de Pouplana, L. Inosine in biology and disease. Genes 2021, 12, 600. [Google Scholar] [CrossRef] [PubMed]




| Metabolites | No. | RT (s) | Related Pathway | H2O2/Con. | 2400 + H2O2/H2O2 | ||
|---|---|---|---|---|---|---|---|
| p Value a | FC b | p Value | FC | ||||
| L-Ornithine | C00077 | 515.79 | Arginine biosynthesis | 4.59 × 10−5 | 0.07 | 5.16 × 10−5 | 0.38 |
| L-Citrulline | C00327 | 402.96 | Arginine biosynthesis | 5.16 × 10−5 | 0.38 | 2.11 × 10−2 | 1.40 |
| N-(L-Arginino)succinate | C03406 | 477.96 | Arginine biosynthesis | 2.22 × 10−3 | 0.86 | 1.55 × 10−4 | 1.69 |
| N-Acetylornithine | C00437 | 403.96 | Arginine biosynthesis | 5.71 × 10−4 | 0.57 | 6.40 × 10−3 | 1.40 |
| N-Acetyl-L-glutamate | C00624 | 318.93 | Arginine biosynthesis | 3.07 × 10−4 | 0.36 | 1.40 × 10−2 | 1.72 |
| Creatine | C00300 | 360.33 | Arginine and proline metabolism | 4.37 × 10−5 | 1.28 | 1.40 × 10−2 | 1.06 |
| Creatinine | C00791 | 174.29 | Arginine and proline metabolism | 6.30 × 10−6 | 1.43 | 1.36 × 10−3 | 1.11 |
| Phosphocreatine | C02305 | 450.62 | Arginine and proline metabolism | 3.41 × 10−6 | 0.29 | 8.53 × 10−5 | 0.39 |
| D-Ribose 5-phosphate | C00117 | 410.74 | Purine metabolism | 3.72 × 10−4 | 3.03 | 8.85 × 10−3 | 1.49 |
| Inosine monophosphate (IMP) | C00130 | 22.17 | Purine metabolism | 2.25 × 10−2 | 0.60 | 4.80 × 10−5 | 1.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, G.; Long, M.; Shan, K.; Xie, C.; Yang, R. Antioxidant Effect of Chrysanthemum morifolium (Chuju) Extract on H2O2-Treated L-O2 Cells as Revealed by LC/MS-Based Metabolic Profiling. Antioxidants 2022, 11, 1068. https://doi.org/10.3390/antiox11061068
Zhan G, Long M, Shan K, Xie C, Yang R. Antioxidant Effect of Chrysanthemum morifolium (Chuju) Extract on H2O2-Treated L-O2 Cells as Revealed by LC/MS-Based Metabolic Profiling. Antioxidants. 2022; 11(6):1068. https://doi.org/10.3390/antiox11061068
Chicago/Turabian StyleZhan, Ge, Men Long, Kai Shan, Chong Xie, and Runqiang Yang. 2022. "Antioxidant Effect of Chrysanthemum morifolium (Chuju) Extract on H2O2-Treated L-O2 Cells as Revealed by LC/MS-Based Metabolic Profiling" Antioxidants 11, no. 6: 1068. https://doi.org/10.3390/antiox11061068
APA StyleZhan, G., Long, M., Shan, K., Xie, C., & Yang, R. (2022). Antioxidant Effect of Chrysanthemum morifolium (Chuju) Extract on H2O2-Treated L-O2 Cells as Revealed by LC/MS-Based Metabolic Profiling. Antioxidants, 11(6), 1068. https://doi.org/10.3390/antiox11061068








