Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action
Abstract
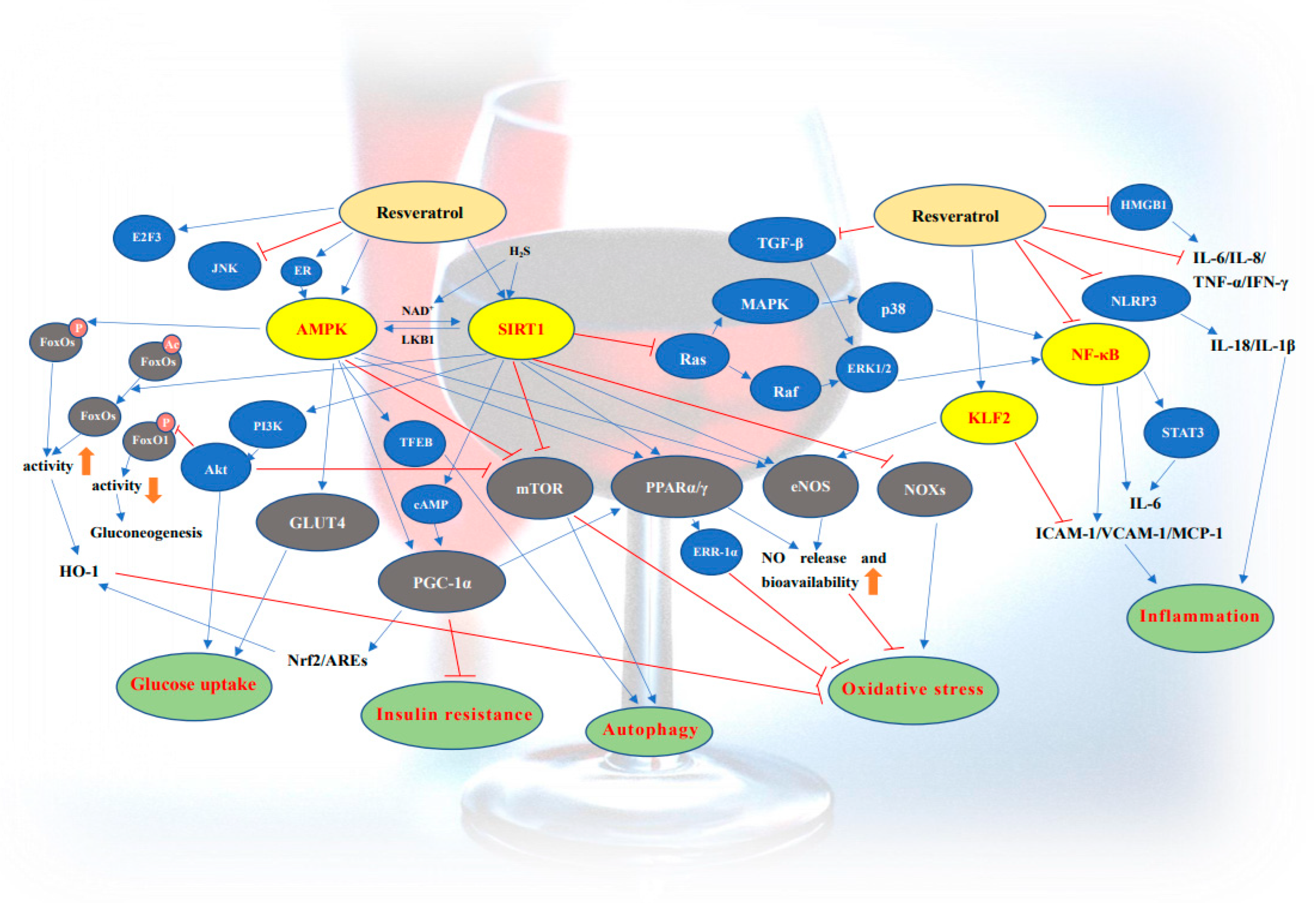
:1. Introduction
2. Resveratrol and Diabetes
2.1. The Activation of SIRT1
2.2. The Activation of AMPK
2.3. Anti-Oxidant Effects
2.4. Improvement of Insulin Resistance
2.5. The Enhancement of Glucose Uptake and Metabolism
2.6. Regulatory Mechanism for Preventing β-Cell Dysfunction
2.7. The Induction of Autophagy
2.8. The Regulation of Lipid Metabolism
3. Resveratrol and Cardiovascular Complications of Diabetes
3.1. Activation of SIRT1
3.2. Activation of AMPK
3.3. Anti-Oxidant Effects
3.4. Anti-Inflammatory Effects
3.5. Improvement of Mitochondrial Function
3.6. Regulation of Lipid Metabolism
3.7. Induction of Autophagy
3.8. Other Molecular Mechanisms
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; De Kretser, D.M.; Linn, T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum. Reprod. Updat. 2018, 24, 86–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.-M.; Liu, N.; Jiang, Y.-P.; Yang, J.-M.; Zheng, J.; Sun, M.; Li, Y.-X.; Sun, T.; Wu, J.; Yu, J.-Q. Vitexin alleviates streptozotocin-induced sexual dysfunction and fertility impairments in male mice via modulating the hypothalamus–pituitary–gonadal axis. Chem. Biol. Interact. 2019, 297, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://diabetesatlas.org (accessed on 23 March 2022).
- Dow, C.; Mancini, F.; Rajaobelina, K.; Boutron-Ruault, M.-C.; Balkau, B.; Bonnet, F.; Fagherazzi, G. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 2018, 33, 141–156. [Google Scholar] [CrossRef]
- Shaikh, A. A Practical Approach to Hypertension Management in Diabetes. Diabetes Ther. 2017, 8, 981–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahrani, A.; Bailey, C.J.; Del Prato, S.; Barnett, A.H. Management of type 2 diabetes: New and future developments in treatment. Lancet 2011, 378, 182–197. [Google Scholar] [CrossRef]
- Pan, M.-H.; Wu, J.-C.; Ho, C.-T.; Lai, C.-S. Antiobesity molecular mechanisms of action: Resveratrol and pterostilbene. BioFactors 2018, 44, 50–60. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Chin, Y.-T.; Ho, Y.; Chen, Y.-R.; Yang, Y.-N.; Yang, Y.-C.; Shih, Y.-J.; Lin, T.-I.; Lin, H.-Y.; Davis, P.J. Resveratrol induces sumoylated COX-2-dependent anti-proliferation in human prostate cancer LNCaP cells. Food Chem. Toxicol. 2018, 112, 67–75. [Google Scholar] [CrossRef]
- Wu, H.; Sheng, Z.-Q.; Xie, J.; Li, R.; Chen, L.; Li, G.-N.; Wang, L.; Xu, B. Reduced HMGB 1-Mediated Pathway and Oxidative Stress in Resveratrol-Treated Diabetic Mice: A Possible Mechanism of Cardioprotection of Resveratrol in Diabetes Mellitus. Oxid. Med. Cell. Longev. 2016, 2016, 9836860. [Google Scholar] [CrossRef] [Green Version]
- Chong, E.; Chang, S.-L.; Hsiao, Y.-W.; Singhal, R.; Liu, S.-H.; Leha, T.; Lin, W.-Y.; Hsu, C.-P.; Chen, Y.-C.; Chen, Y.-J.; et al. Resveratrol, a red wine antioxidant, reduces atrial fibrillation susceptibility in the failing heart by PI3K/AKT/eNOS signaling pathway activation. Heart Rhythm 2015, 12, 1046–1056. [Google Scholar] [CrossRef]
- Alarcón De La Lastra, C.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Su, H.-C.; Hung, L.-M.; Chen, J.-K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1339–E1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palsamy, P.; Subramanian, S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed. Pharmacother. 2008, 62, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Kume, S.; Imaizumi, N.; Koya, D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 2011, 60, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadori, G.; Gautron, L.; Fujikawa, T.; Vianna, C.R.; Elmquist, J.K.; Coppari, R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology 2009, 150, 5326–5333. [Google Scholar] [CrossRef]
- Sharma, S.; Misra, C.S.; Arumugam, S.; Roy, S.; Shah, V.; Davis, J.A.; Shirumalla, R.K.; Ray, A. Antidiabetic activity of resveratrol, a known SIRT1 activator in a genetic model for type-2 diabetes. Phytother. Res. 2011, 25, 67–73. [Google Scholar] [CrossRef]
- Lee, S.-M.; Yang, H.; Tartar, D.M.; Gao, B.; Luo, X.; Ye, S.Q.; Zaghouani, H.; Fang, D. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia 2011, 54, 1136–1146. [Google Scholar] [CrossRef] [Green Version]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Deng, J.-Y.; Hsieh, P.-S.; Huang, J.-P.; Lu, L.-S.; Hung, L.-M. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 2008, 57, 1814–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, T.-C.; Chen, W.-P.; Chi, T.-L.; Kuo, T.-F.; Lee, S.-S.; Cheng, J.-T.; Su, M.-J. Phosphatidylinositol-3-kinase is involved in the antihyperglycemic effect induced by resveratrol in streptozotocin-induced diabetic rats. Life Sci. 2007, 80, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Gao, Y.; Lv, W.; Ma, X.; Hu, J.; Chi, J.; Wang, W.; Wang, Y. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn-Schmiedeberg′s Arch. Pharmacol. 2020, 393, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; Yang, Y. Resveratrol Prevents Retinal Dysfunction by Regulating Glutamate Transporters, Glutamine Synthetase Expression and Activity in Diabetic Retina. Neurochem. Res. 2016, 41, 1050–1064. [Google Scholar] [CrossRef]
- Rehman, K.; Saeed, K.; Munawar, S.M.; Akash, M.S.H. Resveratrol regulates hyperglycemia-induced modulations in experimental diabetic animal model. Biomed. Pharmacother. 2018, 102, 140–146. [Google Scholar] [CrossRef]
- Zhao, W.; Li, A.; Feng, X.; Hou, T.; Liu, K.; Liu, B.; Zhang, N. Metformin and resveratrol ameliorate muscle insulin resistance through preventing lipolysis and inflammation in hypoxic adipose tissue. Cell. Signal. 2016, 28, 1401–1411. [Google Scholar] [CrossRef]
- Hussein, M.M.; Mahfouz, M.K. Effect of resveratrol and rosuvastatin on experimental diabetic nephropathy in rats. Biomed. Pharmacother. 2016, 82, 685–692. [Google Scholar] [CrossRef]
- Huang, S.-S.; Ding, D.-F.; Chen, S.; Dong, C.-L.; Ye, X.-L.; Yuan, Y.-G.; Feng, Y.-M.; You, N.; Xu, J.-R.; Miao, H.; et al. Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy. Sci. Rep. 2017, 7, srep45692. [Google Scholar] [CrossRef]
- Gencoglu, H.; Tuzcu, M.; Hayirli, A.; Sahin, K. Protective effects of resveratrol against streptozotocin-induced diabetes in rats by modulation of visfatin/sirtuin-1 pathway and glucose transporters. Int. J. Food Sci. Nutr. 2015, 66, 314–320. [Google Scholar] [CrossRef]
- Msc, G.M.B.; Bsc, S.M.K.; Brar, N.; Cole, L.K.; Seshadri, N.; Pereira, T.J.; Xiang, B.; Hunt, K.L.; Fonseca, M.A.; Hatch, G.M.; et al. Maternal resveratrol administration protects against gestational diabetes-induced glucose intolerance and islet dysfunction in the rat offspring. J. Physiol. 2019, 597, 4175–4192. [Google Scholar] [CrossRef]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Gambino, R.; Ponzo, V.; Cioffi, I.; Goitre, I.; Evangelista, A.; Ciccone, G.; Cassader, M.; Procopio, M. Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutr. Diabetes 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 103, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Sattarinezhad, A.; Roozbeh, J.; Shiraziyeganeh, B.; Omrani, G.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Alageel, A.; Tomasi, J.; Tersigni, C.; Brietzke, E.; Zuckerman, H.; Subramaniapillai, M.; Lee, Y.; Iacobucci, M.; Rosenblat, J.D.; Mansur, R.B.; et al. Evidence supporting a mechanistic role of sirtuins in mood and metabolic disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Li, K.-X.; Ji, M.-J.; Sun, H.-J. An updated pharmacological insight of resveratrol in the treatment of diabetic nephropathy. Gene 2021, 780, 145532. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Ricardo, S.; Bertram, J.; Nikolic-Paterson, D. Resveratrol inhibits renal fibrosis in the obstructed kidney: Potential role in deacetylation of Smad3. Am. J. Pathol. 2010, 177, 1065–1071. [Google Scholar] [CrossRef]
- Raj, P.; Louis, X.L.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef]
- Xia, X.; Weng, J. Targeting metabolic syndrome: Candidate natural agents. J. Diabetes 2010, 2, 243–249. [Google Scholar] [CrossRef]
- Meng, T.; Qin, W.; Liu, B. SIRT1 Antagonizes Oxidative Stress in Diabetic Vascular Complication. Front. Endocrinol. 2020, 11, 568861. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-N.; Han, L.; Iyer, S.; de Cabo, R.; Zhao, H.; O’Brien, C.A.; Manolagas, S.C.; Almeida, M. Sirtuin1 Suppresses Osteoclastogenesis by Deacetylating FoxOs. Mol. Endocrinol. 2015, 29, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of hepatic foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal Muscle FOXO1 (FKHR) Transgenic Mice Have Less Skeletal Muscle Mass, Down-regulated Type I (Slow Twitch/Red Muscle) Fiber Genes, and Impaired Glycemic Control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef] [Green Version]
- Nakae, J.; Cao, Y.; Oki, M.; Orba, Y.; Sawa, H.; Kiyonari, H.; Iskandar, K.; Suga, K.; Lombes, M.; Hayashi, Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes 2008, 57, 563–576. [Google Scholar] [CrossRef] [Green Version]
- Asadi, S.; Rahimi, Z.; Saidijam, M.; Shabab, N.; Goodarzi, M.T. Effects of Resveratrol on FOXO1 and FOXO3a Genes Expression in Adipose Tissue, Serum Insulin, Insulin Resistance and Serum SOD Activity in Type 2 Diabetic Rats. Int. J. Mol. Cell. Med. 2018, 7, 176–184. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.; Ma, Y.; Shao, Y.; Zhang, J.; Yuan, G.; Guo, X. Resveratrol attenuates dapagliflozin-induced renal gluconeogenesis via activating the PI3K/Akt pathway and suppressing the FoxO1 pathway in type 2 diabetes. Food Funct. 2021, 12, 1207–1218. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Cordis, G.A.; Maulik, N.; Das, D.K. Pharmacological preconditioning with resveratrol: Role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor activation. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H328–H335. [Google Scholar] [CrossRef] [Green Version]
- Abedi-Taleb, E.; Vahabi, Z.; Sekhavati-Moghadam, E.; Khedmat, L.; Jazayeri, S.; Saboor-Yaraghi, A.A. Upregulation of FNDC5 gene expression in C2C12 cells after single and combined treatments of resveratrol and ATRA. Lipids Health Dis. 2019, 18, 181. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.; Park, S.; Kim, M.-J.; Yang, W.K.; Im, D.U.; Yang, K.R.; Hong, J.; Choe, W.; Kang, I.; Kim, S.S.; et al. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014, 281, 4421–4438. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose Restriction Inhibits Skeletal Myoblast Differentiation by Activating SIRT1 through AMPK-Mediated Regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Chen, H.; Li, J.; Li, T.; Zheng, B.; Zheng, Y.; Jin, H.; He, Y.; Gu, Q.; Xu, X. Sirtuin 1–Mediated Cellular Metabolic Memory of High Glucose Via the LKB1/AMPK/ROS Pathway and Therapeutic Effects of Metformin. Diabetes 2012, 61, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Bitterman, J.L.; Chung, J.H. Metabolic effects of resveratrol: Addressing the controversies. Cell. Mol. Life Sci. 2015, 72, 1473–1488. [Google Scholar] [CrossRef]
- Choi, Y.H.; Park, S.; Hockman, S.; Zmuda-Trzebiatowska, E.; Svennelid, F.; Haluzik, M.; Gavrilova, O.; Ahmad, F.; Pepin, L.; Napolitano, M.; et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B–null mice. J. Clin. Investig. 2006, 116, 3240–3251. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Maratos-Flier, E.; Flier, J.S. Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B. Endocrinology 2009, 150, 3076–3082. [Google Scholar] [CrossRef] [Green Version]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative Stress in Diabetes: Implications for Vascular and Other Complications. Int. J. Mol. Sci. 2013, 14, 21525–21550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Kandhare, A.D.; Bodhankar, S.L. Elucidation of protective efficacy of Pentahydroxy flavone isolated from Madhuca indica against arsenite-induced cardiomyopathy: Role of Nrf-2, PPAR-γ, c-fos and c-jun. Environ. Toxicol. Pharmacol. 2017, 56, 172–185. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I.H.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and β-Cell Dysfunction. Oxid. Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef] [Green Version]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef]
- Lee, J.-H.; Song, M.-Y.; Song, E.-K.; Kim, E.-K.; Moon, W.S.; Han, M.-K.; Park, J.-W.; Kwon, K.-B.; Park, B.-H. Overexpression of SIRT1 protects pancreatic β-Cells against cytokine toxicity by suppressing the nuclear factor-κb signaling pathway. Diabetes 2009, 58, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Huang, B.; Qiu, X.; Xiao, L.; Wang, N.; Gao, Q.; Yang, W.; Hao, L. Resveratrol attenuates excessive ethanol exposure induced insulin resistance in rats via improving NAD+/NADH ratio. Mol. Nutr. Food Res. 2017, 61, 1700087. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001, 414, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.-T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [Green Version]
- Schram, M.; Henry, R.M.; Van Dijk, R.A.; Kostense, P.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Bouter, L.; Westerhof, N.; Stehouwer, C.D. Increased Central Artery Stiffness in Impaired Glucose Metabolism and Type 2 Diabetes. Hypertension 2004, 43, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Karaman, A.; Bayram, F.; Gundogan, K.; Ozsan, M.; Karaman, H.; Kelestimur, F. Prevalence of diabetes mellitus and glucose metabolism disorders in the first degree relatives of type 2 diabetic patients. Bratisl Lek List. 2012, 113, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Zhou, L.-J.; Mu, P.-W.; Liu, S.-P.; Chen, S.-J.; Fu, X.-D.; Wang, T.-H. Caveolin-3 is involved in the protection of resveratrol against high-fat-diet-induced insulin resistance by promoting GLUT4 translocation to the plasma membrane in skeletal muscle of ovariectomized rats. J. Nutr. Biochem. 2012, 23, 1716–1724. [Google Scholar] [CrossRef]
- Chen, L.-L.; Zhang, H.-H.; Zheng, J.; Hu, X.; Kong, W.; Hu, D.; Wang, S.-X.; Zhang, P. Resveratrol attenuates high-fat diet–induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation. Metabolism 2011, 60, 1598–1609. [Google Scholar] [CrossRef]
- Do, G.-M.; Jung, U.J.; Park, H.-J.; Kwon, E.-Y.; Jeon, S.-M.; McGregor, R.A.; Choi, M.-S. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef]
- Ruderman, N.; Prentki, M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 2004, 3, 340–351. [Google Scholar] [CrossRef]
- Rogers, N.H.; Witczak, C.A.; Hirshman, M.F.; Goodyear, L.J.; Greenberg, A.S. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem. Biophys. Res. Commun. 2009, 382, 646–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor αa-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordone, L.; Motta, M.C.; Picard, F.; Robinson, A.; Jhala, U.S.; Apfeld, J.; McDonagh, T.; Lemieux, M.; McBurney, M.; Szilvasi, A.; et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol. 2006, 4, e31. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-P.; Chi, T.-C.; Chuang, L.-M.; Su, M.-J. Resveratrol enhances insulin secretion by blocking KATP and KV channels of beta cells. Eur. J. Pharmacol. 2007, 568, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. Resveratrol inhibits insulin secretion from rat pancreatic islets. Eur. J. Pharmacol. 2006, 552, 176–181. [Google Scholar] [CrossRef]
- Szkudelski, T. The insulin-suppressive effect of resveratrol—An in vitro and in vivo phenomenon. Life Sci. 2008, 82, 430–435. [Google Scholar] [CrossRef]
- Henquin, J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Zhou, M.; Zhang, Y.; Liu, Y.; Hou, P.; Zeng, X.; Yi, L.; Mi, M. Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB. Nutr. Metab. 2019, 16, 42. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.J.; Lim, Y. Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation. Metabolism 2014, 63, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Tauriainen, E.; Luostarinen, M.; Martonen, E.; Finckenberg, P.; Kovalainen, M.; Huotari, A.; Herzig, K.-H.; Lecklin, A.; Mervaala, E. Distinct effects of calorie restriction and resveratrol on diet-induced obesity and fatty liver formation. J. Nutr. Metab. 2011, 2011, 525094. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.O.; Paraíso, A.F.; de Oliveira, M.V.M.; Martins, A.; Neto, J.F.; Guimaraes, A.; de Paula, A.M.; Qureshi, M.; Santos, S.H.S. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 2014, 30, 915–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Zorita, S.; Fernandez-Quintela, A.; Lasa, A.; Hijona, E.; Bujanda, L.; Portillo, M.P. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition 2013, 29, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Gubitosi-Klug, R.; Gao, X.; Pop-Busui, R.; de Boer, I.H.; White, N.; Aiello, L.P.; Miller, R.; Palmer, J.; Tamborlane, W.; Wallia, A.; et al. Associations of Microvascular Complications With the Risk of Cardiovascular Disease in Type 1 Diabetes. Diabetes Care 2021, 44, 1499–1505. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Henson, J.; Brady, E.M.; Sargeant, J.A.; Wilmot, E.G.; Athithan, L.; Htike, Z.Z.; Marsh, A.-M.; Biglands, J.D.; Kellman, P.; et al. Cardiovascular Determinants of Aerobic Exercise Capacity in Adults With Type 2 Diabetes. Diabetes Care 2020, 43, 2248–2256. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Zhan, L.; Maulik, G.; Menon, V.P.; Bagchi, D.; Maulik, N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J. Cell. Mol. Med. 2008, 12, 2350–2361. [Google Scholar] [CrossRef] [Green Version]
- Silan, C. The Effects of chronic resveratrol treatment on vascular responsiveness of streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2008, 31, 897–902. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Sun, J.; Chen, G.; Niu, C.; Wang, Y.; Zhao, C.; Sun, J.; Huang, H.; Huang, S.; Liang, Y.; et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FOXO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Front. Pharmacol. 2019, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Liu, B.; Wang, K.; Zhou, S.; Li, W.; Xu, Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-κB pathway. Diabetes Vasc. Dis. Res. 2014, 11, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Wei, J.; Yan, R.; Fan, G.; Lin, L.; Chen, M. Effects of resveratrol on regulation on UCP2 and cardiac function in diabetic rats. J. Physiol. Biochem. 2019, 75, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.; Matta, M.J.; Sunderesan, N.R.; Gupta, M.P.; Periasamy, M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H833–H843. [Google Scholar] [CrossRef] [Green Version]
- Mohammadshahi, M.; Haidari, F.; Soufi, F.G. Chronic resveratrol administration improves diabetic cardiomyopathy in part by reducing oxidative stress. Cardiol. J. 2014, 21, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.-J.; Wang, C.-J.; He, Y.; Zhou, Y.-L.; Peng, X.; Liu, S.-K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol. Sin. 2018, 39, 59–73. [Google Scholar] [CrossRef]
- Ma, S.; Feng, J.; Zhang, R.; Chen, J.; Han, D.; Li, X.; Yang, B.; Li, X.; Fan, M.; Li, C.; et al. SIRT1 Activation by Resveratrol Alleviates Cardiac Dysfunction via Mitochondrial Regulation in Diabetic Cardiomyopathy Mice. Oxid. Med. Cell. Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Kang, L.; Li, C.; Wang, X.; Sun, C.; Li, Q.; Liu, R.; Wang, J. Resveratrol Ameliorates Diabetes-Induced Cardiac Dysfunction Through AT1R-ERK/p38 MAPK Signaling Pathway. Cardiovasc. Toxicol. 2016, 16, 130–137. [Google Scholar] [CrossRef]
- Beaudoin, M.-S.; Perry, C.G.R.; Arkell, A.M.; Chabowski, A.; Simpson, J.A.; Wright, D.C.; Holloway, G.P. Impairments in mitochondrial palmitoyl-CoA respiratory kinetics that precede development of diabetic cardiomyopathy are prevented by resveratrol in ZDF rats. J. Physiol. 2014, 592, 2519–2533. [Google Scholar] [CrossRef] [Green Version]
- Rencber, S.F.; Ozbek, S.K.; Eraldemır, C.; Sezer, Z.; Kum, T.; Ceylan, S.; Guzel, E. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: An experimental study. J. Ovarian Res. 2018, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.; Wang, L.; Li, A.; Qiu, Z.; Qi, L.-W.; Kou, J.; Liu, K.; Liu, B.; Huang, F. The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue. Br. J. Pharmacol. 2016, 173, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Katare, P.B.; Bugga, P.; Dinda, A.K.; Banerjee, S.K. SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM. Cells 2018, 7, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagul, P.K.; Dinda, A.; Banerjee, S.K. Effect of resveratrol on sirtuins expression and cardiac complications in diabetes. Biochem. Biophys. Res. Commun. 2015, 468, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.; Happel, K.; Eelen, G.; Schoors, S.; Oellerich, M.F.; Lim, R.; Zimmermann, B.; Aspalter, I.M.; Franco, C.A.; Boettger, T.; et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 2016, 529, 216–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yang, Q.; Sun, Y.; Xing, Y.; Wang, Y.; Lu, X.; Bai, W.; Liu, X.; Zhao, Y. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J. Cell. Mol. Med. 2014, 18, 1599–1611. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Zhao, L.; Wang, Z.; Liu, H.; Liu, G.; Guan, G. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res. Clin. Pract. 2017, 126, 172–181. [Google Scholar] [CrossRef]
- Yerra, V.G.; Kalvala, A.K.; Kumar, A. Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J. Nutr. Biochem. 2017, 47, 41–52. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lin, C.-C.; Ting, W.-J.; Pai, P.-Y.; Kuo, C.-H.; Ho, T.-J.; Kuo, W.-W.; Chang, C.-H.; Huang, C.-Y.; Lin, W.-T. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. AGE 2014, 36, 9705. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Furukawa-Hibi, Y.; Chen, C.; Horio, Y.; Isobe, K.; Ikeda, K.; Motoyama, N. SIRT1 is critical regulator of FOXO-mediated transcription in re-sponse to oxidative stress. Int. J. Mol. Med. 2005, 16, 237–243. [Google Scholar] [PubMed]
- Kim, M.Y.; Kang, E.S.; Ham, S.A.; Hwang, J.S.; Yoo, T.S.; Lee, H.; Paek, K.S.; Park, C.; Lee, H.T.; Kim, J.-H.; et al. The PPARδ-mediated inhibition of angiotensin II-induced premature senescence in human endothelial cells is SIRT1-dependent. Biochem. Pharmacol. 2012, 84, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Cheang, W.S.; Wong, W.T.; Wang, L.; Cheng, C.K.; Lau, C.W.; Ma, R.C.W.; Xu, A.; Wang, N.; Huang, Y.; Tian, X.Y. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor δ. Pharmacol. Res. 2019, 139, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Hoda, M. Molecular mechanisms of action of resveratrol in modulation of diabetic and non-diabetic cardiomyopathy. Pharmacol. Res. 2020, 161, 105112. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, I.-C.; Cui, X.; Li, Y.-S.; Chien, S.; Shyy, J.Y.-J. Shear stress, SIRT1, and vascular homeostasis. Proc. Natl. Acad. Sci. USA 2010, 107, 10268–10273. [Google Scholar] [CrossRef] [Green Version]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Förstermann, U.; Li, H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, J.T.; Lerin, C.; Gerhart-Hines, Z.; Puigserver, P. Metabolic adaptations through the PGC-1α and SIRT1 pathways. FEBS Lett. 2008, 582, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Lim, J.H.; Kim, M.Y.; Kim, Y.; Hong, Y.A.; Choi, S.R.; Chung, S.; Kim, H.W.; Choi, B.S.; Kim, Y.S.; et al. Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J. Transl. Med. 2016, 14, 176. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, S.; Arimoto, A.; Kuramoto, Y.; Kozako, T.; Honda, S.-I.; Shimeno, H.; Soeda, S. Fasting promotes the expression of SIRT1, an NAD+-dependent protein deacetylase, via activation of PPARα in mice. Mol. Cell. Biochem. 2010, 339, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Poynter, M.; Daynes, R.A. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κb signaling, and reduces inflammatory cytokine production in aging. J. Biol. Chem. 1998, 273, 32833–32841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guellich, A.; Damy, T.; LeCarpentier, Y.; Conti, M.; Claes, V.; Samuel, J.-L.; Quillard, J.; Hébert, J.-L.; Pineau, T.; Coirault, C. Role of oxidative stress in cardiac dysfunction of PPARα−/− mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H93–H102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.; Musi, N.; Fujii, N.; Zou, L.; Luptak, I.; Hirshman, M.F.; Goodyear, L.J.; Tian, R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative α2 subunit of AMP-activated protein kinase. J. Biol. Chem. 2003, 278, 28372–28377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.R.; Li, J.; Coven, D.L.; Pypaert, M.; Zechner, C.; Palmeri, M.; Giordano, F.J.; Mu, J.; Birnbaum, M.J.; Young, L.H. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Investig. 2004, 114, 495–503. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.-F.; Ke, Z.-Q.; Yao, Q.; Guo, S.; Liu, C. Resveratrol Modulates Apoptosis and Autophagy Induced by High Glucose and Palmitate in Cardiac Cells. Cell. Physiol. Biochem. 2018, 46, 2031–2040. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie Restriction Promotes Mitochondrial Biogenesis by Inducing the Expression of eNOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef]
- Kondo, M.; Shibata, R.; Miura, R.; Shimano, M.; Kondo, K.; Li, P.; Ohashi, T.; Kihara, S.; Maeda, N.; Walsh, K.; et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J. Biol. Chem. 2009, 284, 1718–1724. [Google Scholar] [CrossRef] [Green Version]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.J.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Peng, I.-C.; Sun, W.; Su, M.-I.; Hsu, P.-H.; Fu, Y.; Zhu, Y.; DeFea, K.; Pan, S.; Tsai, M.-D.; et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser. Cric. Res. 2009, 104, 496–505. [Google Scholar] [CrossRef]
- Gracia-Sancho, J.; Villarreal, G., Jr.; Zhang, Y.; García-Cardeña, G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 2010, 85, 514–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarreal, G.; Zhang, Y.; Larman, H.B.; Gracia-Sancho, J.; Koo, A.; García-Cardeña, G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 391, 984–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Liu, P.; Xu, S.; Koroleva, M.; Zhang, S.; Si, S.; Jin, Z.G. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci. Rep. 2017, 7, 6686. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.B.; Jain, M.K. Role of krüppel-like transcription factors in endothelial biology. Circ. Res. 2007, 100, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Zhao, X.; Yang, F. Natural Polyphenols Inhibit Lysine-Specific Demethylase-1 in vitro. J. Biochem. Pharmacol. Res. 2013, 1, 56–63. [Google Scholar]
- Wu, H.; Li, G.-N.; Xie, J.; Li, R.; Chen, Q.-H.; Chen, J.-Z.; Wei, Z.-H.; Kang, L.-N.; Xu, B. Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in STZ-induced diabetic mice. BMC Cardiovasc. Disord. 2016, 16, 5. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Song, X.; Zhao, L.; Li, Z.; Liu, B. Resveratrol Prevents Diabetic Cardiomyopathy by Increasing Nrf2 Expression and Transcriptional Activity. BioMed Res. Int. 2018, 2018, 2150218. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Zhu, S.; Chang, S.; Cao, Y.; Dong, J.; Li, J.; Long, R.; Zhou, Y. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in steptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling. Eur. J. Pharmacol. 2013, 720, 147–157. [Google Scholar] [CrossRef]
- Chu, H.; Li, H.; Guan, X.; Yan, H.; Zhang, X.; Cui, X.; Li, X.; Cheng, M. Resveratrol protects late endothelial progenitor cells from TNF-α-induced inflammatory damage by upregulating Kr�ppel-like factor-2. Mol. Med. Rep. 2018, 17, 5708–5715. [Google Scholar] [CrossRef] [Green Version]
- Hannan, N.; Brownfoot, F.C.; Cannon, P.; Deo, M.; Beard, S.; Nguyen, T.V.; Palmer, K.; Tong, S.; Kaitu’U-Lino, T.J. Resveratrol inhibits release of soluble fms-like tyrosine kinase (sFlt-1) and soluble endoglin and improves vascular dysfunction—Implications as a preeclampsia treatment. Sci. Rep. 2017, 7, 1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Song, J.; Hodara, V.; Ford, A.; Wang, X.L.; Shi, Q.; Chen, L.; VandeBerg, J.L. Protective Effects of Resveratrol on TNF-α-Induced Endothelial Cytotoxicity in Baboon Femoral Arterial Endothelial Cells. J. Diabetes Res. 2013, 2013, 185172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Yang, R.; Yang, J.; Yang, J.; Ding, J.; Wu, H.; Zhang, J. Resveratrol pretreatment protects rat hearts from ischemia/reperfusion injury partly via a NALP3 inflammasome pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 8731–8741. [Google Scholar] [PubMed]
- Mattison, J.A.; Wang, M.; Bernier, M.; Zhang, J.; Park, S.-S.; Maudsley, S.; An, S.; Santhanam, L.; Martin, B.; Faulkner, S.; et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014, 20, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, B.; Campen, M.J.; Channell, M.M.; Wherry, S.J.; Varamini, B.; Davis, J.G.; Baur, J.A.; Smoliga, J.M. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 2013, 166, 246–248. [Google Scholar] [CrossRef] [Green Version]
- Lekli, I.; Szabo, G.; Juhasz, B.; Das, S.; Das, M.; Varga, E.; Szendrei, L.; Gesztelyi, R.; Váradi, J.; Bak, I.; et al. Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: The role of GLUT-4 and endothelin. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H859–H866. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.C.; Kaneko, M.; Bakr, S.; Liao, H.; Lu, Y.; Lewis, E.R.; Yan, S.; Ii, S.; Itakura, M.; Rui, L.; et al. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J. 2004, 18, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Xi, J.; Wang, H.; Mueller, R.A.; Norfleet, E.A.; Xu, Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3β and mitochondrial permeability transition pore. Eur. J. Pharmacol. 2009, 604, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Yagyu, H.; Chen, G.; Yokoyama, M.; Hirata, K.; Augustus, A.; Kako, Y.; Seo, T.; Hu, Y.; Lutz, E.P.; Merkel, M.; et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Investig. 2003, 111, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef]
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Mikami, A.; Ogino, A.; Watanabe, T.; Morishita, K.; Okada, H.; Kawasaki, M.; et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 2015, 11, 1146–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbidi, S.; Daiber, A.; Korac, B.; Li, H.; Essop, M.F.; Laher, I. Health Benefits of Fasting and Caloric Restriction. Curr. Diabetes Rep. 2017, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelino, S.; Chang, J.T.; Kumsta, C.; She, X.; Davis, A.; Nguyen, C.; Panowski, S.; Hansen, M. Intestinal Autophagy Improves Healthspan and Longevity in C. elegans during Dietary Restriction. PLoS Genet. 2016, 12, e1006135. [Google Scholar] [CrossRef] [Green Version]
- Pulakat, L.; Chen, H.H. Pro-Senescence and Anti-Senescence Mechanisms of Cardiovascular Aging: Cardiac MicroRNA Regulation of Longevity Drug-Induced Autophagy. Front. Pharmacol. 2020, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yao, W.; Zhu, J.; Mu, K.; Zhang, J.; Zhang, J.-A. Resveratrol Attenuates High Glucose-Induced Vascular Endothelial Cell Injury by Activating the E2F3 Pathway. BioMed Res. Int. 2020, 2020, 6173618. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Najafi, M.; Orouei, S.; Zabolian, A.; Saleki, H.; Azami, N.; Sharifi, N.; Hushmandi, K.; Zarrabi, A.; Ahn, K. Resveratrol Modulates Transforming Growth Factor-Beta (TGF-β) Signaling Pathway for Disease Therapy: A New Insight into Its Pharmacological Activities. Biomedicines 2020, 8, 261. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, F.; Lian, X.F.; Peng, W.Q.; Yin, C.Y. Mesenchymal stem cell-derived exosomes improve diabetes mellitus-induced myo-cardial injury and fibrosis via inhibition of TGF-β1/Smad2 signaling pathway. Cell. Mol. Biol. 2019, 65, 123–126. [Google Scholar] [CrossRef]
- Sierra-Mondragon, E.; Rodríguez-Muñoz, R.; Namorado-Tonix, C.; Molina-Jijon, E.; Romero-Trejo, D.; Pedraza-Chaverri, J.; Reyes, J.L. All-Trans Retinoic Acid Attenuates Fibrotic Processes by Downregulating TGF-β1/Smad3 in Early Diabetic Nephropathy. Biomolecules 2019, 9, 525. [Google Scholar] [CrossRef] [Green Version]
| Study Type | Model | Dose/Dosing Method/Period | Outcome | Proposed Mechanism | Ref. |
|---|---|---|---|---|---|
| In vivo | SD rats (STZ DM model) | RES 0.5 mg/kg, gavage for 8–14 days | ↓Insulin resistance ↑Glucose uptake ↑Hepatic glycogen synthesis | [14] | |
| In vivo | Wistar rats (STZ-NA model) | RES 5 mg/kg, oral for 30 days | ↓Blood glucose ↓Plasma insulin and hemoglobin ↓AST, ALT, ALP | [15] | |
| In vivo | db/db mice (T2DM model) | RES (0.3% mixed in chow) for 8 weeks | ↑Mitochondrial oxidative stress and biogenesis ↓Blood glucose | RES improves oxidative stress and promotes mitochondrial biogenesis through normal Mn-SOD function and glycolipid metabolism. | [16] |
| In vivo | C57BL/6 mice (HFD) | RES 0.03 µg/µL minipump Intracerebroventricularly, 14 weeks | ↓Hyperglycemia ↓Pyruvate-induced hyperglycemia | RES improves hypothalamic NF-κB inflammatory signal transduction by decreasing total and acetylated RelA/P65 protein content. | [17] |
| In vivo | ob/ob mice (T2DM model) | RES 5, 15, 50 mg/kg, oral for 4 weeks | ↓Hyperglycemia ↓Insulin resistance ↓TG, TC, ADPN, FFA | [18] | |
| In vivo | NOD mice (T1DM model) | RES 250 mg/kg oral or subcutaneously inject for 32 weeks | ↓Expression of inflammatory genes ↓Expression of CCR6 | RES blocks CCR6 and CD11b (+) F4/80(hi) macrophages migration from peripheral lymphoid organs to the pancreas. | [19] |
| In vivo | C57BL/6 mice (HFD) | RES (0.04% mixed in chow) for 6 months | ↑Survival ↓Insulin sensitivity ↑Mitochondrial number | RES reduces IGF-I levels and increases AMPK and PGC-1α activity. | [20] |
| In vivo | C57BL/6 mice (HFD) | RES 400 mg/kg, oral for 16 weeks | ↓Insulin resistance ↑Mitochondrial biogenesis ↑Oxidative phosphorylation | RES improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. | [21] |
| In vivo | SD rats (HCF) | RES 1 mg/kg, oral for 15 days or 15 weeks | ↑Glucose uptake ↑Membrane trafficking activity of GLUT4 ↑Phosphorylation of insulin receptor | ER is a key regulator in RES-stimulating insulin-dependent and -independent glucose uptake. | [22] |
| In vivo | Wistar rats (STZ/STZ-NA/ insulin-resistant diabetic model) | RES 3 or 10 mg/kg, oral for 90 min | ↓Blood glucose ↓Insulin resistance ↑GLUT4 expression | RES promotes skeletal muscle glucose uptake through the PI3K-Akt signaling pathway. | [23] |
| In vivo | NOD mice (T1DM model) | RES 200 mg/kg, gavage for 28 days | ↓Blood glucose ↓Inflammatory factors | RES improves renal function not only by its anti-inflammatory effect but also by improving the metabolic memory of hyperglycemia. | [24] |
| In vivo | SD rats (STZ model) | RES 5, 10 mg/kg, gavage for 1–7 months | ↓Blood glucose ↑Weight | RES significantly inhibited the HG-induced decreases in glutamate uptake, GS activity, GLAST, and GS expression. | [25] |
| In vivo | Albino rats (Alloxan model) | RES 30 mg/kg, gavage for 30 days | ↓Hyperglycemia | [26] | |
| In vivo | ICR mice (HFD) | RES 50 mg/kg, oral for 10 days | ↓HIF-1α ↓Inflammation in the adipose tissue ↓Insulin sensitivity | RES reduces cAMP accumulation by preserving PDE3B, thereby preventing PKA/HSL activation and lipolysis, and decreasing FFAs influx and DAG accumulation, thereby improving insulin signaling by inhibiting PKCθ translocation. | [27] |
| In vivo | Wistar rats (STZ model) | RES 5 mg/kg, oral for 8 weeks | ↓Blood glucose ↑Antioxidant status | RES significantly improved the expression of TGF-β1, fibronectin, NF-κB/P65, Nrf2, Sirt1, and FoxO1 in the kidney. | [28] |
| In vivo | db/db, db/m mice (T2DM model) | RES 10 mg/kg, gavage for 12 weeks | ↓Apoptosis of podocytes ↑Autophagy of podocytes | Resveratrol regulates autophagy and apoptosis of podocytes by inhibiting microRNA-383-5p. | [29] |
| In vivo | Wistar albino rats (STZ model) | RES 20 mg/kg, gavage for 8 weeks | ↓Hyperglycemia ↓Serum MDA concentrations | Resveratrol inhibits oxidative stress and increases the potential of extra-hepatic tissues to absorb glucose. | [30] |
| In vivo | SD rats (HFS model) | RES 147.6 mg/kg, oral for 12 weeks | ↓Dysregulated gluconeogenesis ↓Dysregulation of several metabolic genes | [31] | |
| In vivo | ICR mice (STZ model) | RES 50 mg/kg, oral for 7 days | ↓TXNIP/NLRP3 inflammasome activation ↓Cell apoptosis ↓ROS-associated mitochondrial fission | Resveratrol inhibits Drp1 activity to protect mitochondrial integrity and inhibits endoplasmic reticulum stress to prevent NLRP3 inflammasome activation. | [32] |
| Study Type | Model | Dose/Dosing Method/Period | Outcome | Proposed Mechanism | Ref. |
|---|---|---|---|---|---|
| In vivo | SD rats (STZ DM model) | RES 2.5 mg/kg, oral 15 days | ↑Phosphorylation of eNOS ↓Blood glucose | RES improves diabetic myocardial GLUT4 translocation and glucose uptake through the AMPK pathway and by regulating the status of Cav-1 and Cav-3. | [100] |
| In vivo | Wistar rats (STZ DM model) | RES 5 mg/kg, intraperitoneal inject 42 days | ↑Contractile responses to noradrenaline ↑Relaxation response to Ach ↓Blood glucose | [101] | |
| In vivo | C57BL/6 mice (HFD) and db/db mice (T2DM model) | RES 5, 30, 50 mg/kg, oral for 4 weeks | ↓Plasma insulin levels ↓Hyperglycemia↓Fasting BP ↓Angiogenesis ↑Endothelial protection | RES protects diabetic wound healing through its SIRT1-dependent endothelial cell protection and pro-angiogenesis, involving inhibition of FOXO1 and de-inhibition of c-Myc expression. | [102] |
| In vivo | C57BL/6 mice (HFD) and db/db mice (T2DM model) | RES (0.3% mixed in chow) for 8 weeks | ↓Blood glucose, FFA ↓ICAM-1, VCAM-1, MCP-1 ↓NF-κB activity | RES ameliorates diabetic vascular inflammation and macrophage infiltration by inhibiting the NF-κB pathway. | [103] |
| In vivo | SD rats (STZ model/HFD) | RES 10 mg/kg, gavage for 8 months | ↓Insulin sensitivity ↓TG, TC, LDLc ↓ROS | UCP2 mediates RES to improve cardiac function, inhibit myocardial cell apoptosis, and participate in the improvement of mitochondrial function. | [104] |
| In vivo | CD1 mice (STZ T1DM model) | RES 100 mg/kg, oral for 3 months | ↑SERCA2 promoter activity ↑SIRT1 | RES enhances SERCA2a expression and improves cardiac function through activation of SIRT1. | [105] |
| In vivo | SD rats (STZ-NA model) | RES 5 mg/kg, oral for 4 months | ↓Antioxidant enzymes activities ↓Oxidative markers | RES treatment may delay or attenuate the progression of diabetes-related cardiac complications by reducing oxidative stress. | [106] |
| In vivo | SD rats (HFD T2DM model) | RES 50 mg/kg, gavage for 16 weeks | ↓Cardiac dysfunction and hypertrophy ↓SOD activity ↓ATP content | RES activates SIRT1 and increases PGC-1α deacetylation, thereby regulating mitochondrial function and alleviating cardiac injury in diabetic rats. | [107] |
| In vivo | mice (STZ T1DM model) | RES 25 mg/kg, intraperitoneal inject for 5 days | ↓Apoptosis ↑Mitochondrial biogenesis | Activation of SIRT1 by RES ameliorates myocardial injury in DCM through PGC-1α -mediated mitochondrial regulation. | [108] |
| In vivo | SD rats (STZ T1DM model) | RES 80 mg/kg, intraperitoneal inject for 12 weeks | ↑Glucose and lipid metabolism ↑Cardiac function ↓TNF-α, IL-6, IL-1β | Res alleviates cardiac dysfunction caused by diabetes through down-regulation of the AT1R-ERK/P38 MAPK signaling pathway. | [109] |
| In vivo | ZDF rats | RES 200 mg/kg, oral for 6 weeks | ↑The apparent Km to palmitoyl-CoA ↓Mitochondrial reactive oxygen ↓Lipid accumulation | Resveratrol reduces liver fibrosis, p-COA respiratory sensitivity, active lipid accumulation, and mitochondrial reactive oxygen emission rates. | [110] |
| In vivo | Wistar albino rats (DHEA-induced PCOS model) | RES 20 mg/kg, oral for 28 days | ↓Serum testosterone levels ↓Number of TUNEL (+) granulosa cells ↓Number of Graafian follicles ↓Body weights | Resveratrol activates SIRT1 and AMPK to induce antioxidant and anti-inflammatory systems of PCOS. | [111] |
| In vivo | ICR mice (HFD model) | RES 50 mg/kg, gavage for 7 days | ↓Collagen deposition ↓HIF-1α accumulation ↓Fibrosis and inflammation | Resveratrol reduces HIF-1α accumulation by promoting proteasome degradation of HIF-1α by regulating AMPK/SIRT1. | [112] |
| In vivo | SD rats (STZ model) | RES 0.1, 1, 5, 10, 50 μg/kg, intravitreal inject or tail vein injects for 12 weeks | ↑Insulin level ↓AGEs, LDL, Ox-LDL, caspase 3 activity ↓Damage of DR | Resveratrol reduces the inflammatory state and damage of DR through PON1. | [113] |
| In vivo | SD rats (STZ T1DM model) | RES 25 mg/kg, oral for 8 weeks | ↓Cardiac cell size ↓Oxidative stress ↓Fibrosis | Resveratrol activates SIRT3, maintains mitochondrial function, and regulates the acetylation of TFAM. | [114] |
| Identifier No. | Type | Dose/Dosing Method/Period | Phase | Sex | Number Enrolled | Outcome Measures |
|---|---|---|---|---|---|---|
| NCT01038089 | T2DM | RES (90 mg/d and 270 mg/d for 2 weeks) | Not Applicable | All | 20 | Brachial artery flow-mediated dilation Blood markers of inflammation, oxidative stress, insulin resistance |
| NCT01677611 | T2DM | RES (3 g/d for 12 weeks) | Phase 1 | Male | 10 | SIRT1 expression Skeletal muscle AMPK expression Skeletal muscle p-AMPK expression |
| NCT01881347 | T2DM | RES (100 mg/d for 2 weeks and then 300 mg/d for 2 weeks) | Not Applicable | All | 54 | Change from baseline in Brachial artery flow-mediated dilation Change from Baseline in Fingertip peripheral arterial tonometry Change from Baseline in Carotid femoral pulse wave velocity Change from Baseline in Reactive hyperemia |
| NCT01638780 | T2DM | RES (150 mg/kg/d for 30 days) | Not Applicable | Male | 24 | insulin sensitivity (overall, muscle- and liver-specific) muscle mitochondrial oxidative capacity intramyocellular lipid content |
| NCT04449198 | T1DM | RES (500 mg, twice a day for 12 weeks) | Early Phase 1 | All | 24 | Change in AUC for ET-1 + BQ-123 Skeletal Muscle Mitochondrial Function Change in Percentage FMD |
| NCT03436992 | T1DM | RES (1500 mg for 3 months) | Not Applicable | All | 198 | Change in FMD |
| NCT03762096 | T2DM+CAD | RES (1 g, twice a day for 6 weeks) | Not Applicable | All | 40 | Change in endothelial function Effects of resveratrol on caveolar function Effects of resveratrol on molecular signaling |
| NCT01354977 | T2DM+ Insulin Resistance | RES (1000 mg, twice a day for 4 weeks) | Phase 2 | All | 20 | Peripheral Insulin Sensitivity (RD) Measured by the Change in Glucose Rates of Disappearance with Resveratrol or Placebo at Baseline and at 4 weeks. EGP, With Resveratrol or Placebo at Baseline and at 4 weeks. Effects of Resveratrol on Skeletal Muscle Mitochondrial Numbers |
| NCT02244879 | T2DM+ Inflammation+Insulin Resistance | RES (40 mg/d and 500 mg/d for 6 months) | Phase 3 | All | 192 | CRP Metabolic and oxidative markers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Zhao, W.; Xu, S.; Weng, J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants 2022, 11, 1085. https://doi.org/10.3390/antiox11061085
Su M, Zhao W, Xu S, Weng J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants. 2022; 11(6):1085. https://doi.org/10.3390/antiox11061085
Chicago/Turabian StyleSu, Meiming, Wenqi Zhao, Suowen Xu, and Jianping Weng. 2022. "Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action" Antioxidants 11, no. 6: 1085. https://doi.org/10.3390/antiox11061085
APA StyleSu, M., Zhao, W., Xu, S., & Weng, J. (2022). Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants, 11(6), 1085. https://doi.org/10.3390/antiox11061085








