The Potential Role of Everlasting Flower (Helichrysum stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Animals
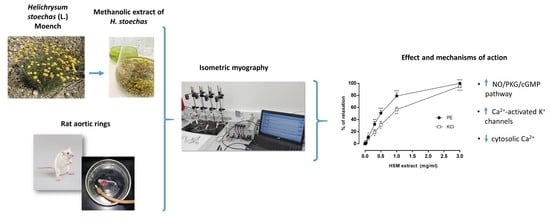
2.3. Preparation of the Aortic Rings and Isometric Myography
2.4. The Experimental Protocols
2.5. Analysis of the Data
3. Results
3.1. The Effects of H. stoechas Extract on PE- and KCl-Induced Contractions
3.2. The Effects of Indomethacin and L-NAME on H. stoechas Extract-Induced Vasorelaxation
3.3. The Effects of PKA and PKG Inhibitors on H. stoechas Extract-Induced Vasorelaxation
3.4. The Effects of H. stoechas Extract on the Contractile Response to PE
3.5. The Effects of Ca2+ Influx on H. stoechas Extract-Induced Vasorelaxation
3.6. Role of Intracellular Ca2+ on the Effect of H. stoechas Extract on the Contractile Response Induced by Phenylephrine
3.7. The Effects of K+ Channels Inhibitors on the H. stoechas Extract-Induced Vasorelaxation
3.8. The Inhibition of H. stoechas Extract-Induced Vasorelaxation by the Combination of TRAM-34 and Apamin with or without L-NAME
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Özaslan, M.; Oguzkan, S.B. Use of Plant Extracts in Alternative Medicine. Pakistan J. Biol. Sci. 2018, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Benítez, G.; González-Tejero, M.R.; Molero-Mesa, J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): Ethnopharmacological synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef]
- Carmona, M.D.; Llorach, R.; Obon, C.; Rivera, D. “Zahraa”, a Unani multicomponent herbal tea widely consumed in Syria: Components of drug mixtures and alleged medicinal properties. J. Ethnopharmacol. 2005, 102, 344–350. [Google Scholar] [CrossRef]
- Orhan, N.; Onaran, M.; Şen, İ.; Işık Gönül, İ.; Aslan, M. Preventive treatment of calcium oxalate crystal deposition with immortal flowers. J. Ethnopharmacol. 2015, 163, 60–67. [Google Scholar] [CrossRef]
- Onaran, M.; Orhan, N.; Farahvash, A.; Ekin, H.N.; Kocabıyık, M.; Gönül, İ.I.; Şen, İ.; Aslan, M. Successful treatment of sodium oxalate induced urolithiasis with Helichrysum flowers. J. Ethnopharmacol. 2016, 186, 322–328. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Bremner, P.; Rivera, D.; Calzado, M.A.; Obón, C.; Inocencio, C.; Beckwith, C.; Fiebich, B.L.; Muñoz, E.; Heinrich, M. Assessing medicinal plants from South-Eastern Spain for potential anti-inflammatory effects targeting nuclear factor-Kappa B and other pro-inflammatory mediators. J. Ethnopharmacol. 2009, 124, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Mykolenko, S.; Liedienov, V.; Kharytonov, M.; Makieieva, N.; Kuliush, T.; Queralt, I.; Marguí, E.; Hidalgo, M.; Pardini, G.; Gispert, M. Presence, mobility and bioavailability of toxic metal(oids) in soil, vegetation and water around a Pb-Sb recycling factory (Barcelona, Spain). Environ. Pollut. 2018, 237, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol α-pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Koeberle, A.; Dehm, F.; Pollastro, F.; Appendino, G.; Northoff, H.; Rossi, A.; Sautebin, L.; Werz, O. Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem. Pharmacol. 2011, 81, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, A.; Deiana, M.; Atzeri, A.; Corona, G.; Incani, A.; Melis, M.P.; Appendino, G.; Dessì, M.A. Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated α-pyrone-phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum. Chem. Biol. Interact. 2007, 165, 117–126. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Nani, A.; Murtaza, B.; Khan, A.S.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Fan, X. Role of Polyphenols as Antioxidant Supplementation in Ischemic Stroke. Oxid. Med. Cell. Longev. 2021, 2021, 5471347. [Google Scholar] [CrossRef]
- Forte, M.; Conti, V.; Damato, A.; Ambrosio, M.; Puca, A.A.; Sciarretta, S.; Frati, G.; Vecchione, C.; Carrizzo, A. Targeting Nitric Oxide with Natural Derived Compounds as a Therapeutic Strategy in Vascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7364138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biegańska-Hensoldt, S.; Rosołowska-Huszcz, D. Polyphenols in preventing endothelial dysfunction. Postepy Hig. Med. Dosw. 2017, 71, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Valero, M.S.; Oliván-Viguera, A.; Garrido, I.; Langa, E.; Berzosa, C.; López, V.; Gómez-Rincón, C.; Murillo, M.D.; Köhler, R. Rock Tea extract (Jasonia glutinosa) relaxes rat aortic smooth muscle by inhibition of L-type Ca2+ channels. J. Physiol. Biochem. 2015, 71, 785–793. [Google Scholar] [CrossRef]
- Borgonetti, V.; Les, F.; López, V.; Galeotti, N. Attenuation of Anxiety-Like Behavior by Helichrysum stoechas (L.) Moench Methanolic Extract through Up-Regulation of ERK Signaling Pathways in Noradrenergic Neurons. Pharmaceuticals 2020, 13, 472. [Google Scholar] [CrossRef]
- Félétou, M.; Köhler, R.; Vanhoutte, P.M. Nitric oxide: Orchestrator of endothelium-dependent responses. Ann. Med. 2012, 44, 694–716. [Google Scholar] [CrossRef]
- Duboscq, C. Vascular endothelium. Hematología 2017, 21, 19–30. [Google Scholar]
- Dogan, M.F.; Yildiz, O.; Arslan, S.O.; Ulusoy, K.G. Potassium channels in vascular smooth muscle: A pathophysiological and pharmacological perspective. Fundam. Clin. Pharmacol. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Köhler, R.; Kaistha, B.P.; Wulff, H. Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin. Ther. Targets 2010, 14, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottolini, M.; Sonkusare, S.K. The Calcium Signaling Mechanisms in Arterial Smooth Muscle and Endothelial Cells. Compr. Physiol. 2021, 11, 1831–1869. [Google Scholar] [CrossRef] [PubMed]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Taubert, D.; Berkels, R.; Klaus, W.; Roesen, R. Nitric oxide formation and corresponding relaxation of porcine coronary arteries induced by plant phenols: Essential structural features. J. Cardiovasc. Pharmacol. 2002, 40, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Gasparotto, A.; dos Reis Piornedo, R.; Assreuy, J.; Da Silva-Santos, J.E. Nitric oxide and Kir6.1 potassium channel mediate isoquercitrin-induced endothelium-dependent and independent vasodilation in the mesenteric arterial bed of rats. Eur. J. Pharmacol. 2016, 788, 328–334. [Google Scholar] [CrossRef]
- Cicala, C.; Morello, S.; Iorio, C.; Capasso, R.; Borrelli, F.; Mascolo, N. Vascular effects of caffeic acid phenethyl ester (CAPE) on isolated rat thoracic aorta. Life Sci. 2003, 73, 73–80. [Google Scholar] [CrossRef]
- Musabayane, C.T.; Munjeri, O.; Mdege, N.D. Effects of Helichrysum ceres extracts on renal function and blood pressure in the rat. Ren. Fail. 2003, 25, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Musabayane, C.T.; Kamadyaapa, D.R.; Gondwe, M.; Moodley, K.; Ojewole, J.A. Cardiovascular effects of Helichrysum ceres S Moore [Asteraceae] ethanolic leaf extract in some experimental animal paradigms. Cardiovasc. J. Afr. 2008, 19, 246–253. [Google Scholar]
- Aslan, M.; Deliorman Orhan, D.; Orhan, N.; Sezik, E.; Yesilada, E. In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin-induced-diabetic rats. J. Ethnopharmacol. 2007, 109, 54–59. [Google Scholar] [CrossRef]
- Aslan, M.; Orhan, D.D.; Orhan, N.; Sezik, E.; Yeşilada, E. A study of antidiabetic and antioxidant effects of Helichrysum graveolens capitulums in streptozotocin-induced diabetic rats. J. Med. Food 2007, 10, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Gan, C.; Zhu, J.; Ma, N.; Wu, L.; Wang, L.; Wang, X. Anti-atherosclerotic activities of flavonoids from the flowers of Helichrysum arenarium L. MOENCH through the pathway of anti-inflammation. Bioorg. Med. Chem. Lett. 2017, 27, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A Review on the Potential Use of Medicinal Plants from Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Dib, I.; Tits, M.; Angenot, L.; Wauters, J.N.; Assaidi, A.; Mekhfi, H.; Aziz, M.; Bnouham, M.; Legssyer, A.; Frederich, M.; et al. Antihypertensive and vasorelaxant effects of aqueous extract of Artemisia campestris L. from Eastern Morocco. J. Ethnopharmacol. 2017, 206, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Millán-Pacheco, C.; Navarrete-Vázquez, G.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Almanza-Pérez, J.C. Antihypertensive and vasorelaxant effect of leucodin and achillin isolated from Achillea millefolium through calcium channel blockade and NO production: In vivo, functional ex vivo and in silico studies. J. Ethnopharmacol. 2021, 273, 113948. [Google Scholar] [CrossRef] [PubMed]
- Salahdeen, H.M.; Idowu, G.O.; Salami, S.A.; Murtala, B.A.; Alada, A.A. Mechanism of vasorelaxation induced by Tridax procumbens extract in rat thoracic aorta. J. Intercult. Ethnopharmacol. 2016, 5, 174–179. [Google Scholar] [CrossRef]
- Ch’ng, Y.S.; Loh, Y.C.; Tan, C.S.; Ahmad, M.; Asmawi, M.Z.; Omar, W.M.W.; Yam, M.F. Vasorelaxant properties of Vernonia amygdalina ethanol extract and its possible mechanism. Pharm. Biol. 2017, 55, 2083–2094. [Google Scholar] [CrossRef] [Green Version]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Módis, K.; Panopoulos, P.; Asimakopoulou, A.; Gerö, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [Green Version]
- Wong, W. Two Gases Required for Vasodilation and Angiogenesis. Sci. Signal. 2012, 5, ec163. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero, M.S.; Nuñez, S.; Les, F.; Castro, M.; Gómez-Rincón, C.; Arruebo, M.P.; Plaza, M.Á.; Köhler, R.; López, V. The Potential Role of Everlasting Flower (Helichrysum stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta. Antioxidants 2022, 11, 1092. https://doi.org/10.3390/antiox11061092
Valero MS, Nuñez S, Les F, Castro M, Gómez-Rincón C, Arruebo MP, Plaza MÁ, Köhler R, López V. The Potential Role of Everlasting Flower (Helichrysum stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta. Antioxidants. 2022; 11(6):1092. https://doi.org/10.3390/antiox11061092
Chicago/Turabian StyleValero, Marta Sofía, Sonia Nuñez, Francisco Les, Marta Castro, Carlota Gómez-Rincón, María Pilar Arruebo, Miguel Ángel Plaza, Ralf Köhler, and Víctor López. 2022. "The Potential Role of Everlasting Flower (Helichrysum stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta" Antioxidants 11, no. 6: 1092. https://doi.org/10.3390/antiox11061092
APA StyleValero, M. S., Nuñez, S., Les, F., Castro, M., Gómez-Rincón, C., Arruebo, M. P., Plaza, M. Á., Köhler, R., & López, V. (2022). The Potential Role of Everlasting Flower (Helichrysum stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta. Antioxidants, 11(6), 1092. https://doi.org/10.3390/antiox11061092