Shape Memory Polymer Foams with Phenolic Acid-Based Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PA Foam Synthesis
2.3. Oxidative Degradation
2.3.1. Mass Loss
2.3.2. Pore Structure
2.3.3. Glass Transition Temperature
2.3.4. Surface Chemistry
2.4. PA Release from SMP Foams
2.5. Cell Culture
2.5.1. Cellular Antioxidant Activity (CAA) Assay
2.5.2. Quantitation of CAA
2.5.3. Cytocompatibility Assay
2.6. Statistics
3. Results and Discussion
3.1. In Vitro Oxidative Degradation
3.1.1. Gravimetric Analysis
3.1.2. Microscopic Analysis
3.1.3. Thermal Characterization
3.1.4. Spectroscopic Characterization and PA Delivery
3.2. PA Release from Foams
3.3. Cellular Antioxidant Activity
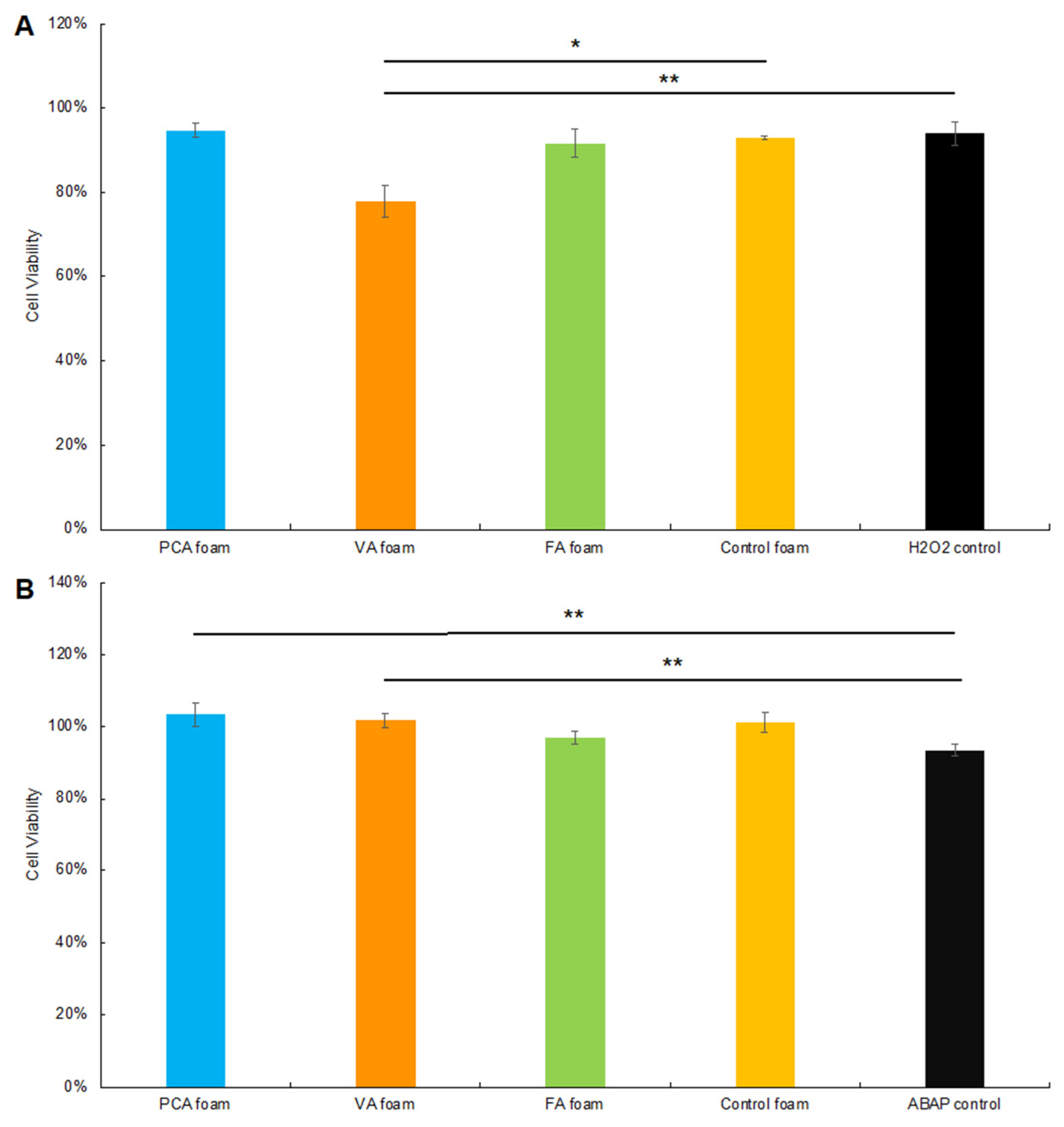
3.3.1. CAA after Hydrogen Peroxide Exposure
3.3.2. CAA after ABAP Exposure
3.3.3. Cytocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Reactive oxygen species (ROS)—A family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur. Cells Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Bayir, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2015, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-T.; Yang, C.-M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Foo, N.-P.; Lin, S.-H.; Lee, Y.-H.; Wu, M.-J.; Wang, Y.-J. α-Lipoic acid inhibits liver fibrosis through the attenuation of ROS-triggered signaling in hepatic stellate cells activated by PDGF and TGF-β. Toxicology 2011, 282, 39–46. [Google Scholar] [CrossRef]
- Gharibi, R.; Yeganeh, H.; Rezapour-Lactoee, A.; Hassan, Z.M. Stimulation of Wound Healing by Electroactive, Antibacterial, and Antioxidant Polyurethane/Siloxane Dressing Membranes: In Vitro and in Vivo Evaluations. ACS Appl. Mater. Interfaces 2015, 7, 24296–24311. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. Herb. Med. 2018, 1, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Nohynek, L.J.; Alakomi, H.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Puupponen-Pimiä, R.H. Berry Phenolics: Antimicrobial Properties and Mechanisms of Action Against Severe Human Pathogens. Nutr. Cancer 2009, 54, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M. Antioxidant activity and antimicrobial effect of berry phenolics—A Finnish perspective. Mol. Nutr. Food Res. 2007, 51, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.-J.E.; Komaitis, M. RP-HPLC Analysis of the Phenolic Compounds of Plant Extracts. Investigation of Their Antioxidant Capacity and Antimicrobial Activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Fischer, J.; Akoh, C.C. Study of Anticancer Activities of Muscadine Grape Phenolics in Vitro. J. Agric. Food Chem. 2005, 53, 8804–8812. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, K.; Figueiredo, C.; Figueredo, T.; Freire, K.; Santos, F.; Alcantara-Neves, N.; Silva, T.; Piuvezam, M. Anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J. Ethnopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014, 62, 885–892. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Sogi, D.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef]
- Al-Saikhan, M.; Howard, L.; Miller, J. Antioxidant Activity and Total Phenolics in Different Genotypes of Potato (Solanum tuberosum, L.). J. Food Sci. 1995, 60, 341–343. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Cotelle, B.S.P.N. Role of Flavonoids in Oxidative Stress. Curr. Top. Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of Phenolic Acid Antimicrobial and Antioxidant Structure–Property Relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Delaey, J.; Dubruel, P.; Van Vlierberghe, S. Shape-Memory Polymers for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909047. [Google Scholar] [CrossRef]
- Monroe, M.B.B.; Easley, A.D.; Grant, K.; Fletcher, G.K.; Boyer, C.; Maitland, D.J. Multifunctional Shape-Memory Polymer Foams with Bio-inspired Antimicrobials. ChemPhysChem 2018, 19, 1999–2008. [Google Scholar] [CrossRef]
- Beaman, H.T.; Shepherd, E.; Satalin, J.; Blair, S.; Ramcharran, H.; Serinelli, S.; Gitto, L.; Dong, K.S.; Fikhman, D.; Nieman, G.; et al. Hemostatic shape memory polymer foams with improved survival in a lethal traumatic hemorrhage model. Acta Biomater. 2021, 137, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Weems, A.; Li, W.; Maitland, D.J.; Calle, L.M. Polyurethane Microparticles for Stimuli Response and Reduced Oxidative Degradation in Highly Porous Shape Memory Polymers. ACS Appl. Mater. Interfaces 2018, 10, 32998–33009. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Arriagada, F.; Correa, O.; Gunther, G.; Nonell, S.; Mura, F.; Olea-Azar, C.; Morales, J. Morin Flavonoid Adsorbed on Mesoporous Silica, a Novel Antioxidant Nanomaterial. PLoS ONE 2016, 11, e0164507. [Google Scholar] [CrossRef] [Green Version]
- Sahiner, N.; Sagbas, S.; Aktas, N. Preparation and characterization of monodisperse, mesoporous natural poly(tannic acid)–silica nanoparticle composites with antioxidant properties. Microporous Mesoporous Mater. 2016, 226, 316–324. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Essential oil mediated synthesis of silver nanocrystals for environmental, anti-microbial and antioxidant applications. Mater. Sci. Eng. C 2016, 61, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Marulasiddeshwara, M.B.; Dakshayani, S.S.; Kumar, M.N.S.; Chethana, R.; Kumar, P.R.; Devaraja, S. Facile-one pot-green synthesis, antibacterial, antifungal, antioxidant and antiplatelet activities of lignin capped silver nanoparticles: A promising therapeutic agent. Mater. Sci. Eng. C 2017, 81, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef]
- de Cristo Soares Alves, A.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C 2016, 60, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, R.; Lipka, E.; Howsam, M.; Betbeder, D. Evolution of availability of curcumin inside poly-lactic-co-glycolic acid nanoparticles: Impact on antioxidant and antinitrosant properties. Int. J. Nanomed. 2015, 10, 5355. [Google Scholar] [CrossRef] [Green Version]
- Perni, S.; Prokopovich, P. Poly-beta-amino-esters nano-vehicles based drug delivery system for cartilage. Nanomed. Nanotechnol. Biol. Med. 2016, 13, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Liu, J.; Fikhman, D.A.; Dong, K.S.; Monroe, M.B.B. Shape Memory Polymer Foams with Phenolic Acid-Based Antioxidant and Antimicrobial Properties for Traumatic Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 168. [Google Scholar] [CrossRef]
- Weems, A.C.; Wacker, K.T.; Carrow, J.K.; Boyle, A.J.; Maitland, D.J. Shape memory polyurethanes with oxidation-induced degradation: In vivo and in vitro correlations for endovascular material applications. Acta Biomater. 2017, 59, 33–44. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Kellett, M.E.; Greenspan, P.; Pegg, R.B. Modification of the cellular antioxidant activity (CAA) assay to study phenolic antioxidants in a Caco-2 cell line. Food Chem. 2018, 244, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.K.; Carranza, C.; Chawla, C.P.; Gray, P.; Eoh, J.H.; Cereceres, S.; Cosgriff-Hernandez, E.M. Comparative analysis of in vitro oxidative degradation of poly (carbonate urethanes) for biostability screening. J. Biomed. Mater. Res. Part A 2014, 102, 3649–3665. [Google Scholar] [CrossRef] [PubMed]
- Vakil, A.U.; Petryk, N.M.; Shepherd, E.; Monroe, M.B.B. Biostable Shape Memory Polymer Foams for Smart Biomaterial Applications. Polymers 2021, 13, 4084. [Google Scholar] [CrossRef] [PubMed]
- Vakil, A.U.; Petryk, N.M.; Shepherd, E.; Beaman, H.T.; Ganesh, P.S.; Dong, K.S.; Monroe, M.B.B. Shape Memory Polymer Foams with Tunable Degradation Profiles. ACS Appl. Bio Mater. 2021, 4, 6769–6779. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating Antioxidant Functionality into Polymer Materials: Fundamentals, Strategies, and Applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef] [Green Version]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef]
- Song, P.; Xu, Z.; Guo, Q. Bioinspired Strategy to Reinforce PVA with Improved Toughness and Thermal Properties via Hydrogen-Bond Self-Assembly. ACS Macro Lett. 2013, 2, 1100–1104. [Google Scholar] [CrossRef]
- Ravichandran, S.; Radhakrishnan, J.; Jayabal, P.; Venkatasubbu, G.D. Antibacterial screening studies of electrospun Polycaprolactone nano fibrous mat containing Clerodendrum phlomidis leaves extract. Appl. Surf. Sci. 2019, 484, 676–687. [Google Scholar] [CrossRef]
- Gaikwad, A.; Hlushko, H.; Karimineghlani, P.; Selin, V.; Sukhishvili, S.A. Hydrogen-Bonded, Mechanically Strong Nanofibers with Tunable Antioxidant Activity. ACS Appl. Mater. Interfaces 2020, 12, 11026–11035. [Google Scholar] [CrossRef]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Correlation between Structures and Antioxidant Activities of Polyvinylpyrrolidone/Garcinia mangostana L. Extract Composite Nanofiber Mats Prepared Using Electrospinning. J. Nanomater. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, J.L.; Subianto, S.; Dutta, N.K.; Choudhury, N.R. Induced insolubility of electrospun poly(N-vinylcaprolactam) fibres through hydrogen bonding with Tannic acid. Polymer 2016, 87, 194–201. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Gu, J.; Jia, S.; Guan, Y.; Zhang, Y. Zero-order release of polyphenolic drugs from dynamic, hydrogen-bonded LBL films. Soft Matter 2015, 12, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xu, R.; Wang, H.; Guan, Y.; Zhang, Y. Precise and tunable time-controlled drug release system using layer-by-layer films as erodible coatings. Mater. Sci. Eng. C 2020, 116, 111244. [Google Scholar] [CrossRef]
- Tan, J.Y.; Chua, C.K.; Leong, K.F. Indirect fabrication of gelatin scaffolds using rapid prototyping technology. Virtual Phys. Prototyp. 2010, 5, 45–53. [Google Scholar] [CrossRef]
- Duguay, D.G.; Labow, R.S.; Santerre, J.; McLean, D.D. Development of a mathematical model describing the enzymatic degradation of biomedical polyurethanes. 1. Background, rationale and model formulation. Polym. Degrad. Stab. 1995, 47, 229–249. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Bat, E.; Zhang, Z.; Feijen, J.; Grijpma, D.W.; Poot, A.A. Biodegradable elastomers for biomedical applications and regenerative medicine. Regen. Med. 2014, 9, 385–398. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Watanabe, T.; Murahashi, S.-I. Oxidation of Primary Amines to Oximes with Molecular Oxygen using 1,1-Diphenyl-2-picrylhydrazyl and WO3/Al2O3 as Catalysts. J. Org. Chem. 2013, 78, 2301–2310. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Thao-Heu, S.; Lavédrine, B. Assessment of the degradation of polyurethane foams after artificial and natural ageing by using pyrolysis-gas chromatography/mass spectrometry and headspace-solid phase microextraction-gas chromatography/mass spectrometry. J. Chromatogr. A 2011, 1218, 4498–4508. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and Cinnamic Acid Derivatives as Antioxidants: Structure−Activity Relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Fikhman, D.A.; Monroe, M.B.B. Shape Memory Polymer Foams with Phenolic Acid-Based Antioxidant Properties. Antioxidants 2022, 11, 1105. https://doi.org/10.3390/antiox11061105
Du C, Fikhman DA, Monroe MBB. Shape Memory Polymer Foams with Phenolic Acid-Based Antioxidant Properties. Antioxidants. 2022; 11(6):1105. https://doi.org/10.3390/antiox11061105
Chicago/Turabian StyleDu, Changling, David Anthony Fikhman, and Mary Beth Browning Monroe. 2022. "Shape Memory Polymer Foams with Phenolic Acid-Based Antioxidant Properties" Antioxidants 11, no. 6: 1105. https://doi.org/10.3390/antiox11061105