Antioxidative Effects of Standardized Aronia melanocarpa Extract on Reproductive and Metabolic Disturbances in a Rat Model of Polycystic Ovary Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Experimental Protocol
2.3.1. Assessment of Estrous Cycle
2.3.2. PCOS Induction
2.3.3. Grouping and Treatments
Aronia melanocarpa Standardized Extract
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Ultrasound Examination of Ovaries
2.6. Sacrificing the Animals and Sample Collection
Ovarian Tissue Homogenization
2.7. Biochemical Analysis
2.7.1. Hormonal Assays
2.7.2. Lipid Profile
2.7.3. OS Parameters
TBARS Determination
Determination of NO2−
Determination of H2O2
Determination of O2−
Determination of CAT
Determination of SOD
Determination of GSH
2.7.4. Determination of Total Protein Content
2.8. Ovarian Histology
2.9. Statistical Analysis
3. Results
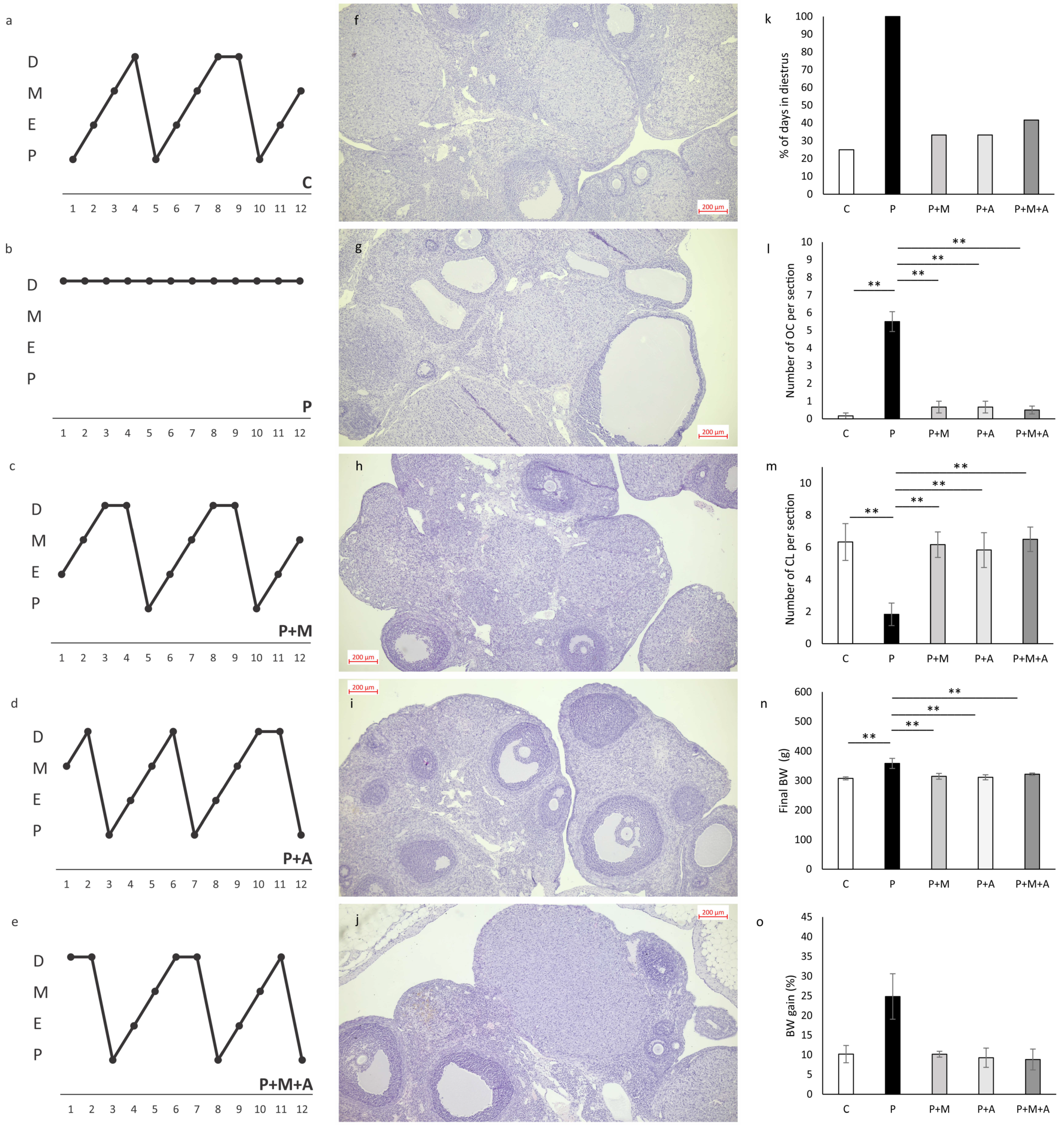
3.1. Effect of MET, SEA, and Their Combination on PCOS-Related Anthropometric Indices, Estrus Cycle, and Ovarian Histology
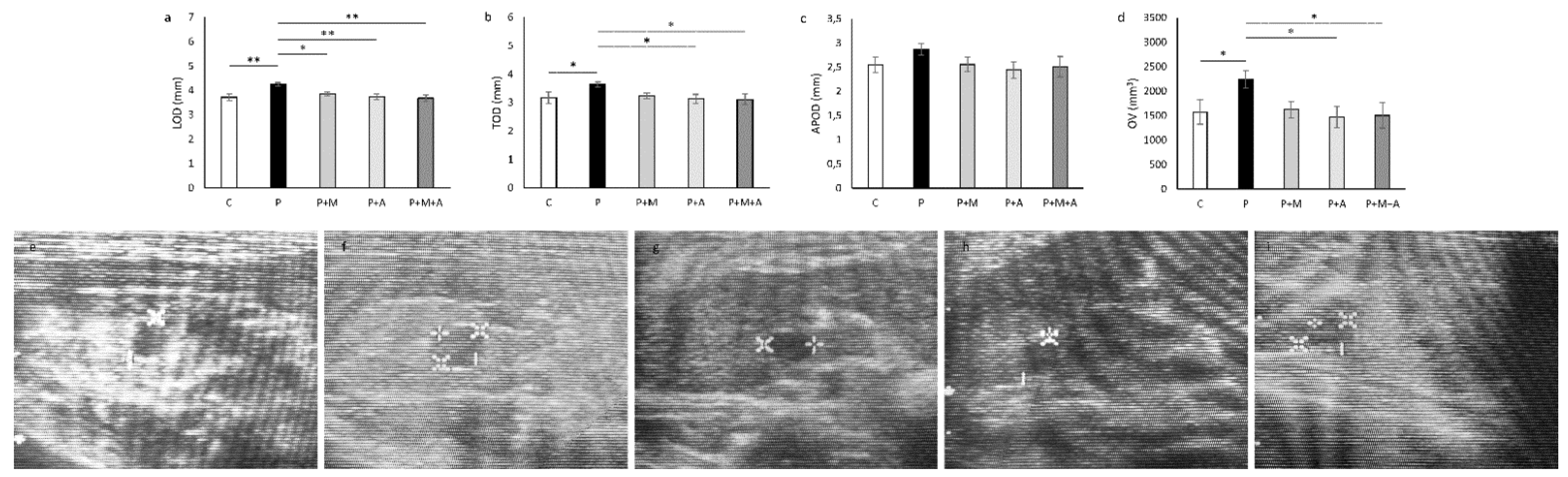
3.2. Effect of MET, SEA, and Their Combination on PCOS-Related Ovarian Dimensions Assessed by Ultrasonographical Analysis
3.3. Effect of MET, SEA, and Their Combination on PCOS-Related Changes in Serum Sex-Hormone Levels
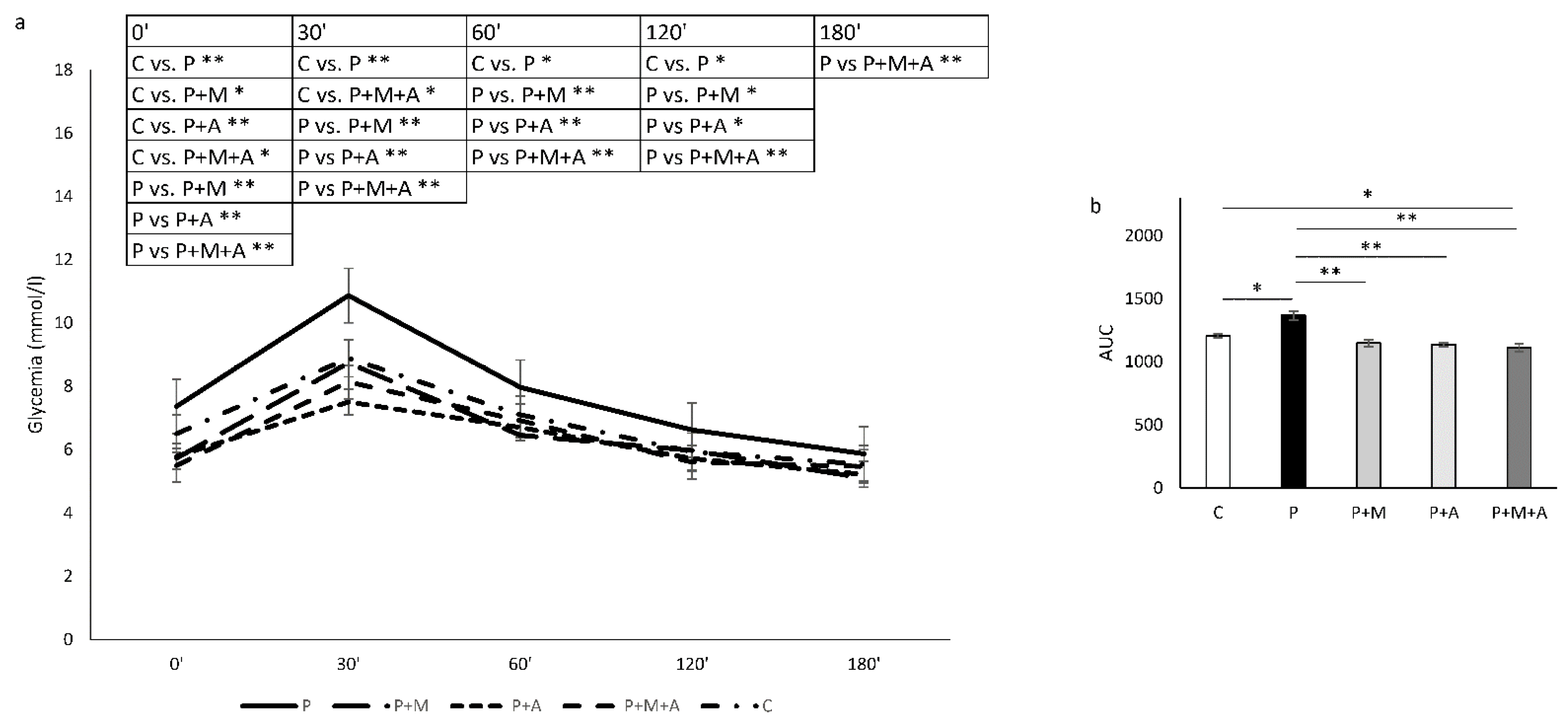
3.4. Effect of MET, SEA, and Their Combination on PCOS-Related Alterations in Glycoregulation
3.5. Effect of MET, SEA, and Their Combination on PCOS-Related Changes in Serum Lipid Profile
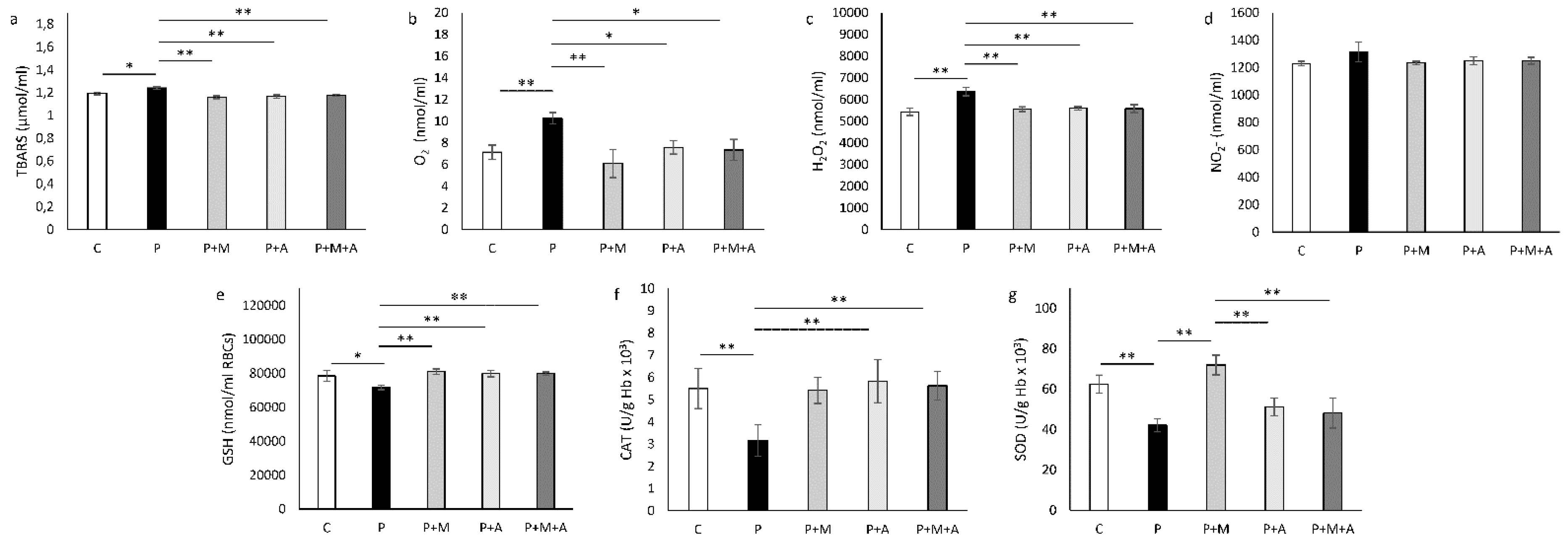
3.6. Effect of MET, SEA, and Their Combination on PCOS-Related Alteration of Systemic Oxidative Status
3.7. Effect of MET, SEA, and Their Combination on PCOS-Related Alteration of Ovarian Oxidative Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic ovary syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Abdel Khalek Abdel Razek, A.; Abou Elatta, H. Differentiation Between Phenotypes of Polycystic Ovarian Syndrome With Sonography. J. Diagn. Med. Sonogr. 2021, 37, 337–344. [Google Scholar] [CrossRef]
- Mathur, R.; Alexander, C.J.; Yano, J.; Trivax, B.; Azziz, R. Use of metformin in polycystic ovary syndrome. Am. J. Obstet. Gynecol. 2008, 199, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Dunaif, A. Should all women with PCOS be treated for insulin resistance? Fertil. Steril. 2012, 97, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Kalra, B.; Kalra, S.; Sharma, J.B. The inositols and polycystic ovary syndrome. Indian J. Endocrinol. Metab. 2016, 20, 720–724. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydłowska, I.; Nawrocka-Rutkowska, J.; Ziętek, M.; Verbanac, D.; Saso, L. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome-Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef]
- Unfer, V.; Facchinetti, F.; Orrù, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Aversa, A.; La Vignera, S.; Rago, R.; Gambineri, A.; Nappi, R.E.; Calogero, A.E.; Ferlin, A. Fundamental Concepts and Novel Aspects of Polycystic Ovarian Syndrome: Expert Consensus Resolutions. Front. Endocrinol. 2020, 11, 516. [Google Scholar] [CrossRef]
- Arentz, S.; Abbott, J.A.; Smith, C.A.; Bensoussan, A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement. Altern. Med. 2014, 14, 511. [Google Scholar] [CrossRef] [Green Version]
- Mancini, A.; Bruno, C.; Vergani, E.; d’Abate, C.; Giacchi, E.; Silvestrini, A. Oxidative Stress and Low-Grade Inflammation in Polycystic Ovary Syndrome: Controversies and New Insights. Int. J. Mol. Sci. 2021, 22, 1667. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia melanocarpa L.-A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [Green Version]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Dobričević, N.; Pliestić, S.; Galić, A.; Bilić, D.P.; Voća, S. Antioxidant Potential of Fruit Juice with Added Chokeberry Powder (Aronia melanocarpa). Molecules 2017, 22, 2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotła, E.; Oniszczuk, A. The Efficacy of Black Chokeberry Fruits against Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef]
- Jakovljevic, V.; Milic, P.; Bradic, J.; Jeremic, J.; Zivkovic, V.; Srejovic, I.; Nikolic Turnic, T.; Milosavljevic, I.; Jeremic, N.; Bolevich, S.; et al. Standardized Aronia melanocarpa Extract as Novel Supplement against Metabolic Syndrome: A Rat Model. Int. J. Mol. Sci. 2018, 20, 6. [Google Scholar] [CrossRef] [Green Version]
- Milosavljevic, I.; Jakovljevic, V.; Petrovic, D.; Draginic, N.; Jeremic, J.; Mitrovic, M.; Zivkovic, V.; Srejovic, I.; Stojic, V.; Bolevich, S.; et al. Standardized Aronia melanocarpa extract regulates redox status in patients receiving hemodialysis with anemia. Mol. Cell. Biochem. 2021, 476, 4167–4175. [Google Scholar] [CrossRef]
- Tasic, N.; Jakovljevic, V.L.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black chokeberry Aronia melanocarpa extract reduces blood pressure, glycemia and lipid profile in patients with metabolic syndrome: A prospective controlled trial. Mol. Cell. Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef]
- Joksimovic Jovic, J.; Sretenovic, J.; Jovic, N.; Rudic, J.; Zivkovic, V.; Srejovic, I.; Mihajlovic, K.; Draginic, N.; Andjic, M.; Milinkovic, M.; et al. Cardiovascular Properties of the Androgen-Induced PCOS Model in Rats: The Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 8862878. [Google Scholar] [CrossRef]
- Jovic, J.J.; Jovic, N.; Sretenovic, J.; Zivkovic, V.; Nikolic, M.; Rudic, J.; Milošević, V.; Ristić, N.; Andric, K.; Tomic, T.D.; et al. Normotensive rats with PCOS exhibit the hypertensive pattern: Focus on oxidative stress. Reproduction 2021, 163, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.J.; Jang, M.; Choi, J.H.; Park, K.S.; Cho, I.H. An Improved Dehydroepiandrosterone-Induced Rat Model of Polycystic Ovary Syndrome (PCOS): Post-pubertal Improve PCOS’s Features. Front. Endocrinol. 2018, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, M.; Meng, F.; Sun, X.; Xu, H.; Zhang, J.; Cui, P.; Morina, N.; Li, X.; Li, W.; et al. Metformin Ameliorates Uterine Defects in a Rat Model of Polycystic Ovary Syndrome. EBioMedicine 2017, 18, 157–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardo, L.G.; Buckett, W.M.; Khullar, V. Determination of the best-fitting ultrasound formulaic method for ovarian volume measurement in women with polycystic ovary syndrome. Fertil. Steril. 2003, 79, 632–633. [Google Scholar] [CrossRef]
- Furat Rencber, S.; Kurnaz Ozbek, S.; Eraldemır, C.; Sezer, Z.; Kum, T.; Ceylan, S.; Guzel, E. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: An experimental study. J. Ovarian Res. 2018, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Jeremic, J.N.; Jakovljevic, V.L.; Zivkovic, V.I.; Srejovic, I.M.; Bradic, J.V.; Milosavljevic, I.M.; Mitrovic, S.L.; Jovicic, N.U.; Bolevich, S.B.; Svistunov, A.A.; et al. Garlic Derived Diallyl Trisulfide in Experimental Metabolic Syndrome: Metabolic Effects and Cardioprotective Role. Int. J. Mol. Sci. 2020, 21, 9100. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Auclair, C.; Voisin, E. Nitroblue tetrazolium reduction. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 123–132. [Google Scholar]
- Beutler, E. Catalase. In Red Cell Metabolism. A Manual of Biochemical Methods; Beutler, E., Ed.; Grune and Stratton: New York, NY, USA, 1982; pp. 105–106. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170. [Google Scholar] [CrossRef]
- Beutler, E. Reduced glutathione (GSH). In Red Cell Metabolism. A Manual of Biochemical Methods; Beutler, E., Ed.; Grune and Stratton: New York, NY, USA, 1975; pp. 112–114. [Google Scholar]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Wen, L.; Lin, W.; Li, Q.; Chen, G.; Wen, J. Effect of Sleeve Gastrectomy on Kisspeptin Expression in the Hypothalamus of Rats with Polycystic Ovary Syndrome. Obesity 2020, 28, 1117–1128, Erratum in Obesity 2020, 28, 2242. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Li, S.; Hu, M.; Di, F.; Liu, J.; Du, Y. Decreased brain and muscle ARNT-like protein 1 expression mediated the contribution of hyperandrogenism to insulin resistance in polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2020, 18, 32. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Chen, Y.; Ma, M.; Li, M. Mechanism of quercetin on the improvement of ovulation disorder and regulation of ovarian CNP/NPR2 in PCOS model rats. J. Formos. Med. Assoc. 2021, 121, 1081–1092. [Google Scholar] [CrossRef]
- Kabel, A.M.; Ashour, A.M.; Omar, M.S.; Estfanous, R.S. Effect of fish oil and telmisartan on dehydroepiandrosterone-induced polycystic ovarian syndrome in rats: The role of oxidative stress, transforming growth factor beta-1, and nuclear factor kappa B. Food Sci. Nutr. 2020, 8, 5149–5159. [Google Scholar] [CrossRef]
- Peng, F.; Hu, Y.; Peng, S.; Zeng, N.; Shi, L. Apigenin exerts protective effect and restores ovarian function in dehydroepiandrosterone induced polycystic ovary syndrome rats: A biochemical and histological analysis. Ann. Med. 2022, 54, 578–587. [Google Scholar] [CrossRef]
- Çelik, L.S.; Kuyucu, Y.; Yenilmez, E.D.; Tuli, A.; Dağlıoğlu, K.; Mete, U.Ö. Effects of vitamin D on ovary in DHEA-treated PCOS rat model: A light and electron microscopic study. Ultrastruct. Pathol. 2018, 42, 55–64. [Google Scholar] [CrossRef]
- Jensterle, M.; Kravos, N.A.; Ferjan, S.; Goricar, K.; Dolzan, V.; Janez, A. Long-term efficacy of metformin in overweight-obese PCOS: Longitudinal follow-up of retrospective cohort. Endocr. Connect. 2020, 9, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Tohma, Y.A.; Onalan, G.; Tepeoglu, M.; Bayraktar, N.; Colak, E.; Ozcimen, E.E.; Zeyneloglu, H.B. Phosphodiesterase 4 inhibitor plus metformin is superior to metformin alone for the treatment of polycystic ovary syndrome: A rat model study. Exp. Ther. Med. 2019, 17, 4013–4022. [Google Scholar] [CrossRef] [Green Version]
- Dallas, C.; Gerbi, A.; Elbez, Y.; Caillard, P.; Zamaria, N.; Cloarec, M. Clinical study to assess the efficacy and safety of a citrus polyphenolic extract of red orange, grapefruit, and orange (Sinetrol-XPur) on weight management and metabolic parameters in healthy overweight individuals. Phytother. Res. 2014, 28, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J. Nutr. 2015, 145, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, B.; Anderson, R.A. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, S.; McCarthy, M.I.; Hardy, K. Development of polycystic ovary syndrome: Involvement of genetic and environmental factors. Int. J. Androl. 2006, 29, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Kara Bozkurt, D.; Kurban, D.; Takmaz, T.; Sevket, O.; Ozcan, P. 2-D and 3-D ultrasonographic characteristics of the ovary in women with PCOS and multifollicular ovaries. J. Obstet. Gynaecol. 2021, 41, 920–926. [Google Scholar] [CrossRef]
- Kalhori, Z.; Mehranjani, M.S.; Azadbakht, M.; Shariatzadeh, M.A. L-Carnitine improves endocrine function and folliculogenesis by reducing inflammation, oxidative stress and apoptosis in mice following induction of polycystic ovary syndrome. Reprod. Fertil. Dev. 2019, 31, 282–293. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, D.; Chen, Y.; Liu, D.; Chu, S.; Zhang, S. Beneficial effects of Heqi san on rat model of polycystic ovary syndrome through the PI3K/AKT pathway. DARU J. Pharm. Sci. 2017, 25, 21. [Google Scholar] [CrossRef]
- Kim, N.H.; Jegal, J.; Kim, Y.N.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. The Effects of Aronia melanocarpa Extract on Testosterone-Induced Benign Prostatic Hyperplasia in Rats, and Quantitative Analysis of Major Constituents Depending on Extract Conditions. Nutrients 2020, 12, 1575. [Google Scholar] [CrossRef]
- Schmitt, E.; Stopper, H. Estrogenic activity of naturally occurring anthocyanidins. Nutr. Cancer. 2001, 41, 145–149. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Maeda, H. Phytoestrogenic Activity of Blackcurrant Anthocyanins Is Partially Mediated through Estrogen Receptor Beta. Molecules 2017, 23, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, R.K.; Balaji, B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm. Biol. 2017, 55, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, S.; Pellatt, L.; Ramanathan, K.; Whitehead, S.A.; Mason, H.D. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology 2009, 150, 4794–4801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, S.A.; Al-Zaid, M.; Towers, P.A.; Scott, C.J.; Al-Shoumer, K.A. Irregular cycles and steroid hormones in polycystic ovary syndrome. Hum. Reprod. 2005, 20, 2402–2408. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Orisaka, M.; Tajima, K.; Hattori, K.; Kotsuji, F. Luteinizing hormone-induced Akt phosphorylation and androgen production are modulated by MAP Kinase in bovine theca cells. J. Ovarian Res. 2009, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Mahamed, R.R.; Maganhin, C.C.; Sasso GR, S.; de Jesus Simões, M.; Baracat MC, P.; Baracat, E.C. Metformin improves ovarian follicle dynamics by reducing theca cell proliferation and CYP-17 expression in an androgenized rat model. J. Ovarian Res. 2018, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Legro, R.S. Is there a male phenotype in polycystic ovary syndrome families? J. Pediatr. Endocrinol. Metab. 2000, 13 (Suppl. 5), 1307–1309. [Google Scholar]
- Di Guardo, F.; Ciotta, L.; Monteleone, M.; Palumbo, M. Male Equivalent Polycystic Ovarian Syndrome: Hormonal, Metabolic, and Clinical Aspects. Int. J. Fertil. Steril. 2020, 14, 79–83. [Google Scholar] [CrossRef]
- Yamane, T.; Kozuka, M.; Konda, D.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Improvement of blood glucose levels and obesity in mice given aronia juice by inhibition of dipeptidyl peptidase IV and α-glucosidase. J. Nutr. Biochem. 2016, 31, 106–112. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, J.; Zhang, Y.; Zhou, W. Metformin and sitagliptin combination therapy ameliorates polycystic ovary syndrome with insulin resistance through upregulation of lncRNA H19. Cell Cycle 2019, 18, 2538–2549. [Google Scholar] [CrossRef]
- Tang, T.; Lord, J.M.; Norman, R.J.; Yasmin, E.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2012, 5, CD003053. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.A. Dyslipidemia in PCOS. Steroids 2012, 77, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef] [Green Version]
- Daskalova, E.; Delchev, S.; Vladimirova-Kitova, L.; Kitov, S.; Denev, P. Black Chokeberry (Aronia melanocarpa) Functional Beverages Increase HDL-Cholesterol Levels in Aging Rats. Foods 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Jahan, S.; Razak, S.; Pirzada, M.; Ullah, H.; Almajwal, A.; Rauf, N.; Afsar, T. Protective effects of GABA against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats. J. Ovarian Res. 2017, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Enechukwu, C.I.; Onuegbu, A.J.; Olisekodiaka, M.J.; Eleje, G.U.; Ikechebelu, J.I.; Ugboaja, J.O.; Amah, U.K.; Okwara, J.E.; Igwegbe, A.O. Oxidative stress markers and lipid profiles of patients with polycystic ovary syndrome in a Nigerian tertiary hospital. Obstet. Gynecol. Sci. 2019, 62, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, X.; Ma, L.; Chen, Y.; He, S. Soy isoflavones alleviate polycystic ovary syndrome in rats by regulating NF-κB signaling pathway. Bioengineered 2021, 12, 7215–7223. [Google Scholar] [CrossRef] [PubMed]


| Group | Testosterone | Estradiol | Progesterone |
|---|---|---|---|
| C | 0.14 ± 0.05 | 78.78 ± 8.76 | 24.56 ± 7.40 |
| P | 2.95 ± 0.49 a | 118.00 ± 17.93 | 11.74 ± 1.02 A |
| P + M | 0.12 ± 0.04 b | 64.78 ± 7.88 B | 28.15 ± 6.72 B |
| P + A | 0.13 ± 0.05 b | 53.38 ± 7.84 B | 27.63 ± 6.83 b |
| P + M + A | 0.15 ± 0.09 b | 61.50 ± 11.03 B | 27.17 ± 80 B |
| Group | Cholesterol | Triglycerides | HDL | LDL |
|---|---|---|---|---|
| C | 2.07 ± 0.19 | 0.46 ± 0.09 | 0.77 ± 0.03 | 1.18 ± 0.07 |
| P | 2.82 ± 0.07 a | 1.05 ± 0.10 a | 0.68 ± 0.09 | 1.67 ± 0.05 a |
| P + M | 1.88 ± 0.20 b | 0.53 ± 0.12 b | 0.75 ± 0.12 | 1.00 ± 0.16 b |
| P + A | 1.92 ± 0.27 B | 0.50 ± 0.12 b | 0.95 ± 0.06 | 1.03 ± 0.11 b |
| P + M + A | 2.18 ± 0.21 b | 0.43 ± 0.11 b | 0.90 ± 0.08 | 0.14 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudic, J.; Jakovljevic, V.; Jovic, N.; Nikolic, M.; Sretenovic, J.; Mitrovic, S.; Bolevich, S.; Bolevich, S.; Mitrovic, M.; Raicevic, S.; et al. Antioxidative Effects of Standardized Aronia melanocarpa Extract on Reproductive and Metabolic Disturbances in a Rat Model of Polycystic Ovary Syndrome. Antioxidants 2022, 11, 1099. https://doi.org/10.3390/antiox11061099
Rudic J, Jakovljevic V, Jovic N, Nikolic M, Sretenovic J, Mitrovic S, Bolevich S, Bolevich S, Mitrovic M, Raicevic S, et al. Antioxidative Effects of Standardized Aronia melanocarpa Extract on Reproductive and Metabolic Disturbances in a Rat Model of Polycystic Ovary Syndrome. Antioxidants. 2022; 11(6):1099. https://doi.org/10.3390/antiox11061099
Chicago/Turabian StyleRudic, Jovan, Vladimir Jakovljevic, Nikola Jovic, Maja Nikolic, Jasmina Sretenovic, Slobodanka Mitrovic, Sergey Bolevich, Stefani Bolevich, Miroslav Mitrovic, Sasa Raicevic, and et al. 2022. "Antioxidative Effects of Standardized Aronia melanocarpa Extract on Reproductive and Metabolic Disturbances in a Rat Model of Polycystic Ovary Syndrome" Antioxidants 11, no. 6: 1099. https://doi.org/10.3390/antiox11061099
APA StyleRudic, J., Jakovljevic, V., Jovic, N., Nikolic, M., Sretenovic, J., Mitrovic, S., Bolevich, S., Bolevich, S., Mitrovic, M., Raicevic, S., Andric, K., Dimkic Milenkovic, A., Rakic, D., & Joksimovic Jovic, J. (2022). Antioxidative Effects of Standardized Aronia melanocarpa Extract on Reproductive and Metabolic Disturbances in a Rat Model of Polycystic Ovary Syndrome. Antioxidants, 11(6), 1099. https://doi.org/10.3390/antiox11061099









