Evolutionary Adaptations of Parasitic Flatworms to Different Oxygen Tensions
Abstract
1. Introduction
2. Parasitic Flatworms and Oxygen Availability
2.1. General Information about Flatworms
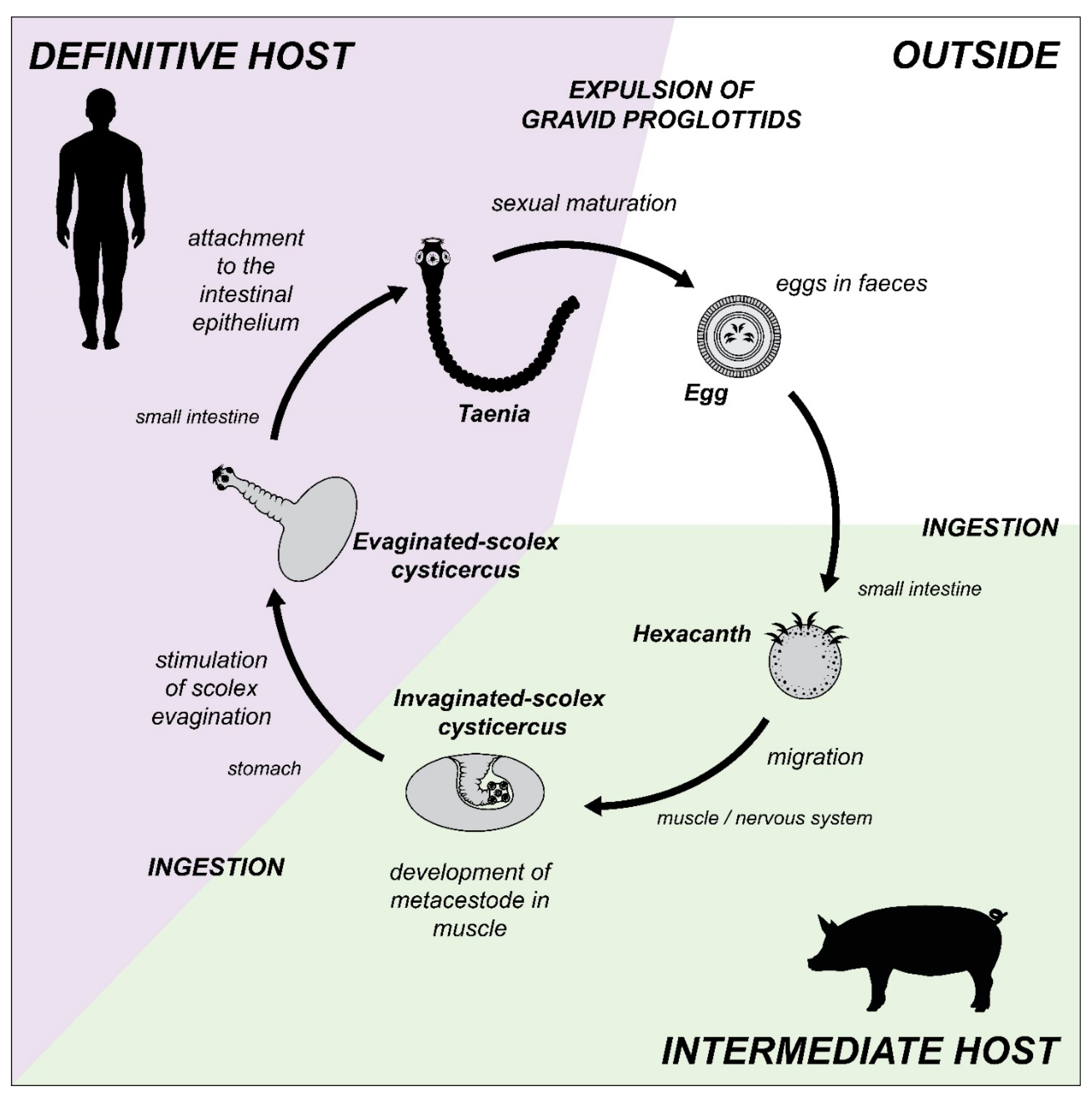
2.2. The Complex Life Cycles of the Trematoda and Cestoda
3. Adaptations of Parasitic Flatworms to Changes in Oxygen Tension
3.1. Ultrastructural Adaptations
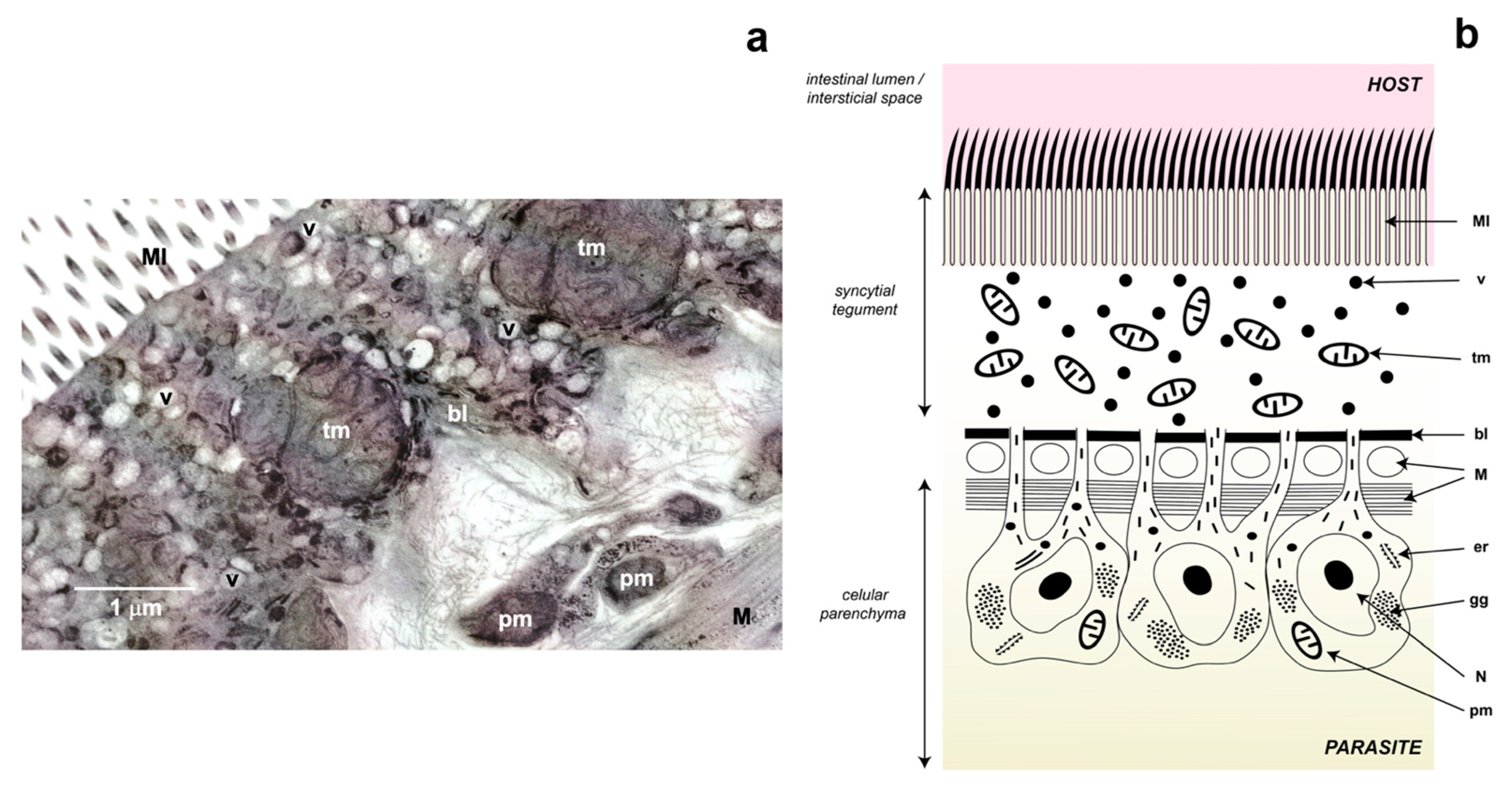
3.1.1. Body Wall (Tegument)
3.1.2. Diversity of Mitochondria
3.2. Metabolic Adaptations
3.2.1. Oxygen Carriers and Storage
3.2.2. HIF and the Detection of Oxygen in the Environment
3.2.3. Aerobic Metabolism
3.2.4. Anaerobic Metabolism
- It occurs in two cell compartments: cytosol and mitochondrion
- It is coupled to the generation of the proton gradient produced in complex I of the ETC, when the NADH coming from the formation of Acetyl-CoA and the one resulting from the activity of mME are oxidized (Figure 1).
- At the end of the pathway, several reduced compounds are obtained, including succinate, acetate, and propionate [119].
- The need for a final electron acceptor molecule
- Maintenance of redox balance
- Both are carried out in the mitochondrial compartments, which allows the formation of a proton gradient and therefore the synthesis of ATP.
3.3. Molecular Adaptations
3.3.1. Immune Response and Oxidative Stress in Parasitic Flatworms
Sources of Exposure to Reactive Oxygen Species
Functioning and Localization of Enzymatic Antioxidant Systems in Parasitic Flatworms
- (1)
- Superoxide Dismutase and Peroxidases
- (2)
- Thioredoxin-Glutathione Reductase
- (3)
- Glutathione-S-Transferase and Other Antioxidant Molecules
- (a)
- Cytochrome p450. This cytochrome has monooxygenase activity, which allows it to oxidize multiple exogenous molecules and contributes to their detoxification. Both in the flukes of S. mansoni [176] and Opisthorchis felineus [177], as well as in the genomes of the cestodes, only one copy of the gene has been found [92].
- (b)
- Phytochelatin synthase (PCS). This enzyme works together with GST in the detoxification of xenobiotics and in the uptake of potentially harmful transition metals [178] through the formation of glutathione biopolymers [179]. Originally reported in plants, the presence of a functional PCS was reported in S. mansoni [180] and its presence was later confirmed in the genomes of cestodes [92] as well as in the parasitic nematode Ancylostoma ceylanicum [181]. Previously, we reported the presence of three unknown thiols in an extract of low molecular weight thiols obtained from the cysticercus of T. crassiceps [165] and whose retention patterns coincide with those found in S. mansoni and are associated with phytochelatins of different sizes [180]. Significantly, both phytochelatins and the PSC gene are absent in the mammalian hosts, suggesting that it is an adaptation to parasitic life [182], although its specific function is still under discussion [183].
- (c)
- Myoglobin (Mb). We have previously talked about the capacity of Mb to store O2. Other activities that Mb presents are peroxidase/dioxygenase, having the ability to interact with O2 molecules such as NO, CO, and H2O2 [184]. Ren et al. reported that a globin from C. sinensis (CsMb) showed peroxidase activity and that it may be important for ROS detoxification because of its overexpression after incubation with exogenous H2O2 [185]. This was later corroborated by Kim et al., who showed that incubation of C. sinensis flukes under aerobic conditions or in the presence of nitric oxide or nitrite is sufficient to induce the expression of the gene encoding CsMb [72]. Interestingly, overexpression of CsMb was also observed when flukes were co-incubated with human cholangiocytes (bile epithelial cells).
- (d)
- Other enzymes. Under conditions of oxidative stress, hydroxyl groups can be nonspecifically oxidized to their aldehyde form. Similarly, reactions with radicals can lead to the formation of reactive carbonyls. As part of the characterization of the response of E. granulosus protoscolex to oxidative stress by exogenous H2O2, Cancela et al. reported high levels of a type of aldo-keto reductase (AKR), estradiol-17-beta dehydrogenase, and the enzyme carbonyl reductase 1 (CBR) [31]. AKRs are NADPH-dependent enzymes that can reduce aldehydes to alcohols [186]. On the other hand, CBR is an enzyme necessary to detoxify reactive carbonyls [187].
- (4)
- Complexity of the Antioxidant Response
- -
- Time elapsed since the establishment of the infection. Skrzycki et al. compared oxidative stress markers and the presence of antioxidant enzymes in two populations of the adult H. diminuta cestode, one with a short experimental infection time and another with a well-consolidated infection [126]. They found a high activity of the enzymes SOD, Ph-GPx, and Prx in the anterior end (close to the intestinal epithelium) comparable to that of both tapeworms. However, in older tapeworms they found higher GST activity and lower GSH concentration, which suggests that adults also face a constant detoxification process. As the tapeworm size increases and occupies the ileum, oxidant indicators increase with a progressive decrease in antioxidant enzymes (except GST); however, at the posterior end of the parasite, where the proglottids are sexually mature, antioxidant enzymes increase again. This suggests that the production and storage of eggs, which occurs in the mature and gravid proglottids located at the posterior end of tapeworms, requires the participation of antioxidant systems. In the case of old tapeworms, a similar pattern of antioxidant enzyme activity is observed, but contrary to expectations, oxidative stress markers always remained below the levels reached by their young counterparts. This suggests that by consolidating the infection, old tapeworms have managed to modulate the immune response, which leads to less exposure to ROS. Finally, the only enzyme that does not significantly reduce its activity is GST, which implies that the parasite is always ready to purge toxic metabolites.
- -
- Sexual dimorphism of the parasite. Oliveira et al. compared the contribution of nutrients and gender of unpaired adults of S. mansoni on O2 consumption pathways and susceptibility to oxidative stress [85]. In general, they found a greater contribution of glutamine to respiration in females, which contrasts with a greater contribution of glucose in the case of males. The O2 consumption rate was higher in males compared to females, regardless of the respiratory substrate. In contrast, the rate of ROS production and the expression of antioxidant enzymes was higher in females than in males. This suggests that the physiological process of egg production is related to an increase in endogenous ROS. Finally, females were more tolerant to exogenous oxidative stress than males, possibly due to basal overexpression of their antioxidant systems.
3.3.2. Parasite-Host Relationships
4. Conclusions
- An energy metabolism that transits between aerobiosis and anaerobiosis depending on the availability of oxygen.
- This is possible because they have an enzymatic repertoire with common metabolic pathways involving enzymes that catalyze reversible reactions.
- In anaerobiosis, in addition to lactic fermentation, they have another fermentation pathway known as malate dismutation that allows them to obtain a greater amount of energy even in the absence of oxygen. Additionally, this pathway allows them to maintain their redox balance by eliminating the electrons in molecules that are secreted into the medium, mainly succinate, acetate, and propionate.
- Due to the absence of a circulatory system, they developed a tegument through which O2 diffuses.
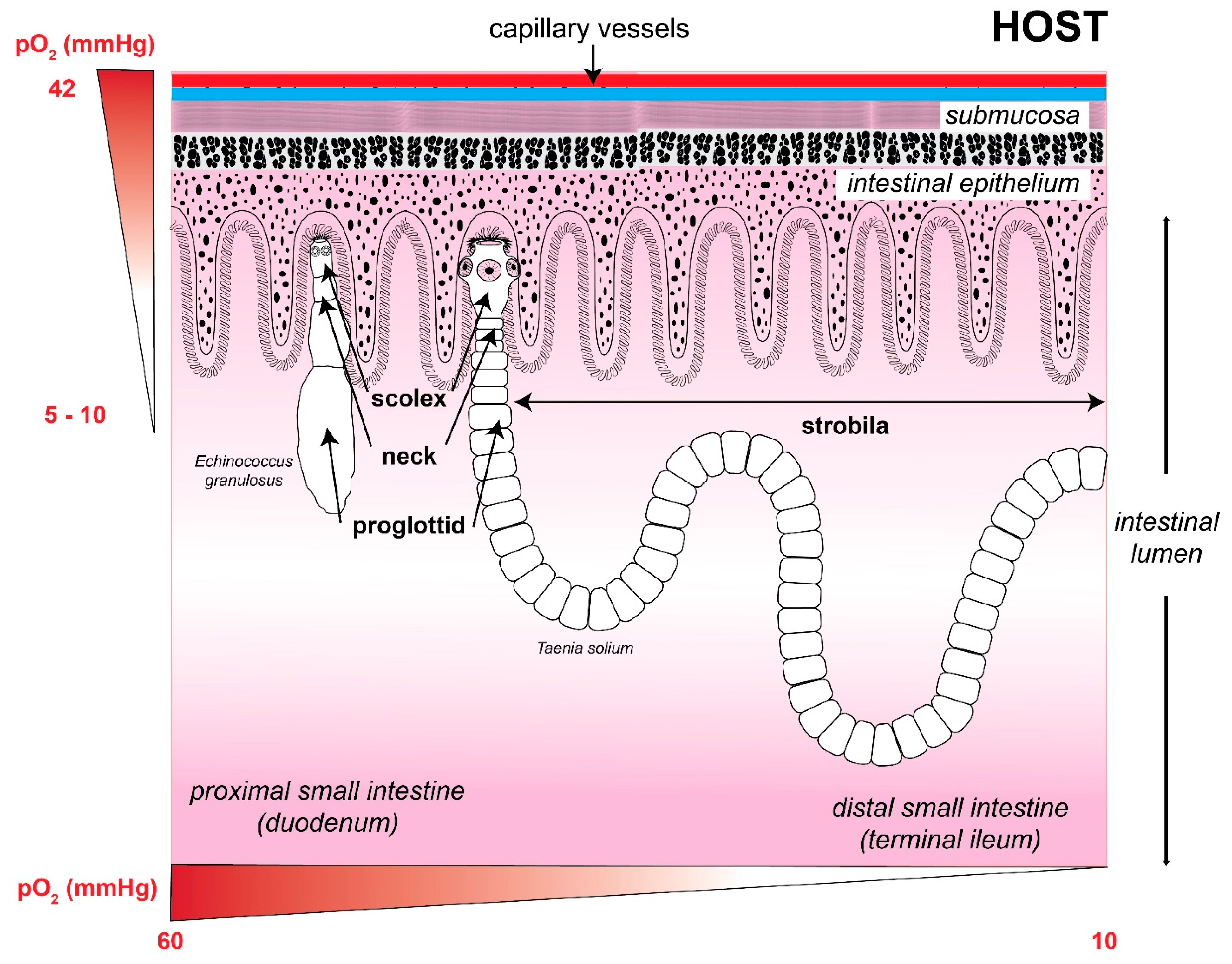
- The diffusion of oxygen generates the formation of a concentration gradient, its presence being greater in the tegument than in the parenchyma.
- Two populations of mitochondria, aerobic and anaerobic, have been described; the first located mainly in the tegument.
- Finally, the exposure of the tegument to a higher concentration of O2 implies a greater production of ROS in it, as indirectly demonstrated by a significant presence of antioxidant enzymes in this region (SOD, GPx, Prx).
Funding
Acknowledgments
Conflicts of Interest
References
- Hamilton, T.L.; Bryant, D.A.; Macalady, J.L. The role of biology in planetary evolution: Cyanobacterial primary production in low-oxygen Proterozoic oceans. Environ. Microbiol. 2016, 18, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Fisher, W.W.; Hemp, J.; Valentine, J.S. How did life survive earth’s great oxygenation? Curr. Opin. Chem. Biol. 2016, 31, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Lenton, T.M.; Dahl, T.W.; Daines, S.J.; Mills, B.J.W.; Ozaki, K.; Saltzman, M.R.; Porada, P. Earliest land plants created modern levels of atmospheric oxygen. Proc. Natl. Acad. Sci. USA 2016, 113, 9704–9709. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Puttick, M.N.; Clark, J.D.; Donoghue, P.C.J. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. USA 2018, 115, E2274–E2283. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The rise of oxygen in Earth´s ocean and atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zimorski, V.; Mental, M.; Tielens, A.G.M.; Martin, W.F. Energy metabolism in anaerobic eukaryotes and Earth’s late oxygenation. Free Radic. Biol. Med. 2019, 140, 279–294. [Google Scholar] [CrossRef]
- Mentel, M.; Martin, W. Energy metabolism among eukaryotic anaerobes in light of Proterozoic ocean chemistry. Phil. Trans. R. Soc. B Biol. Sci. 2008, 363, 2717–2729. [Google Scholar] [CrossRef]
- Mentel, M.; Rottger, M.; Leys, S.; Tielens, A.G.M.; Martin, W.F. Of early animals, anaerobic mitochondria, and a modern sponge. Bioassays 2014, 36, 924–932. [Google Scholar] [CrossRef]
- Muller, M. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 2012, 76, 453–474. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; Rotte, C.; van Hellemond, J.J.; Martin, W. Mitochondria as we don’t know them. Trends Biochem. Sci. 2002, 27, 564–572. [Google Scholar] [CrossRef]
- Meléndez-Hevia, E.; Montero-Gómez, N.; Montero, F. From prebiotic chemistry to cellular metabolism-The chemical evolution of metabolism before Darwinian natural selection. J. Theor. Biol. 2008, 252, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.H.; Conway, T. Evolution of carbohydrate metabolic pathways. Res. Microbiol. 1996, 147, 448–455. [Google Scholar] [CrossRef]
- Van Der Giezen, M.; Lenton, T.M. The rise of oxygen and complex life. J. Eukaryot. Microbiol. 2012, 59, 111–113. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.T.; Valentine, J.S. How Super is Superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Collins, J.J., 3rd. Platyhelminthes. Curr. Biol. 2017, 27, R252–R256. [Google Scholar] [CrossRef]
- Bobes, R.J.; Fragoso, G.; Fleury, A.; García-Varela, M.; Sciutto, E.; Larralde, C.; Laclette, J.P. Evolution, molecular epidemiology and perspectives on the research of taeniid parasites with special emphasis on Taenia solium. Infect. Genet. Evol. 2014, 23, 150–160. [Google Scholar] [CrossRef]
- Egger, B.; Steinke, D.; Tarui, H.; De Mulder, K.; Arendt, D.; Borgonie, G.; Funayama, N.; Gschwentner, R.; Hartenstein, V.; Hobmayer, B.; et al. To be or not to be a flatworm: The acoel controversy. PLoS ONE 2009, 4, e5502. [Google Scholar] [CrossRef]
- Riutort, M.; Álvarez-Presas, M.; Lázaro, E.; Solà, E.; Paps, J. Evolutionary history of the Tricladida and the Platyhelminthes: An up-to-date phylogenetic and systematic account. Int. J. Dev. Biol. 2012, 56, 5–17. [Google Scholar] [CrossRef]
- Cheng, L.C.; Tu, K.C.; Seidel, C.W.; Robb, S.; Guo, F.; Sánchez Alvarado, A. Cellular, ultrastructural and molecular analyses of epidermal cell development in the planarian Schmidtea mediterranea. Dev. Biol. 2018, 433, 357–373. [Google Scholar] [CrossRef]
- Hahn, C.; Fromm, B.; Bachmann, L. Comparative genomics of flatworms (platyhelminthes) reveals shared genomic features of ecto- and endoparastic neodermata. Genome Biol. Evol. 2014, 6, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Perkins, E.M.; Donnellan, S.C.; Bertozzi, T.; Whittington, I.D. Closing the mitochondrial circle on paraphyly of the Monogenea (Platyhelminthes) infers evolution in the diet of parasitic flatworms. Int. J. Parasitol. 2010, 40, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.; Lamberton, P.; McGregor, A. Human liver flukes. Lancet Gastroenterol. Hepatol. 2017, 2, 680–689. [Google Scholar] [CrossRef]
- Toledo, A.; Osorio, R.; Matus, C.; Martinez Lopez, Y.; Ramirez Cruz, N.; Sciutto, E.; Fragoso, G.; Arauz, A.; Carrillo-Mezo, R.; Fleury, A. Human Extraparenchymal Neurocysticercosis: The Control of Inflammation Favors the Host…but Also the Parasite. Front. Immunol. 2018, 9, 2652. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.D.; Skuce, P.J.; McNeilly, T.N. The influence of liver fluke infection on production in sheep and cattle: A meta-analysis. Int. J. Parasitol. 2021, 51, 913–924. [Google Scholar] [CrossRef]
- Cwiklinski, K.; O’Neill, S.M.; Donnelly, S.; Dalton, J.P. A prospective view of animal and human Fasciolosis. Parasite Immunol. 2016, 38, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Devleesschauwer, B.; Schmidt, V.; Winkler, A.S.; Harrison, W.; Johansen, M.V. The societal cost of Taenia solium cysticercosis in Tanzania. Acta Trop. 2017, 165, 141–154. [Google Scholar] [CrossRef]
- Neves, L.X.; Wilson, R.A.; Brownridge, P.; Harman, V.M.; Holman, S.W.; Beynon, R.J.; Eyers, C.E.; DeMarco, R.; Castro-Borges, W. Quantitative Proteomics of Enriched Esophageal and Gut Tissues from the Human Blood Fluke Schistosoma mansoni Pinpoints Secreted Proteins for Vaccine Development. J. Proteome Res. 2020, 19, 314–326. [Google Scholar] [CrossRef]
- Morley, N.J. Ecology of free-living metacercariae (Trematoda). Adv. Parasitol. 2015, 89, 1–78. [Google Scholar]
- Li, W.H.; Yang, Y.; Zhang, N.Z.; Wang, J.K.; Liu, Y.J.; Li, L.; Yan, H.B.; Jia, W.Z.; Fu, B. Comparative Transcriptome Analyses of the Developmental Stages of Taenia multiceps. Front. Vet. Sci. 2021, 8, 677045. [Google Scholar] [CrossRef]
- Cancela, M.; Paes, J.A.; Moura, H.; Barr, J.R.; Zaha, A.; Ferreira, H.B. Unraveling oxidative stress response in the cestode parasite Echinococcus granulosus. Sci. Rep. 2019, 9, 15876. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Behm, C.A. Biochemistry of Parasites and Host Parasite Relationships; Van den Bossche, H., Ed.; North Holland: Amsterdam, The Netherland, 1976; pp. 89–94. [Google Scholar]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- De Santis, V.; Singer, M. Tissue oxygen tension monitoring of organ perfusion: Rationale, methodologies, and literature review. Br. J. Anaesth. 2015, 115, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.S.; Bittinger, K.; Esipova, T.V.; Hou, L.; Chau, L.; Jiang, J.; Mesaros, C.; Lund, P.J.; Liang, X.; FitzGerald, G.A.; et al. Microbes vs. chemistry in the origin of the anaerobic gut lumen. Proc. Natl. Acad. Sci. USA 2018, 115, 4170–4175. [Google Scholar] [CrossRef]
- Mori, M.P.; Penjweini, R.; Knutson, J.R.; Wang, P.; Hwang, P.M. Mitochondria and oxygen homeostasis. FEBS J. 2021. [Google Scholar] [CrossRef]
- Komuniecki, R.; Tielens, A.G.M. Carbohydrate and energy metabolism helminths. In Molecular Medical Parasitology; Marr, J.J., Nilsen, T.W., Komuniecki, R.W., Eds.; Academic Press: London, UK, 2003; pp. 339–358. [Google Scholar]
- Ward, J.B.J.; Keely, S.J.; Keely, S.J. Oxygen in the regulation of intestinal epithelial transport. J. Phisiol. 2014, 592, 2473–2489. [Google Scholar] [CrossRef]
- Harada, S.; Inaoka, D.K.; Ohmori, J.; Kita, K. Diversity of parasite complex II. BBA 2013, 1827, 658–667. [Google Scholar] [CrossRef]
- Van Hellemond, J.J.; Tielens, A.G.M. Expression and functional properties of fumarate reductase. Biochem. J. 1994, 304, 321–333. [Google Scholar] [CrossRef]
- Kita, K.; Nihei, C.; Tomitsuka, E. Parasite Mitochondria as Drug Target: Diversity and Dynamic Changes During the Life Cycle. Curr. Med. Chem. 2003, 10, 2535–2548. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; van den Heuvel, J.M.; van den Berg, S.G. Differences in intermediary energy metabolism between juvenile and adult Fasciola hepatica. Mol. Biochem. Parasitol. 1987, 24, 273–281. [Google Scholar] [CrossRef]
- Tielens, A.G.M. The carbohydrate metabolism of Fasciola hepatica, an example of biochemical adaptations in parasitic helminths. Acta Parasitol. 2000, 45, 59–66. [Google Scholar]
- Poddubnaya, L.G.; Scholz, T.; Kuchta, R.; Levron, C.; Brunanská, M. Ultrastructure of the proglottid tegument (neodermis) of the cestode Echinophallus wageneri (Pseudophyllidea: Echinophallidae), a parasite of the bathypelagic fish Centrolophus niger. Parasitol. Res. 2007, 101, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Wendt, G.R.; Collins, J.N.; Pei, J.; Pearson, M.S.; Bennett, H.M.; Loukas, A.; Berriman, M.; Grishin, N.V.; Collins, J.J., 3rd. Flatworm-specific transcriptional regulators promote the specification of tegumental progenitors in Schistosoma mansoni. Elife 2018, 7, e33221. [Google Scholar] [CrossRef] [PubMed]
- Sotillo, J.; Pearson, M.; Becker, L.; Mulvenna, J.; Loukas, A. A quantitative proteomic analysis of the tegumental proteins from Schistosoma mansoni schistosomula reveals novel potential therapeutic targets. Int. J. Parasitol. 2015, 45, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, S.; Fukuda, K.; Nakamura, T.; Aoki, T.; Sugiyama, H. Paragonimus westermani possesses aerobic and anaerobic mitochondria in different tissues, adapting to fluctuating oxygen tension in microaerobic habitats. Int. J. Parasitol. 2010, 40, 1651–1658. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; van den Heuvel, J.M.; van den Bergh, S.G. The energy metabolism of Fasciola hepatica during its development in the final host. Mol. Biochem. Parasitol. 1984, 13, 301–307. [Google Scholar] [CrossRef]
- Starling, J.A. Tegumental carbohydrate transport in intestinal helminths: Correlation between mechanisms of membrane transport and the biochemical environment of absorptive surfaces. Trends Am. Microsc. Soc. 1975, 94, 508–523. [Google Scholar] [CrossRef]
- Brehm, K.; Koziol, U. Echinococcus-Host Interactions at Cellular and Molecular Levels. Adv. Parasitol. 2017, 95, 147–212. [Google Scholar]
- Van Hellemond, J.J.; Retra, K.; Brouwers, J.F.H.M.; van Balkom, M.Y.; Shoemarker, C.B.; Tielens, A.G.T. Functions of the tegument of schistosomes: Clues from the proteome and lipidome. Int. J. Parasitol. 2006, 36, 691–699. [Google Scholar] [CrossRef]
- Leow, C.Y.; Willis, C.; Hofmann, A.; Jones, M.K. Structure-function analysis of apical membrane-associated molecules of the tegument of schistosome parasites of humans: Prospects for identification of novel targets for parasite control. Br. J. Pharmacol. 2015, 172, 1653–1663. [Google Scholar] [CrossRef]
- Tovar, J.; Fischer, A.; Clark, C.G. The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol. Microbiol. 1999, 32, 1013–1021. [Google Scholar] [CrossRef]
- Makiuchi, T.; Nozaki, T. Highly divergent mitochondrion-related organelles in anaerobic parasitic protozoa. Biochimie 2014, 100, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Palade, G.E. An electron microscope study of the mitochondrial structure. J. Histochem. Chem. Cytochem. 1953, 1, 188–211. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, I.E. Structure and morphology. Integration into the cell. In Mitochondria, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 18–59. [Google Scholar]
- Kita, K.; Takamiya, S. Electron-transfer complexes in Ascaris mitochondria. Adv. Parasitol. 2002, 51, 95–131. [Google Scholar] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.P.; Geary, T.G. The Structure and Function of Helminth Surfaces: Structural. In Biochemistry and Molecular Biology of Parasites; Marr, J.J., Müller, M., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 203–232. [Google Scholar]
- Tielens, A.G.M. Energy generation in parasitic helminths. Parasitol. Today 1994, 10, 346–352. [Google Scholar] [CrossRef]
- Lumsden, R.D. Ultrastructure of mitochondria in a cestode, Lacistorrhynchus tenuis (V. Benden, 1858). J. Parasitol. 1967, 53, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Del Arenal, M.I.P.; Cea, B.A.; Moreno-Sanchez, R.; Escamilla, J.E. A method for the isolation of tegument syncytium mitochondria from Taenia crassiceps cysticerci and partial characterization of their aerobic metabolism. J. Parasitol. 1998, 84, 461–468. [Google Scholar] [CrossRef]
- Takamiya, S.; Wang, H.; Hiraishi, A.; Yu, Y.; Hamajima, F. Respiratory chain of the lung Paragonimus westermani: Facultative anaerobic mitochondria. Arch. Biochem. Biophys. 1994, 312, 142–150. [Google Scholar] [CrossRef]
- Roppongi, T.; Mizuno, N.; Miyagawa, Y.; Kobayashi, T.; Nakagawa, K.; Adachi, S. Solubility and mass transfer coefficient of oxygen through gas- and water-lipid interfaces. J. Food Sci. 2021, 86, 867–873. [Google Scholar] [CrossRef]
- Terwilliger, N.B. Functional adaptations of oxygen-transport proteins. J. Exp. Biol. 1998, 201, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Gell, D.A. Structure and function of haemoglobins. Blood Cells Mol. Dis. 2018, 70, 13–42. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Opazo, J.C.; Hoffmann, F.G. Gene duplication, genome duplication, and the functional diversification of vertebrate globins. Mol. Phylogenet. Evol. 2013, 66, 469–478. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, J.V.; Yu, H.S.; Jeong, H.J.; Hong, Y.C.; Kim, J.; Kong, H.H.; Chung, D.I. Molecular characterization of two myoglobins of Paragonimus westermani. J. Parasitol. 2007, 93, 97–103. [Google Scholar] [CrossRef]
- Goldberg, D.E. The enigmatic oxygen-avid hemoglobin of Ascaris. Bioessays 1995, 17, 177–182. [Google Scholar] [CrossRef]
- Kiger, L.; Rashid, A.K.; Griffon, N.; Haque, M.; Moens, L.; Gibson, Q.H.; Poyart, C.; Marden, M.C. Trematode hemoglobins show exceptionally high oxygen affinity. Biophys. J. 1998, 75, 990–998. [Google Scholar] [CrossRef]
- González, R.; Mendoza-Hernández, G.; Plancarte, A. Purification of Taenia solium cysticerci superoxide dismutase and myoglobin copurification. Parasitol. Res. 2002, 88, 881–887. [Google Scholar]
- Kim, S.H.; Yang, D.; Bae, Y.A. Hypoxic and nitrosative stress conditions modulate expression of myoglobin genes in a carcinogenic hepatobiliary trematode, Clonorchis sinensis. PLoS Negl. Trop. Dis. 2021, 15, e0009811. [Google Scholar] [CrossRef]
- Burmester, T.; Hankeln, T. Function and evolution of vertebrate globins. Acta Physiol. 2014, 211, 501–514. [Google Scholar] [CrossRef]
- McManus, D.P. Intermediary metabolism in parasitic helminths. Int. J. Parasitol. 1987, 17, 79–95. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; van de Pas, F.A.; van den Heuvel, J.M.; van den Bergh, S.G. The aerobic energy metabolism of Schistosoma mansoni miracidia. Mol. Biochem. Parasitol. 1991, 46, 181–184. [Google Scholar] [CrossRef]
- Young, N.D.; Nagarajan, N.; Lin, S.J.; Korhonen, P.K.; Jex, A.R.; Hall, R.S.; Safavi-Hemami, H.; Kaewkong, W.; Bertrand, D.; Gao, S.; et al. The Opisthorchis viverrini genome provides insights into life in the bile duct. Nat. Commun. 2014, 5, 4378. [Google Scholar] [CrossRef] [PubMed]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer. 2008, 8, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Rytkonen, K.T.; Storz, J.F. Evolutionary origins of oxygen sensing in animals. EMBO Rep. 2011, 12, 2–4. [Google Scholar] [CrossRef]
- Goto, M.; Amino, H.; Nakajima, M.; Tsuji, N.; Sakamoto, K.; Kita, K. Cloning and characterization of hypoxia-inducible factor-1 subunits from Ascaris suum-a parasitic nematode highly adapted to changes of oxygen conditions during its life cycle. Gene 2013, 516, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Oh, G.S.; Sohn, W.M.; Lee, K.; Yang, H.J.; Bae, Y.A. Molecular characteristics and induction profiles of hypoxia-inducible factor-1α and other basic helix-loop-helix and Per-Arnt-Sim domain-containing proteins identified in a carcinogenic liver fluke Clonorchis sinensis. Parasitology 2019, 146, 176–186. [Google Scholar] [CrossRef]
- Cui, S.J.; Xu, L.L.; Zhang, T.; Xu, M.; Yao, J.; Fang, C.Y.; Feng, Z.; Yang, P.Y.; Hu, W.; Liu, F. Proteomic characterization of larval and adult developmental stages in Echinococcus granulosus reveals novel insight into host-parasite interactions. J. Proteom. 2013, 84, 158–175. [Google Scholar] [CrossRef]
- Boyunaga, H.; Schmitz, M.G.; Brouwers, J.F.; van Hellemond, J.J.; Tielens, A.G.M. Fasciola hepatica miracidia are dependent on respiration and endogenous glycogen degradations for their energy generation. Parasitology 2001, 122, 169–173. [Google Scholar] [CrossRef]
- Parkinson, J.; Wasmuth, J.D.; Salinas, G.; Bizarro, C.V.; Sanford, C.; Berriman, M.; Ferreira, H.B.; Zaha, A.; Blaxter, M.L.; Maizels, R.M.; et al. A transcriptomic analysis of Echinococcus granulosus larval stages: Implications for parasite biology and host adaptation. PLoS Negl. Trop. Dis. 2012, 6, e1897. [Google Scholar] [CrossRef]
- De Almeida Leandro, L.; Fraga, C.M.; de Souza Lino, R., Jr.; Vinaud, M.C. Partial reverse of the TCA cycle is enhanced in Taenia crassiceps experimental neurocysticercosis after in vivo treatment with anthelminthic drugs. Parasitol. Res. 2014, 113, 1313–1317. [Google Scholar] [CrossRef]
- Oliveira, M.P.; Correa Soares, J.B.; Oliveira, M.F. Sexual Preferences in Nutrient Utilization Regulate Oxygen Consumption and Reactive Oxygen Species Generation in Schistosoma mansoni: Potential Implications for Parasite Redox Biology. PLoS ONE 2016, 11, e0158429. [Google Scholar] [CrossRef] [PubMed]
- Ritler, D.; Rufener, R.; Li, J.V.; Kämpfer, U.; Müller, J.; Bühr, C.; Schürch, S.; Lundström-Stadelmann, B. In vitro metabolomic footprint of the Echinococcus multilocularis metacestode. Sci. Rep. 2019, 9, 19438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Comparative Transcriptomic Analysis of the Larval and Adult Stages of Taenia pisiformis. Genes 2019, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Willms, K.; Robert, L.; Caro, J.A. Ultrastructure of smooth muscle, gap junctions and glycogen distribution in Taenia solium tapeworms from experimentally infected hamsters. Parasitol. Res. 2003, 89, 308–316. [Google Scholar] [CrossRef]
- Valkounová, J.; Zdárská, Z.; Slais, J. Histochemistry of the racemose form of Cysticercus cellulosae. Folia Parasitol. 1992, 39, 207–226. [Google Scholar]
- Chekulayev, V.; Mado, K.; Shevchuk, I.; Koit, A.; Kaldma, A.; Klepinin, A.; Timohhina, N.; Tepp, K.; Kandashvili, M.; Ounpuu, L.; et al. Metabolic remodeling in human colorectal cancer and surrounding tissues: Alterations in regulation of mitochondrial respiration and metabolic fluxes. Biochem. Biophys. Rep. 2015, 4, 111–125. [Google Scholar] [CrossRef]
- Skelly, P.J.; Shoemaker, C.B. A molecular genetic study of the variations in metabolic function during schistosome development. Mem. Ins. Oswaldo Cruz. 1995, 90, 281–284. [Google Scholar] [CrossRef]
- Tsai, I.J.; Zarowiecki, M.; Holroyd, N.; Garciarrubio, A.; Sánchez-Flores, A.; Brooks, K.L.; Tracey, A.; Bobes, R.J.; Fragoso, G.; Sciutto, E.; et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature 2013, 496, 57–63. [Google Scholar] [CrossRef]
- Fraga, C.M.; Costa, T.L.; Bezerra, J.C.; de Souza Lino, R., Jr.; Vinaud, M.C. Taenia crassiceps: Host treatment alters glycolisis and tricarboxilic acid cycle in cysticerci. Exp. Parasitol. 2012, 130, 146–151. [Google Scholar] [CrossRef]
- Del Arenal, I.P.; Rubio, M.E.; Ramírez, J.; Rendón, J.L.; Escamilla, J.E. Cyanide-resistant respiration in Taenia crassiceps metacestode (cysticerci) is explained by the H2O2-producing side-reaction of respiratory complex I with O2. Parasitol. Int. 2005, 54, 185–193. [Google Scholar] [CrossRef]
- Bennet, E.M.; Behm, C.A.; Bryant, C. The role of the host in the regulation of end-product formation in two strains of the rat tapeworm, Hymenolepis diminuta. Int. J. Parasitol. 1990, 20, 841–848. [Google Scholar] [CrossRef]
- Bryant, C. Organic acid excretion by helminths. Parasitol. Today 1993, 9, 58–60. [Google Scholar] [CrossRef]
- Campbell, T.; Rubin, N.; Komuniecki, R. Succinate-dependent energy generation in Ascaris suum mitochondria. Mol. Biochem. Parasitol. 1989, 33, 1–12. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; van Hellemond, J.J. The electron transport chain in anaerobically functioning eukaryotes. Biochim. Biophys. Acta 1998, 1365, 71–78. [Google Scholar] [CrossRef]
- Matsumoto, J.; Sakamoto, K.; Shinjyo, N.; Kido, Y.; Yamamoto, N.; Yagi, K.; Miyoshi, H.; Nonaka, N.; Katakura, K.; Kita, K.; et al. Anaerobic NADH-fumarate reductase system is predominant in the respiratory chain of Echinococcus multilocularis, providing a novel target for the chemotherapy of Alveolar Echinococcosis. Antimicrob. Agents Chemother. 2008, 52, 164–170. [Google Scholar] [CrossRef]
- Ovington, K.S.; Bryant, C. The role of carbon dioxide in the formation of end-products Hymenolepis diminuta. Int. J. Parasitol. 1981, 11, 221–228. [Google Scholar] [CrossRef]
- Fioravanti, C.F.; Vandock, K.P. Transhydrogenase and the anaerobic mitochondrial metabolism of adult Hymenolepis diminuta. Parasitology 2010, 137, 395–410. [Google Scholar] [CrossRef]
- Van Hellemond, J.J.; van der Klei, A.; van Weelden, S.W.H.; Tielens, A.G.M. Biochemical and evolutionary aspects of anaerobically functioning mitochondria. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 213–215. [Google Scholar] [CrossRef]
- Lima, N.F.; Picanço, G.A.; Costa, T.L.; de Souza Lino Junior, R.; Vinaud, M.C. In Vivo Treatment with the Combination of Nitazoxanide and Flubendazole Induces Gluconeogenesis and Protein Catabolism in Taenia crassiceps cysticerci. Acta Parasitol. 2021, 66, 98–103. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; van Grinsven, K.; Henze, K.; van Hellemond, J.J.; Martin, W. Acetate formation in the energy metabolism of parasitic helminths and protists. Int. J. Parasitol. 2010, 40, 387–397. [Google Scholar] [CrossRef]
- Van Grinsven, K.W.A.; van Hellemond, J.J.; Tielens, A.G.M. Acetate: Succinate CoA-transferase in the anaerobic mitochondria of Fasciola hepatica. Mol. Biochem. Parasitol. 2009, 164, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.W.; Folkers, K.; Coenzyme, Q. LXII. Structure and Synthesis of Rhodoquinone, a Natural Aminoquinone of the Coenzyme Q Group. J. Am. Chem. Soc. 1965, 87, 1409–1410. [Google Scholar] [CrossRef] [PubMed]
- Van Hellemond, J.J.; Klockiewicz, M.; Gaasenbeek, C.P.; Roos, M.H.; Tielens, A.G.M. Rhodoquinone and complex II of the electron transport chain in anaerobically functioning eukaryotes. J. Biol. Chem. 1995, 270, 31065–31070. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, C.F.; Kim, Y. Rhodoquinone requirement of the Hymenolepis diminuta mitochondrial electron transport system. Mol. Biochem. Parasitol. 1988, 28, 129–134. [Google Scholar] [CrossRef]
- Boveris, A.; Hertig, C.; Turrens, J. Fumarate reductase and other mitochondrial activities in trypanosoma cruzi. Mol. Biochem. Parasitol. 1986, 19, 163–169. [Google Scholar] [CrossRef]
- Arrigoni, O.; Singer, T.P. Limitations of the phenazine methosulfate assay for succinic and related dehydrogenases. Nature 1992, 193, 1256–1258. [Google Scholar] [CrossRef]
- Kuramochi, T.; Hirawake, H.; Kojima, S.; Takamiya, S.; Furushima, R.; Aoki, T.; Komuniecki, R.; Kita, K. Sequence comparison between the flavoprotein subunit of the fumarate reductase (complex II) of the anaerobic parasitic nematode, Ascaris suum and the succinate dehydrogenase of the aerobic, free-living nematode, Caenorhabditis elegans. Mol. Biol. Parasitol. 1994, 68, 177–187. [Google Scholar] [CrossRef]
- Amino, H.; Osanai, A.; Miyadera, H.; Shinjyo, N.; Tomitsuka, E.; Taka, H.; Mineki, R.; Murayama, K.; Takamiya, S.; Aoki, T.; et al. Isolation and characterization of the stage-specific cytochrome b small subunit (CybS) of Ascaris suum complex II from the aerobic respiratory chain of larval mitochondria. Mol. Biochem. Parasitol. 2003, 128, 175–186. [Google Scholar] [CrossRef]
- Amino, H.; Wang, H.; Hirawake, H.; Saruta, F.; Mizuchi, D.; Mineki, R.; Shindo, N.; Murayama, K.; Takamiya, S.; Aoki, T.; et al. Stage specific isoforms of Ascaris suum complex II: The fumarate reductase of the parasitic adult and the succinate dehydrogenase of free-living larvae share a common iron-sulfur subunit. Mol. Biochem. Parasitol. 2000, 106, 63–76. [Google Scholar] [CrossRef]
- Salinas, G.; Langelaan, D.N.; Shepherd, J.N. Rhodoquinone in bacteria and animals: Two distinct pathways for biosynthesis of this key electron transporter used in anaerobic bioenergetics. BBA Bioenerg. 2020, 1861, 1–14. [Google Scholar] [CrossRef]
- Sakai, C.; Tomitsuka, E.; Esumi, H.; Harada, S.; Kita, K. Mitochondrial fumarate reductase as a target of chemotherapy: From parasites to cancer cells. BBA 2012, 1820, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Tomitsuka, E.; Kita, K.; Esumi, H. An anticancer agent, pyrvinium pamoate inhibits the NADH-fumarate reductase system-a unique mitochondrial energy metabolism in tumor microenvironments. J. Biochem. 2012, 152, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Saz, H.J.; deBruyn, B.; de Mata, Z. Acyl-CoA transferase activities in homogenates of Fasciola hepatica adults. J. Parasitol. 1996, 82, 694–696. [Google Scholar] [CrossRef]
- Rivière, L.; van Weelden, S.W.; Glass, P.; Vegh, P.; Coustou, V.; Biran, M.; van Hellemond, J.J.; Bringaud, F.; Tielens, A.G.M.; Boshart, M. Acetyl: Succinate CoA-transferase in procyclic Trypanosoma brucei. Gene identification and role in carbohydrate metabolism. J. Biol. Chem. 2004, 279, 45337–45346. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.M.; De Castro, A.M.; Reynoso-Ducoing, O.; Ambrosio, J.; Hernández-Campos, A.; Castillo, R.; Vinaud, M.C. Alternative energy production pathways in Taenia crassiceps in vitro exposed to a benzimidazole derivative (RCB20). Parasitology 2016, 143, 88–493. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Archie, E.A.; Craft, M.E.; Hawley, D.M.; Martin, L.B.; Moore, J.; White, L. Host behaviour-parasite feedback: An essential link between animal behaviour and disease ecology. Proc. Biol. Sci. 2016, 283, 20153078. [Google Scholar] [CrossRef]
- Zarowiecki, M.; Berriman, M. What helminth genomes have taught us about parasite evolution. Parasitology 2015, 142, S85–S97. [Google Scholar] [CrossRef]
- Fragoso, G.; Bobes, R.J.; Espinoza, B.; Martínez, M.L.; Pérez-Morales, D.; Rosas, G.; Sciutto, E.; Laclette, J.P. Changes in cyst’s nuclear chromatin resulting after experimental manipulation of Taenia crassiceps mice infections: Biological implications. Exp. Parasitol. 2012, 130, 423–429. [Google Scholar] [CrossRef]
- Escobedo, G.; Larralde, C.; Chavarria, A.; Cerbón, M.A.; Morales-Montor, J. Molecular mechanisms involved in the differential effects of sex steroids on the reproduction and infectivity of Taenia crassiceps. J. Parasitol. 2004, 90, 1235–1244. [Google Scholar] [CrossRef]
- Larralde, C.; Morales, J.; Terrazas, I.; Govezensky, T.; Romano, M.C. Sex hormone changes induced by the parasite lead to feminization of the male host in murine Taenia crassiceps cysticercosis. J. Steroid Biochem. Mol. Biol. 1995, 52, 575–580. [Google Scholar] [CrossRef]
- Mourão, M.; Dinguirard, N.; Franco, G.R.; Yoshino, T.P. Role of the endogenous antioxidant system in the protection of Schistosoma mansoni primary sporocysts against exogenous oxidative stress. PLoS Negl. Trop. Dis. 2009, 3, e550. [Google Scholar] [CrossRef] [PubMed]
- Skrzycki, M.; Majewska, M.; Podsiad, M.; Czeczot, H.; Salamatin, R.; Twarowska, J.; Grytner-Zięcina, B. Hymenolepis diminuta: Experimental studies on the antioxidant system with short and long term infection periods in the rats. Exp. Parasitol. 2011, 129, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Suttiprapa, S.; Sotillo, J.; Smout, M.; Suyapoh, W.; Chaiyadet, S.; Tripathi, T.; Laha, T.; Loukas, A. Opisthorchis viverrini Proteome and Host-Parasite Interactions. Adv. Parasitol. 2018, 102, 45–72. [Google Scholar] [PubMed]
- Zheng, Y. Proteomic analysis of Taenia hydatigena cyst fluid reveals unique internal microenvironment. Acta Trop. 2017, 176, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Dorey, A.; Cwiklinski, K.; Rooney, J.; De Marco Verissimo, C.; López Corrales, J.; Jewhurst, H.; Fazekas, B.; Calvani, N.; Hamon, S.; Gaughan, S.; et al. Autonomous Non Antioxidant Roles for Fasciola hepatica Secreted Thioredoxin-1 and Peroxiredoxin-1. Front. Cell Infect. Microbiol. 2021, 11, 667272. [Google Scholar] [CrossRef]
- Al-Shehri, S.S. Reactive oxygen and nitrogen species and innate immune response. Biochimie 2021, 181, 52–64. [Google Scholar] [CrossRef]
- Vinogradov, A.D.; Grivennikova, V.G. Oxidation of NADH and ROS production by respiratory complex I. Biochim. Biophys. Acta 2016, 1857, 863–871. [Google Scholar] [CrossRef]
- Moné, Y.; Ribou, A.C.; Cosseau, C.; Duval, D.; Théron, A.; Mitta, G.; Gourbal, B. An example of molecular co-evolution: Reactive oxygen species (ROS) and ROS scavenger levels in Schistosoma mansoni/Biomphalaria glabrata interactions. Int. J. Parasitol. 2011, 41, 721–730. [Google Scholar] [CrossRef]
- Berriman, M.; Haas, B.J.; LoVerde, P.T.; Wilson, R.A.; Dillon, G.P.; Cerqueira, G.C.; Mashiyama, S.T.; Al-Lazikani, B.; Andrade, L.F.; Ashton, P.D.; et al. The genome of the blood fluke Schistosoma mansoni. Nature 2009, 460, 352–358. [Google Scholar] [CrossRef]
- Zhang, H.C.; Ma, K.X.; Yang, Y.J.; Shi, C.Y.; Chen, G.W.; Liu, D.Z. Molecular cloning, characterization, expression and enzyme activity of catalase from planarian Dugesia japonica in response to environmental pollutants. Ecotoxicol. Environ. Saf. 2018, 165, 88–95. [Google Scholar] [CrossRef]
- Hernández-Santoyo, A.; Landa, A.; González-Mondragón, E.; Pedraza-Escalona, M.; Parra-Unda, R.; Rodríguez-Romero, A. Crystal structure of Cu/Zn superoxide dismutase from Taenia solium reveals metal-mediated self-assembly. FEBS J. 2011, 278, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fu, Y.; Wu, X.; Xie, Y.; Nie, H.; Chen, L.; Nong, X.; Gu, X.; Wang, S.; Peng, X.; et al. Annotation of the transcriptome from Taenia pisiformis and its comparative analysis with three Taeniidae species. PLoS ONE 2012, 7, e32283. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; LoVerde, P.T. Schistosoma mansoni: The developmental regulation and immunolocalization of antioxidant enzymes. Exp. Parasitol. 1997, 86, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Toppo, S.; Vanin, S.; Bosello, V.; Tosatto, S.C. Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid. Redox Signal. 2008, 10, 1501–1514. [Google Scholar] [CrossRef]
- Changklungmoa, N.; Chaithirayanon, K.; Cheukamud, W.; Chaiwichien, A.; Osotprasit, S.; Samrit, T.; Sobhon, P.; Kueakhai, P. Expression and characterization of glutathione peroxidase of the liver fluke. Parasitol. Res. 2018, 117, 3487–3495. [Google Scholar] [CrossRef]
- Fan, J.; Wu, H.; Li, K.; Liu, X.; Tan, Q.; Cao, W.; Liang, B.; Ye, B. Transcriptomic Features of Echinococcus granulosus Protoscolex during the Encystation Process. Korean J. Parasitol. 2020, 58, 287–299. [Google Scholar] [CrossRef]
- Zelck, U.E.; Von Janowsky, B. Antioxidant enzymes in intramolluscan Schistosoma mansoni and ROS-induced changes in expression. Parasitology 2004, 128, 493–501. [Google Scholar] [CrossRef]
- Cai, G.B.; Bae, Y.A.; Kim, S.H.; Sohn, W.M.; Lee, Y.S.; Jiang, M.S.; Kim, T.S.; Kong, Y. Vitellocyte-specific expression of phospholipid hydroperoxide glutathione peroxidases in Clonorchis sinensis. Int. J. Parasitol. 2008, 38, 1613–1623. [Google Scholar] [CrossRef]
- Low, F.M.; Hampton, M.B.; Winterbourn, C.C. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid. Redox Signal. 2008, 10, 1621–1630. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zhang, C.; Guo, B.; Wei, Q.; Li, L.; Yang, N.; Peter McManus, D.; Gao, X.; Zhang, W.; et al. Echinococcus granulosus sensu stricto: Silencing of thioredoxin peroxidase impairs the differentiation of protoscoleces into metacestodes. Parasite 2018, 25, 57. [Google Scholar] [CrossRef]
- Kumagai, T.; Osada, Y.; Kanazawa, T. 2-Cys peroxiredoxins from Schistosoma japonicum: The expression profile and localization in the life cycle. Mol. Biochem. Parasitol. 2006, 149, 135–143. [Google Scholar] [CrossRef]
- Threadgold, L.T.; Arme, C.; Read, C.P. Ultrastructure localization of a peroxidase in the tapeworm, Hymenolepis diminuta. J. Parasitol. 1968, 54, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Redox biology of the intestine. Free Radic. Res. 2011, 45, 1245–1266. [Google Scholar] [CrossRef]
- Sun, Q.A.; Kirnarsky, L.; Sherman, S.; Gladyshev, V.N. Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systems. Proc. Natl. Acad. Sci. USA 2001, 98, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Novoselov, S.V.; Sun, Q.A.; Moustafa, M.E.; Zhou, Y.; Oko, R.; Hatfield, D.L.; Gladyshev, V.N. Mammalian selenoprotein thioredoxin-glutathione reductase. Roles in disulfide bond formation and sperm maturation. J. Biol. Chem. 2005, 280, 26491–26498. [Google Scholar] [CrossRef] [PubMed]
- Alger, H.M.; Williams, D.L. The disulfide redox system of Schistosoma mansoni and the importance of a multifunctional enzyme, thioredoxin glutathione reductase. Mol. Biochem. Parasitol. 2002, 121, 129–139. [Google Scholar] [CrossRef]
- Agorio, A.; Chalar, C.; Cardozo, S.; Salinas, G. Alternative mRNAs arising from trans-splicing code for mitochondrial and cytosolic variants of Echinococcus granulosus thioredoxin Glutathione reductase. J. Biol. Chem. 2003, 278, 12920–12928. [Google Scholar] [CrossRef]
- Rendón, J.L.; del Arenal, I.P.; Guevara-Flores, A.; Uribe, A.; Plancarte, A.; Mendoza-Hernández, G. Purification, characterization and kinetic properties of the multifunctional thioredoxin-glutathione reductase from Taenia crassiceps metacestode (cysticerci). Mol. Biochem. Parasitol. 2004, 133, 61–69. [Google Scholar] [CrossRef]
- Otero, L.; Bonilla, M.; Protasio, A.V.; Fernández, C.; Gladyshev, V.N.; Salinas, G. Thioredoxin and glutathione systems differ in parasitic and free-living platyhelminths. BMC Genom. 2010, 11, 237. [Google Scholar] [CrossRef]
- Martínez-González, J.J.; Guevara-Flores, A.; Alvarez, G.; Rendón-Gómez, J.L.; Del Arenal, I.P. In vitro killing action of auranofin on Taenia crassiceps metacestode (cysticerci) and inactivation of thioredoxin-glutathione reductase (TGR). Parasitol. Res. 2010, 107, 227–231. [Google Scholar] [CrossRef]
- Prast-Nielsen, S.; Huang, H.H.; Williams, D.L. Thioredoxin glutathione reductase: Its role in redox biology and potential as a target for drugs against neglected diseases. Biochim. Biophys. Acta 2011, 1810, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, J.; Xie, S.; Qian, C.; Wang, J.; Zhang, W.; Yin, X.; Hua, Z.; Yu, C. Thioredoxin glutathione reductase as a novel drug target: Evidence from Schistosoma japonicum. PLoS ONE 2012, 7, e31456. [Google Scholar] [CrossRef] [PubMed]
- Eweas, A.F.; Allam, G. Targeting thioredoxin glutathione reductase as a potential antischistosomal drug target. Mol. Biochem. Parasitol. 2018, 225, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Shukla, H.; Kalita, P.; Tripathi, T. Structural insights into natural compounds as inhibitors of Fasciola gigantica thioredoxin glutathione reductase. J. Cell Biochem. 2018, 119, 3067–3080. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Flores, A.; Martínez-González, J.J.; Herrera-Juárez, Á.M.; Rendón, J.L.; González-Andrade, M.; Torres Durán, P.V.; Enríquez-Habib, R.G.; Del Arenal Mena, I.P. Effect of curcuminoids and curcumin derivate products on thioredoxin-glutathione reductase from Taenia crassiceps cysticerci. Evidence suggesting a curcumin oxidation product as a suitable inhibitor. PLoS ONE 2019, 14, e0220098. [Google Scholar]
- Lyu, H.; Petukhov, P.A.; Banta, P.R.; Jadhav, A.; Lea, W.A.; Cheng, Q.; Arnér, E.; Simeonov, A.; Thatcher, G.; Angelucci, F.; et al. Characterization of Lead Compounds Targeting the Selenoprotein Thioredoxin Glutathione Reductase for Treatment of Schistosomiasis. ACS Infec. Dis. 2020, 6, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Faixová, D.; Hrčková, G.; Mačák Kubašková, T.; Mudroňová, D. Antiparasitic Effects of Selected Isoflavones on Flatworms. Helminthologia 2021, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinski, K.; Dalton, J.P.; Dufresne, P.J.; La Course, J.; Williams, D.J.; Hodgkinson, J.; Paterson, S. The Fasciola hepatica genome: Gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biol. 2015, 16, 71. [Google Scholar] [CrossRef]
- Bonilla, M.; Denicola, A.; Novoselov, S.V.; Turanov, A.A.; Protasio, A.; Izmendi, D.; Gladyshev, V.N.; Salinas, G. Platyhelminth mitochondrial and cytosolic redox homeostasis is controlled by a single thioredoxin glutathione reductase and dependent on selenium and glutathione. J. Biol. Chem. 2008, 283, 17898–17907. [Google Scholar] [CrossRef]
- Guevara-Flores, A.; Del Arenal, I.P.; Mendoza-Hernández, G.; Pardo, J.P.; Flores-Herrera, O.; Rendón, J.L. Mitochondrial Thioredoxin-Glutathione Reductase from Larval Taenia crassiceps (Cysticerci). J. Parasitol. Res. 2010, 2010, 719856. [Google Scholar] [CrossRef]
- Martínez-González, J.J.; Guevara-Flores, A.; Rendón, J.L.; Arenal, I. Auranofin-induced oxidative stress causes redistribution of the glutathione pool in Taenia crassiceps cysticerci. Mol. Biochem. Parasitol. 2015, 201, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, A.N.; Davioud-Charvet, E.; Sayed, A.A.; Califf, L.L.; Dessolin, J.; Arnér, E.S.; Williams, D.L. Thioredoxin glutathione reductase from Schistosoma mansoni: An essential parasite enzyme and a key drug target. PLoS Med. 2007, 4, e206. [Google Scholar]
- Angelucci, F.; Miele, A.E.; Boumis, G.; Dimastrogiovanni, D.; Brunori, M.; Bellelli, A. Glutathione reductase and thioredoxin reductase at the crossroad: The structure of Schistosoma mansoni thioredoxin glutathione reductase. Proteins 2008, 72, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, A.W. The glutathione S-transferases: Their role in the transport of organic anions from blood to bile. Int. Rev. Physiol. 1980, 21, 150–169. [Google Scholar]
- Nguyen, H.A.; Bae, Y.A.; Lee, E.G.; Kim, S.H.; Diaz-Camacho, S.P.; Nawa, Y.; Kang, I.; Kong, Y. A novel sigma-like glutathione transferase of Taenia solium metacestode. Int. J. Parasitol. 2010, 40, 1097–1106. [Google Scholar] [CrossRef]
- Pearson, W.R. Phylogenies of glutathione transferase families. Methods Enzymol. 2005, 401, 186–204. [Google Scholar]
- Wu, B.; Dong, D. Human cytosolic glutathione transferases: Structure, function, and drug discovery. Trends Pharmacol. Sci. 2012, 33, 656–668. [Google Scholar] [CrossRef]
- Bae, Y.A.; Kim, J.G.; Kong, Y. Phylogenetic characterization of Clonorchis sinensis proteins homologous to the sigma-class glutathione transferase and their differential expression profiles. Mol. Biochem. Parasitol. 2016, 206, 46–55. [Google Scholar] [CrossRef]
- Iriarte, A.; Arbildi, P.; La-Rocca, S.; Musto, H.; Fernández, V. Identification of novel glutathione transferases in Echinococcus granulosus. An evolutionary perspective. Acta Trop. 2012, 123, 208–216. [Google Scholar] [CrossRef]
- Kim, J.G.; Ahn, C.S.; Kim, S.H.; Bae, Y.A.; Kwon, N.Y.; Kang, I.; Yang, H.J.; Sohn, W.M.; Kong, Y. Clonorchis sinensis omega-class glutathione transferases play major roles in the protection of the reproductive system during maturation and the response to oxidative stress. Parasites Vectors 2016, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Ziniel, P.D.; Karumudi, B.; Barnard, A.H.; Fisher, E.M.; Thatcher, G.R.; Podust, L.M.; Williams, D.L. The Schistosoma mansoni Cytochrome P450 (CYP3050A1) Is Essential for Worm Survival and Egg Development. PLoS Negl. Trop. Dis. 2015, 9, e0004279. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, M.Y.; Vavilin, V.A.; Sripa, B.; Laha, T.; Brindley, P.J.; Mordvinov, V.A. Functional Analysis of the Unique Cytochrome P450 of the Liver Fluke Opisthorchis felineus. PLoS Negl. Trop. Dis. 2015, 9, e0004258. [Google Scholar] [CrossRef]
- Pal, R.; Rai, J.P. Phytochelatins: Peptides involved in heavy metal detoxification. Appl. Biochem. Biotechnol. 2010, 160, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Rea, P.A. Phytochelatin synthase: Of a protease a peptide polymerase made. Physiol. Plant. 2012, 145, 154–164. [Google Scholar] [CrossRef]
- Ray, D.; Williams, D.L. Characterization of the phytochelatin synthase of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2011, 5, e1168. [Google Scholar] [CrossRef] [PubMed]
- Rigouin, C.; Vermeire, J.J.; Nylin, E.; Williams, D.L. Characterization of the phytochelatin synthase from the human parasitic nematode Ancylostoma ceylanicum. Mol. Biochem. Parasitol. 2013, 191, 1–6. [Google Scholar] [CrossRef]
- Williams, D.L.; Bonilla, M.; Gladyshev, V.N.; Salinas, G. Thioredoxin glutathione reductase-dependent redox networks in platyhelminth parasites. Antioxid. Redox Signal. 2013, 19, 735–745. [Google Scholar] [CrossRef]
- Bundy, J.G.; Kille, P.; Liebeke, M.; Spurgeon, D.J. Metallothioneins may not be enough--the role of phytochelatins in invertebrate metal detoxification. Environ. Sci. Technol. 2014, 48, 885–886. [Google Scholar] [CrossRef]
- Mannino, M.H.; Patel, R.S.; Eccardt, A.M.; Perez Magnelli, R.A.; Robinson, C.; Janowiak, B.E.; Warren, D.E.; Fisher, J.S. Myoglobin as a versatile peroxidase: Implications for a more important role for vertebrate striated muscle in antioxidant defense. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 234, 9–17. [Google Scholar] [CrossRef]
- Ren, M.; He, L.; Huang, Y.; Mao, Q.; Li, S.; Qu, H.; Bian, M.; Liang, P.; Chen, X.; Ling, J.; et al. Molecular characterization of Clonorchis sinensis secretory myoglobin: Delineating its role in anti-oxidative survival. Parasites Vectors 2014, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. The aldo-keto reductases (AKRs): Overview. Chem. Biol. Interact. 2015, 234, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Forrest, G.L.; González, B. Carbonyl reductase. Chem. Biol. Interact. 2000, 129, 21–40. [Google Scholar] [CrossRef]
- Escobedo, G.; Romano, M.C.; Morales-Montor, J. Differential in vitro effects of insulin on Taenia crassiceps and Taenia solium cysticerci. J. Helminthol. 2009, 83, 403–412. [Google Scholar] [CrossRef]
- Adalid-Peralta, L.; Rosas, G.; Arce-Sillas, A.; Bobes, R.J.; Cárdenas, G.; Hernández, M.; Trejo, C.; Meneses, G.; Hernández, B.; Estrada, K.; et al. Effect of Transforming Growth Factor-β upon Taenia solium and Taenia crassiceps Cysticerci. Sci. Rep. 2017, 7, 12345. [Google Scholar] [CrossRef]
- Navarrete-Perea, J.; Moguel, B.; Mendoza-Hernández, G.; Fragoso, G.; Sciutto, E.; Bobes, R.J.; Laclette, J.P. Identification and quantification of host proteins in the vesicular fluid of porcine Taenia solium cysticerci. Exp. Parasitol. 2014, 143, 11–17. [Google Scholar] [CrossRef]
- Ahn, C.S.; Han, X.; Bae, Y.A.; Ma, X.; Kim, J.T.; Cai, H.; Yang, H.J.; Kang, I.; Wang, H.; Kong, Y. Alteration of immunoproteome profile of Echinococcus granulosus hydatid fluid with progression of cystic echinococcosis. Parasites Vectors 2015, 8, 10. [Google Scholar] [CrossRef]
- Ahn, C.S.; Kim, J.G.; Han, X.; Kang, I.; Kong, Y. Comparison of Echinococcus multilocularis and Echinococcus granulosus hydatid fluid proteome provides molecular strategies for specialized host-parasite interactions. Oncotarget 2017, 8, 97009–97024. [Google Scholar] [CrossRef]
- Flores-Bautista, J.; Navarrete-Perea, J.; Fragoso, G.; Flisser, A.; Soberón, X.; Laclette, J.P. Fate of uptaken host proteins in Taenia solium and Taenia crassiceps cysticerci. Biosci. Rep. 2018, 38, BSR20180636. [Google Scholar] [CrossRef]
- Ahn, C.S.; Kim, J.G.; Bae, Y.A.; Kim, S.H.; Shin, J.H.; Yang, Y.; Kang, I.; Kong, Y. Fasciclin-calcareous corpuscle binary complex mediated protein-protein interactions in Taenia solium metacestode. Parasites Vectors 2017, 10, 438. [Google Scholar] [CrossRef]
- Loos, J.A.; Caparros, P.A.; Nicolao, M.C.; Denegri, G.M.; Cumino, A.C. Identification and pharmacological induction of autophagy in the larval stages of Echinococcus granulosus: An active catabolic process in calcareous corpuscles. Int. J. Parasitol. 2014, 44, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinski, K.; Robinson, M.W.; Donnelly, S.; Dalton, J.P. Complementary transcriptomic and proteomic analyses reveal the cellular and molecular processes that drive growth and development of Fasciola hepatica in the host liver. BMC Genom. 2021, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Becerro-Recio, D.; González-Miguel, J.; Ucero, A.; Sotillo, J.; Martínez-Moreno, Á.; Pérez-Arévalo, J.; Cwiklinski, K.; Dalton, J.P.; Siles-Lucas, M. Recognition Pattern of the Fasciola hepatica Excretome/Secretome during the Course of an Experimental Infection in Sheep by 2D Immunoproteomics. Pathogens 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Virginio, V.G.; Monteiro, K.M.; Drumond, F.; de Carvalho, M.O.; Vargas, D.M.; Zaha, A.; Ferreira, H.B. Excretory/secretory products from in vitro-cultured Echinococcus granulosus protoscoleces. Mol. Biochem. Parasitol. 2012, 183, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.S.; Fang, B.B.; Li, Z.D.; Li, L.; Bi, X.J.; Li, W.D.; Zhang, N.; Lin, R.Y.; Wen, H. Thioredoxin peroxidase secreted by Echinococcus granulosus (sensu stricto) promotes the alternative activation of macrophages via PI3K/AKT/mTOR pathway. Parasites Vectors 2019, 12, 542. [Google Scholar] [CrossRef]
- García-Montoya, G.M.; Mesa-Arango, J.A.; Isaza-Agudelo, J.P.; Agudelo-Lopez, S.P.; Cabarcas, F.; Barrera, L.F.; Alzate, J.F. Transcriptome profiling of the cysticercus stage of the laboratory model Taenia crassiceps, strain ORF. Acta Trop. 2016, 154, 50–62. [Google Scholar] [CrossRef]
- Greenberg, R.M. ABC multidrug transporters in schistosomes and other parasitic flatworms. Parasitol. Int. 2013, 62, 647–653. [Google Scholar] [CrossRef]


| Specie | Biologic Form | Host | Localization in Host | Oxygen Concentration | References | |
|---|---|---|---|---|---|---|
| pO2 | mmHg | |||||
| T. solium | Egg | Enviroment | NA | 21.1 | 160 | [33] |
| Oncosphera | Pig | Duodenum | 5.9 | 45 | [34] | |
| Blood capillaries * | 5.3–13.2 | 40–100 | [33] | |||
| Cysticerci | Muscle | 4.9 | 37.5 | [34] | ||
| Brain | 3.9 | 30 | [34] | |||
| Taenia | Human | Duodenum ** | 7.9 | 60 | [35] | |
| Ileum ** | 1.3 | 10 | [35] | |||
| Cecum ** | 0 | 0 | [35] | |||
| Cysticerci | Muscle (in rest) | 3.6–3.9 | 27–30 | [36] | ||
| Brain (grey matter) | 2.1–5.3 | 16–40 | [36] | |||
| Brain (white matter) | 3.2–4.4 | 24–33 | [36] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-González, J.d.J.; Guevara-Flores, A.; del Arenal Mena, I.P. Evolutionary Adaptations of Parasitic Flatworms to Different Oxygen Tensions. Antioxidants 2022, 11, 1102. https://doi.org/10.3390/antiox11061102
Martínez-González JdJ, Guevara-Flores A, del Arenal Mena IP. Evolutionary Adaptations of Parasitic Flatworms to Different Oxygen Tensions. Antioxidants. 2022; 11(6):1102. https://doi.org/10.3390/antiox11061102
Chicago/Turabian StyleMartínez-González, José de Jesús, Alberto Guevara-Flores, and Irene Patricia del Arenal Mena. 2022. "Evolutionary Adaptations of Parasitic Flatworms to Different Oxygen Tensions" Antioxidants 11, no. 6: 1102. https://doi.org/10.3390/antiox11061102
APA StyleMartínez-González, J. d. J., Guevara-Flores, A., & del Arenal Mena, I. P. (2022). Evolutionary Adaptations of Parasitic Flatworms to Different Oxygen Tensions. Antioxidants, 11(6), 1102. https://doi.org/10.3390/antiox11061102





