Soluble Free, Esterified and Insoluble-Bound Phenolic Antioxidants from Chickpeas Prevent Cytotoxicity in Human Hepatoma HuH-7 Cells Induced by Peroxyl Radicals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Extraction of Free and Esterified Phenolics
2.3. Insoluble-Bound Phenolic Compounds Extraction
2.4. Total Phenolic Content
2.5. Ferric Reducing Antioxidant Power
2.6. Oxygen Radical Absorbance Capacity
2.7. UPLC-MS/MS Analysis
2.8. Cytotoxicity and Hepatoprotective Activity
2.9. Statistical Analysis
3. Results
3.1. Total Phenolic Content (TPC) and Ferric Reducing Antioxidant Power (FRAP)
3.2. Identification of Phenolics Compounds by LC-MS/MS
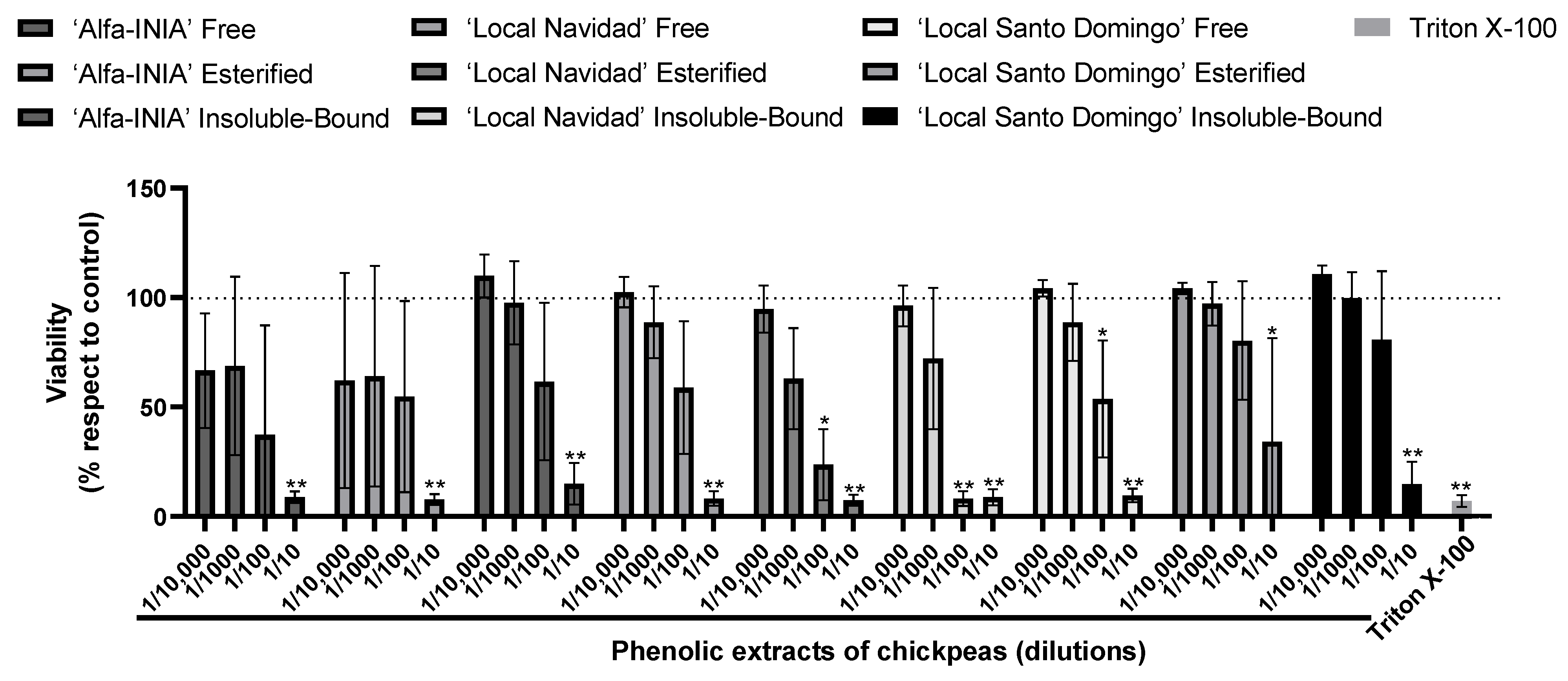
3.3. Cytotoxicity and Hepatoprotective Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallace, T.; Murray, R.; Zelman, K. The nutritional value and health benefits of chickpeas and hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations, FAOSTAT. Chickpeas. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 9 February 2022).
- Pinheiro, A.; Ivanovic, C.; Rodríguez, L. Legume consumption in Chile: Perspectives and challenges. Rev. Chil. Nutr. 2018, 45, 14–20. [Google Scholar] [CrossRef]
- Pye, C.; Sutherland, S.; Martín, P.S. Consumo de frutas, verduras y legumbres en adultos de Santiago Oriente, Chile: ¿Ha influido el confinamiento por COVID-19? Rev. Chil. Nutr. 2021, 48, 374–380. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Sanda, G.; Niculescu, L.; Deleanu, M.; Sima, A.; Stancu, C. Phenolic compounds exerting lipid-regulatory, anti-inflammatory and epigenetic effects as complementary treatments in cardiovascular diseases. Biomolecules 2020, 10, 641. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xue, L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Quintero, M.; Saracho, A.; Chavez, J.; Garzon, J.; Pineda, K.; Delgado, F.; Lopez, J. Phenolic profiles and their contribution to the antioxidant activity of selected chickpea genotypes from Mexico and ICRISAT collections. Plant Foods Hum. Nutr. 2018, 73, 122–129. [Google Scholar] [CrossRef]
- Perez, L.; Huerta, J.; Ruiz, S.; Cinco, F.; Wong, F.; Rascón, L.; Robles, M.; González, R.; Rosas, E.; Corella, M.; et al. Evaluation of quality, antioxidant capacity, and digestibility of chickpea (Cicer arietinum L. cv Blanoro) stored under N2 and CO2 atmospheres. Molecules 2021, 26, 2773. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Llorach, R.; Favari, C.; Alonso, D.; Garcia-Aloy, M.; Andres-Lacueva, C.; Urpi-Sarda, M. Comparative metabolite fingerprinting of legumes using LC-MS-based untargeted metabolomics. Food Res. Int. 2019, 126, 108666. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Badani, H.; Kapulnik, Y.; Shomer, I.; Oren, M.; Galili, S. Determination of polyphenols, flavonoids, and antioxidant capacity in colored chickpea (Cicer arietinum L.). J. Food Sci. 2010, 75, S115–S119. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Heiras, M.; Ochoa, M.; Gutiérrez, R.; López, J.; Mora, S.; Milán, J.; Garzón, J.; Reyes, C. Technological properties, antioxidant activity and total phenolic and flavonoid content of pigmented chickpea (Cicer arietinum L.) cultivars. Int. J. Food Sci. Nutr. 2013, 64, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Chen, G.; Yu, L.; Yang, L.; Gao, Y. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Falcão, H.; Ladeira, C.; Ramos, M.B.; de Camargo, A.C.; Shahidi, F.; Kurozawa, L.E.; Iouko, E. Soybean ultrasound pre-treatment prior to soaking affects β-glucosidase activity, isoflavone profile and soaking time. Food Chem. 2018, 269, 404–412. [Google Scholar] [CrossRef]
- Falcão, H.; Ramos Silva, M.B.; de Camargo, A.C.; Shahidi, F.; Franchin, M.; Rosalen, P.L.; Matias, S.; Kurozawa, L.; Iouko, E. Optimizing the potential bioactivity of isoflavones from soybeans via ultrasound pretreatment: Antioxidant potential and NF-κB activation. J. Food Biochem. 2019, 43, e13018. [Google Scholar] [CrossRef]
- Yoshiara, L.Y.; Mandarino, J.M.G.; Carrão-Panizzi, M.C.; Madeira, T.B.; Silva, J.B. da, Camargo, A.C. de, Shahidi, F.; Ida, E.I. Germination changes the isoflavone profile and increases the antioxidant potential of soybean. J. Food Bioact. 2018, 3, 144–150. [Google Scholar] [CrossRef] [Green Version]
- de Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Geraldi, M.V.; Maróstica Júnior, M.R.; Shahidi, F.; Schwember, A.R. Is chickpea a potential substitute for soybean? Phenolic bioactives and potential health benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef] [Green Version]
- de Camargo, A.C.; Speisky, H.; Bridi, R.; Núñez Pizarro, P.; Larena, A.; Pinaffi-Langley, A.C.d.C.; Shahidi, F.; Schwember, A.R. Chickpeas from a Chilean region affected by a climate-related catastrophe: Effects of water stress on grain yield and flavonoid composition. Molecules 2022, 27, 691. [Google Scholar] [CrossRef]
- de Rezende, N.; de Camargo, A.C.; de Alencar, S.M.; Danielski, R.; Shahidi, F.; Bervelieri, T.M.; Yoko, E.I.; Spinosa, W.A.; Grossmann, M.V.E. Phenolics and alkaloids of raw cocoa nibs and husk: The role of soluble and insoluble-bound antioxidants. Food Biosci. 2021, 42, 101085. [Google Scholar]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 1. Extraction and purification procedure. J. Agric. Food. Chem. 1982, 30, 330–334. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Bridi, R.; Atala, E.; Pizarro, P.; Núñez, G. Honeybee pollen load: Phenolic composition and antimicrobial activity and antioxidant capacity. J. Nat. Prod. 2019, 82, 559–565. [Google Scholar] [CrossRef]
- Sar, F.; Leroy, P.; Nicolas, A.; Archimbault, P. Development and optimization of a liquid chromatographic method for the determination of gentamicin in calf tissues. Anal. Chim. Acta 1993, 275, 285–293. [Google Scholar] [CrossRef]
- Leroy, P.; Decolin, D.; Nicolas, A.; Archimbault, P. Determination of josamycin residues in porcine tissues using high-performance liquid chromatography with pre-column derivatization and spectrofluorimetric detection. Analyst 1994, 119, 2743–2747. [Google Scholar] [CrossRef]
- Linde, S.; Welinder, B.S. Non-ideal behaviour of silica-based stationary phases in trifluoroacetic acid-acetonitrile-based reversed-phase high-performance liquid chromatographic separations of insulins and proinsulins. J. Chromatogr. 1991, 536, 43–55. [Google Scholar] [CrossRef]
- Dimitrova, D.; Lashev, L.D.; Yanev, S.G.; Pandova, B. Pharmacokinetics of enrofloxacin in turkeys. Res. Vet. Sci. 2007, 82, 392–397. [Google Scholar] [CrossRef]
- Giordano, A.; Retamal, M.; Leyton, F.; Martínez, P.; Bridi, R.; Velásquez, P.; Montenegro, G. Bioactive polyphenols and antioxidant capacity of Azara petiolaris and Azara integrifolia Honeys. CyTA J. Food 2018, 16, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.; Moravec, R.A. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev. Technol. 2004, 2, 51–62. [Google Scholar] [CrossRef]
- Bridi, R.; Lino von Poser, G.; Gómez, M.; Andia, M.E.; Oyarzún, J.E.; Núñez, P.; Vasquez, A.J.; Espinosa, C. Hepatoprotective species from the Chilean medicinal flora: Junellia spathulata (Verbenaceae). J. Ethnopharmacol. 2021, 267, 113543. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, J.; Andia, M.E.; Uribe, S.; Núñez, P.; Núñez, G.; Montenegro, G.; Bridi, R. Honeybee pollen extracts reduce oxidative stress and steatosis in hepatic cells. Molecules 2020, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Walsh, K.; Bhattarai, S.; Naiker, M. Partitioning of nutritional and bioactive compounds between the kernel, hull and husk of five new chickpea genotypes grown in Australia. Future Foods 2021, 4, 100065. [Google Scholar] [CrossRef]
- Troncoso, J.; Costello, A.C.; Kim, J.H.; Johnson, G.V.W. Metal-catalyzed oxidation of bovine neuroflaments in vitro. Free Radical Bio. Med. 1995, 18, 891–899. [Google Scholar] [CrossRef]
- Braughler, J.; Duncan, L.A.; Chase, R.L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J. Biol. Chem. 1986, 261, 10282–10289. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef]
- Yonekura, L.; Martins, C.A.; Sampaio, G.R.; Monteiro, M.P.; Cesar, L.A.M.; Mioto, B.M.; Mori, C.S.; Mendes, T.M.N.; Ribeiro, M.L.; Arcari, D.P.; et al. Bioavailability of catechins from guarana (Paullinia cupana) and its effect on antioxidant enzymes and other oxidative stress markers in healthy human subjects. Food Funct. 2016, 7, 2970–2978. [Google Scholar] [CrossRef]
- Munteanu, I.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Alshikh, N.; de Camargo, A.C.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Domínguez, D.; Cuevas, E.; Milán, J.; León, L.; Gutiérrez, R.; Reyes, C. Optimal germination condition impacts on the antioxidant activity and phenolic acids profile in pigmented desi chickpea (Cicer arietinum L.) seeds. J. Food Sci. Technol. 2018, 55, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, S.; Choudhary, D.; Ahmad, N.; Karvande, A.; Kumar, A.; Banala, V.; Mishra, P.; Trivedi, R. Dietary flavonoid kaempferol inhibits glucocorticoid-induced bone loss by promoting osteoblast survival. Nutrition 2018, 53, 64–76. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Q.; Zhao, T. Preventive effects of kaempferol on high-fat diet-induced obesity complications in C57BL/6 mice. Biomed. Res. Int. 2020, 2020, 453248. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, R.; Kalia, K. Kaempferol in ameliorating diabetes-induced fibrosis and renal damage: An in vitro and in vivo study in diabetic nephropathy mice model. Phytomedicine 2020, 16, 153235. [Google Scholar] [CrossRef]
- Shrestha, R.; Mohankumar, K.; Martin, G.; Hailemariam, A.; Lee, S.; Jin, U.; Burghardt, R.; Safe, S. Flavonoids kaempferol and quercetin are nuclear receptor 4A1 (NR4A1, Nur77) ligands and inhibit rhabdomyosarcoma cell and tumor growth. J. Exp. Clin. Cancer Res. 2021, 40, 392. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.; Zhang, Z.; Rahman, K.; Wang, S.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yu, H.; Han, F.; Wang, M.; Luo, Y.; Guo, X. Biochanin A induces S phase arrest and apoptosis in lung cancer cells. Biomed. Res. Int. 2018, 2018, 3545376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Qin, H.; Li, Y.; Li, J.; Fu, L.; Li, M.; Jiang, C.; Yun, J.; Liu, Z.; Feng, Y.; et al. Biochanin A protect against lipopolysaccharide-induced acute lung injury in mice by regulating TLR4/NF-κB and PPAR-γ pathway. Microb. Pathog. 2020, 138, 103846. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ye, Z.N.; Zhuang, Z.; Gao, Y.; Tang, C.; Zhou, C.H.; Wang, C.X.; Zhang, X.S.; Xie, G.B.; Liu, J.P.; et al. Biochanin A reduces inflammatory injury and neuronal apoptosis following subarachnoid hemorrhage via suppression of the TLRs/TIRAP/MyD88/NF-κB pathway. Behav. Neurol. 2018, 2018, 1960106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfraz, A.; Javeed, M.; Ajmal, M.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- Moon, Y.; Sagawa, K.; Frederick, K.; Zhang, S.; Morris, M.E. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J. 2006, 8, E433–E442. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, Y.; Li, X.; Zhang, T.; Mitani, T.; Yasuda, M.; Nanba, F.; Toda, T.; Yamashita, Y.; Ashida, H. Black soybean seed coat polyphenols prevent AAPH-induced oxidative DNA-damage in HepG2 cells. J. Clin. Biochem. Nutr. 2017, 60, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Khanna, K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 247–254. [Google Scholar] [CrossRef]
- de Camargo, A.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Qiu, X.-Q.; Shu, Z.-H.; Liu, Q.-C.; Hu, M.-B.; Han, T.; Rahman, K.; Qin, L.-P.; Cheng, Z. Hepato-protective activity of total iridoid glycosides isolated from Paederia scandens (lour.) Merr. Var. tomentosa. J. Ethnopharmacol. 2015, 174, 317–321. [Google Scholar] [CrossRef]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Rahman, T.; Hodgson, H.J. Animal models of acute hepatic failure. Int. J. Exp. Pathol. 2000, 81, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Compound | MRM Transition 1 | DP * | CE | CXP | MRM Transition 2 | DP | CE | CXP |
|---|---|---|---|---|---|---|---|---|
| m-Hydroxybenzoic acid | 137.0 > 92.9 | −50 | −16 | −7 | 137.0 > 64.9 | −50 | −32 | −11 |
| Cinnamic acid | 147.0 > 103.1 | −55 | −14 | −7 | 147.0 > 76.9 | −55 | −28 | −7 |
| p-Coumaric acid | 162.9 > 119 | −70 | −20 | −5 | 162.9 > 119 | −70 | −38 | −25 |
| Ferulic acid | 193.0 > 134.0 | −55 | −20 | −7 | 193.0 > 177.9 | −55 | −16 | −15 |
| Syringic acid | 197.0 > 181.9 | −65 | −18 | −5 | 197.0 > 122.9 | −65 | −30 | −7 |
| Sinapic acid | 223.0 > 207.9 | −75 | −18 | −7 | 223.0 > 148.8 | −75 | −26 | −13 |
| Daidzein | 252.9 > 131.7 | −105 | −50 | −9 | 252.9 > 207.7 | −105 | −44 | −1 |
| Formononetin | 267.1 > 251.6 | −110 | −26 | −9 | 267.1 > 222.9 | −110 | −46 | −9 |
| Genistein | 268.8 > 133.0 | −170 | −38 | −43 | 268.8 > 181.0 | −170 | −34 | −13 |
| Biochanin A | 282.9 > 267.9 | −80 | −32 | −5 | 282.9 > 211.1 | −80 | −46 | −5 |
| Luteolin | 285.0 > 133.0 | −125 | −42 | −5 | 285.0 > 150.9 | −125 | −34 | −11 |
| Kaempferol | 285.0 > 184.9 | −135 | −36 | −15 | 285.0 > 116.9 | −135 | −48 | −3 |
| Taxifolin | 302.9 > 285.0 | −105 | −14 | −5 | 302.9 > 125.0 | −105 | −30 | −7 |
| Isorhamnetin | 315.0 > 299.9 | −130 | −32 | −15 | 315.0 > 150.9 | −130 | −40 | −11 |
| Rutin | 609.0 > 299.8 | −170 | −50 | −13 | 609.0 > 300.5 | −170 | −42 | −9 |
| Free | Esterified | Insoluble-Bound | Total | |
|---|---|---|---|---|
| TPC | ||||
| ‘California-INIA’ | 7.3 ± 0.2 b | 4.5 ± 0.3 c | 13.3 ± 0.3 a | 25.1 |
| ‘Alfa-INIA’ | 8.9 ± 0.7 a | 1.6 ± 0.1 c | 6.8 ± 0.4 b | 17.3 |
| ‘Local Navidad’ | 7.7 ± 0.4 b | 3.0 ± 0.3 c | 12.2 ± 1.0 a | 22.9 |
| ‘Local Santo Domingo’ | 10.2 ± 0.7 b | 4.6 ± 0.2 c | 16.7 ± 0.4 a | 31.5 |
| FRAP | ||||
| ‘California-INIA’ | 14.6 ± 0.4 b | 9.9 ± 0.4 c | 19.6 ± 0.7 a | 44.1 |
| ‘Alfa-INIA’ | 16.3 ± 0.9 b | 7.7 ± 0.3 c | 28.7 ± 0.8 a | 52.7 |
| ‘Local Navidad’ | 16.2 ± 0.6 a | 9.0 ± 0.6 b | 8.3 ± 0.2 b | 33.4 |
| ‘Local Santo Domingo’ | 19.4 ± 0.3 b | 9.1 ± 0.4 c | 41.8 ± 0.4 a | 70.4 |
| ORAC | ||||
| ‘California-INIA’ | 181.8 ± 12.1 b | 69.0 ± 2.9 c | 1798.4 ± 38.1 a | 2049.3 |
| ‘Alfa-INIA’ | 221.4 ± 9.9 b | 23.5 ± 0.7 c | 461.6 ± 57.2 a | 706.5 |
| ‘Local Navidad’ | 257.0 ±13.8 b | 60.7 ± 6.4 c | 1243.1 ± 34.9 a | 1560.8 |
| ‘Local Santo Domingo’ | 328.2 ± 21.9 b | 103.5 ± 4.6 c | 391.6 ± 1.9 a | 823.2 |
| Free | Esterified | Insoluble-Bound | Total | |
|---|---|---|---|---|
| Phenolic acids | ||||
| m-Hydroxybenzoic acid ** | ||||
| ‘California-INIA’ | 81.4 ± 2.0 b | 67.0 ± 2.0 b | 309.7 ± 11.6 a | 458.0 |
| ‘Alfa-INIA’ | 66.0 ± 2.8 b | 38.4 ± 4.0 b | 294.2 ± 27.1 a | 398.6 |
| ‘Local Navidad’ | 64.4 ± 0.9 b | 59.3 ± 1.4 b | 319.1 ± 29.5 a | 442.8 |
| ‘Local Santo Domingo’ | 23.3 ± 2.4 c | 50.7 ± 3.9 b | 143.2 ± 3.5 a | 217.2 |
| Cinnamic acid ** | ||||
| ‘California-INIA’ | 2.2 ± 0.2 | nd | nd | 2.2 |
| ‘Alfa-INIA’ | 2.8 ± 0.1 | nd | nd | 2.8 |
| ‘Local Navidad’ | 1.6 ± 0.1 | nd | nd | 1.6 |
| ‘Local Santo Domingo’ | tr | nd | nd | tr |
| p-Coumaric acid | ||||
| ‘California-INIA’ | 16.3 ± 0.4 a | 8.7 ± 0.8 b | 17.3 ± 1.9 a | 42.3 |
| ‘Alfa-INIA’ | 8.6 ± 0.6 a | 0.6 ± 0.1 c | 6.0 ± 0.1 b | 15.2 |
| ‘Local Navidad’ | 9.6 ± 0.5 a | 1.7 ± 0.1 c | 4.1 ± 0.1 b | 15.4 |
| ‘Local Santo Domingo’ | 22.7 ± 0.4 a | 10.4 ± 1.0 c | 6.6 ± 0.4 b | 39.7 |
| Ferulic acid | ||||
| ‘California-INIA’ | 5.8 ± 0.1 a | 2.7 ± 0.2 b | 5.4 ± 0.7 a | 13.8 |
| ‘Alfa-INIA’ | 3.4 ± 0.2 a | 0.9 ± 0.0 b | tr | 4.3 |
| ‘Local Navidad’ | 2.1 ± 0.1 a | 0.6 ± 0.0 b | tr | 2.7 |
| ‘Local Santo Domingo’ | nd | 2.3 ± 0.2 | tr | 2.3 |
| Syringic acid | ||||
| ‘California-INIA’ | 1.2 ± 0.0 c | 4.0 ± 0.1 b | 7.5 ± 0.8 a | 12.8 |
| ‘Alfa-INIA’ | 1.2 ± 0.0 b | 1.3 ± 0.2 b | 2.3 ± 0.1 a | 4.8 |
| ‘Local Navidad’ | 1.4 ± 0.1 c | 4.0 ± 0.2 b | 5.8 ± 0.6 a | 11.2 |
| ‘Local Santo Domingo’ | 0.6 ± 0.0 c | 4.6 ± 0.2 a | 2.1 ± 0.0 b | 7.3 |
| Sinapic acid | ||||
| ‘California-INIA’ | 12.9 ± 0.7 | nd | nd | 12.9 |
| ‘Alfa-INIA’ | 49.4 ± 0.6 | nd | nd | 49.4 |
| ‘Local Navidad’ | 33.3 ± 2.0 | nd | nd | 33.3 |
| ‘Local Santo Domingo’ | 23.6 ± 0.8 | nd | nd | 23.6 |
| Flavonoids | ||||
| Luteolin | ||||
| ‘California-INIA’ | tr | tr | nd | tr |
| ‘Alfa-INIA’ | tr | nd | nd | tr |
| ‘Local Navidad’ | tr | nd | nd | tr |
| ‘Local Santo Domingo’ | tr | tr | nd | tr |
| Kaempferol | ||||
| ‘California-INIA’ | tr | tr | tr | tr |
| ‘Alfa-INIA’ | tr | tr | tr | tr |
| ‘Local Navidad’ | tr | tr | tr | tr |
| ‘Local Santo Domingo’ | tr | tr | tr | tr |
| Taxifolin ** | ||||
| ‘California-INIA’ | 5.3 ± 0.3 c | 14.5 ± 1.4 b | 22.9 ± 2.1 a | 42.6 |
| ‘Alfa-INIA’ | 9.4 ± 0.5 | nd | nd | 9.4 |
| ‘Local Navidad’ | 4.6 ± 0.1 b | 3.5 ± 0.1 b | 45.5 ± 1.2 a | 53.6 |
| ‘Local Santo Domingo’ | 89.0 ± 2.0 a | 9.9 ± 0.4 c | 56.6 ± 3.9 b | 155.5 |
| Isorhamnetin | ||||
| ‘California-INIA’ | tr | tr | tr | tr |
| ‘Alfa-INIA’ | tr | tr | tr | tr |
| ‘Local Navidad’ | tr | tr | tr | tr |
| ‘Local Santo Domingo’ | tr | tr | tr | tr |
| Rutin | ||||
| ‘California-INIA’ | tr | tr | tr | tr |
| ‘Alfa-INIA’ | tr | tr | tr | tr |
| ‘Local Navidad’ | tr | tr | tr | tr |
| ‘Local Santo Domingo’ | tr | tr | tr | tr |
| Isoflavonoids | ||||
| Daidzein | ||||
| ‘California-INIA’ | tr | nd | nd | tr |
| ‘Alfa-INIA’ | tr | nd | nd | tr |
| ‘Local Navidad’ | tr | nd | nd | tr |
| ‘Local Santo Domingo’ | tr | nd | nd | tr |
| Formononetin ** | ||||
| ‘California-INIA’ | 140.8 ± 9.0 | nd | tr | 140.8 |
| ‘Alfa-INIA’ | 59.6 ± 3.8 | nd | tr | 59.6 |
| ‘Local Navidad’ | 128.6 ± 7.0 | nd | tr | 128.6 |
| ‘Local Santo Domingo’ | 51.9 ± 2.9 | nd | tr | 51.9 |
| Genistein | ||||
| ‘California-INIA’ | nd | tr | tr | tr |
| ‘Alfa-INIA’ | nd | tr | tr | tr |
| ‘Local Navidad’ | nd | tr | tr | tr |
| ‘Local Santo Domingo’ | nd | tr | tr | tr |
| Biochanin A ** | ||||
| ‘California-INIA’ | 7586.4 ± 632.3 a | 286.5 ± 13.0 b | 507.1 ± 57.7 b | 8380.0 |
| ‘Alfa-INIA’ | 5401.1 ± 418.4 a | 139.1 ± 5.2 c | 841.9 ± 85.1 b | 6382.1 |
| ‘Local Navidad’ | 6048.0 ± 290.4 a | 130.2 ± 6.8 b | 117.9 ± 17.1 b | 6296.1 |
| ‘Local Santo Domingo’ | 7544.0 ± 15.2 a | 233.1 ± 8.7 c | 583.7 ± 14.5 b | 8360.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Camargo, A.C.; Concepción Alvarez, A.; Arias-Santé, M.F.; Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Núñez Pizarro, P.; Bustos, S.M.; Schwember, A.R.; Shahidi, F.; et al. Soluble Free, Esterified and Insoluble-Bound Phenolic Antioxidants from Chickpeas Prevent Cytotoxicity in Human Hepatoma HuH-7 Cells Induced by Peroxyl Radicals. Antioxidants 2022, 11, 1139. https://doi.org/10.3390/antiox11061139
de Camargo AC, Concepción Alvarez A, Arias-Santé MF, Oyarzún JE, Andia ME, Uribe S, Núñez Pizarro P, Bustos SM, Schwember AR, Shahidi F, et al. Soluble Free, Esterified and Insoluble-Bound Phenolic Antioxidants from Chickpeas Prevent Cytotoxicity in Human Hepatoma HuH-7 Cells Induced by Peroxyl Radicals. Antioxidants. 2022; 11(6):1139. https://doi.org/10.3390/antiox11061139
Chicago/Turabian Stylede Camargo, Adriano Costa, Alina Concepción Alvarez, María Fernanda Arias-Santé, Juan Esteban Oyarzún, Marcelo E. Andia, Sergio Uribe, Paula Núñez Pizarro, Simón M. Bustos, Andrés R. Schwember, Fereidoon Shahidi, and et al. 2022. "Soluble Free, Esterified and Insoluble-Bound Phenolic Antioxidants from Chickpeas Prevent Cytotoxicity in Human Hepatoma HuH-7 Cells Induced by Peroxyl Radicals" Antioxidants 11, no. 6: 1139. https://doi.org/10.3390/antiox11061139
APA Stylede Camargo, A. C., Concepción Alvarez, A., Arias-Santé, M. F., Oyarzún, J. E., Andia, M. E., Uribe, S., Núñez Pizarro, P., Bustos, S. M., Schwember, A. R., Shahidi, F., & Bridi, R. (2022). Soluble Free, Esterified and Insoluble-Bound Phenolic Antioxidants from Chickpeas Prevent Cytotoxicity in Human Hepatoma HuH-7 Cells Induced by Peroxyl Radicals. Antioxidants, 11(6), 1139. https://doi.org/10.3390/antiox11061139









