A Greater Improvement of Intrahepatic Fat Contents after 6 Months of Lifestyle Intervention Is Related to a Better Oxidative Stress and Inflammatory Status in Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
- Conventional diet (CD) group: These participants followed the recommendations of American Association for the Study of Liver Disease (AASLD) [30], with energy restrictions to loss of at least 3–5% of the body weight to improve steatosis and 7–10% to improve most of the histopathological features of NASH, following the general guidelines of the U.S. Department of Health and Human Services and U.S. Department of Agriculture (20–35% fat, 10–35% protein, 45–65% carbohydrate) [31].
- Mediterranean diet high meal frequency (MD-HMF) group: This group was instructed to follow a Mediterranean diet characterized by a distribution of macronutrients of 40–45% carbohydrates (50–70% of carbohydrates should be low glycemic and rich in fiber), 30–35% fat, and 25% protein. This dietary pattern was previously observed to decrease fat mass and overall weight and improve the oxidative status in subjects with metabolic syndrome [32,33]. Total daily caloric intake was distributed over seven meals, with the highest calorie meals eaten early during the morning.
- Mediterranean diet physical activity (MD-PA) group: This group consumed an energy-restricted Mediterranean diet with a meal frequency of four to five meals per day, including snacks. Total calorie intake for this group came from 35–40% from fat (8–10% of saturated fatty acids, >20% of monounsaturated fatty acids, >10% of polyunsaturated fatty acids, and <300 mg/day of cholesterol), about 20% from proteins, and 40–45% or more from carbohydrates (mainly with low glycemic index). Sodium chloride should not reach 6 g/day (2.4 g of sodium), and dietary fiber should be no less than 30–35 g/day [34].
2.2. Anthropometric Characterization
2.3. Blood Collection and Analysis
2.4. Enzymatic Determinations
2.5. Malondialdehyde Assay
2.6. Protein Carbonyl Determination
2.7. Immunoassay Kits
2.8. Statistics
3. Results
3.1. Anthropometric, Biochemical, and Hematological Parameters
3.2. Oxidative Stress and Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finck, B.N. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes 2018, 67, 2485–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huaang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef]
- Ni Than, N.A.; Newsome, P.N. Non-alcoholic fatty liver disease: When to intervene and with what. Clin. Med. 2015, 15, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef]
- Al-Dayyat, H.M.; Rayyan, Y.M.; Tayyem, R.F. Non-alcoholic fatty liver disease and associated dietary and lifestyle risk factors. Diabetes Metab. Syndr. 2018, 12, 569–575. [Google Scholar] [CrossRef]
- Liu, Z.; Suo, C.; Zhao, R.; Yuan, H.; Jin, L.; Zhang, T.; Chen, X. Genetic predisposition, lifestyle risk, and obesity associate with the progression of nonalcoholic fatty liver disease. Dig. Liver Dis. 2021, 53, 1435–1442. [Google Scholar] [CrossRef]
- Noureddin, M.; Zelber-Sagi, S.; Wilkens, L.R.; Porcel, J.; Boushey, C.J.; Marchand, L.; Le Rosen, H.R.; Setiawan, V.W. Diet Associations With Nonalcoholic Fatty Liver Disease in an Ethnically Diverse Population: The Multiethnic Cohort. Hepatology 2020, 71, 1940–1952. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative stress and pro-inflammatory status in patients with non-alcoholic fatty liver disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Dixon, L.J.; Feldstein, A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ucar, F.; Sezer, S.; Erdogan, S.; Akyol, S.; Armutcu, F.; Akyol, O. The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013, 18, 127–133. [Google Scholar] [CrossRef]
- Carter-Kent, C.; Zein, N.N.; Feldstein, A.E. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: Implications for treatment. Am. J. Gastroenterol. 2008, 103, 1036–1042. [Google Scholar] [CrossRef]
- El-Din, S.H.S.; Sabra, A.-N.A.; Hammam, O.A.; Ebeid, F.A.; El-Lakkany, N.M. Pharmacological and antioxidant actions of garlic and.or onion in non-alcoholic fatty liver disease (NAFLD) in rats. J. Egypt. Soc. Parasitol. 2014, 44, 295–308. [Google Scholar]
- Hajighasem, A.; Farzanegi, P.; Mazaheri, Z. Effects of combined therapy with resveratrol, continuous and interval exercises on apoptosis, oxidative stress, and inflammatory biomarkers in the liver of old rats with non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 2019, 125, 142–149. [Google Scholar] [CrossRef]
- Patterson, R.E.; Kalavalapalli, S.; Williams, C.M.; Nautiyal, M.; Mathew, J.T.; Martinez, J.; Reinhard, M.K.; McDougall, D.J.; Rocca, J.R.; Yost, R.A.; et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E484–E494. [Google Scholar] [CrossRef] [Green Version]
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients 2019, 11, 677. [Google Scholar] [CrossRef] [Green Version]
- Dallio, M.; Romeo, M.; Gravina, A.G.; Masarone, M.; Larussa, T.; Abenavoli, L.; Persico, M.; Loguercio, C.; Federico, A. Nutrigenomics and Nutrigenetics in Metabolic- (Dysfunction) Associated Fatty Liver Disease: Novel Insights and Future Perspectives. Nutrients 2021, 13, 1679. [Google Scholar] [CrossRef]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cigrovski Berkovic, M.; Bilic-Curcic, I.; Mrzljak, A.; Cigrovski, V. NAFLD and Physical Exercise: Ready, Steady, Go! Front. Nutr. 2021, 8, 734859. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [PubMed] [Green Version]
- Byers, T.; Sedjo, R.L. Body fatness as a cause of cancer: Epidemiologic clues to biologic mechanisms. Endocr. Relat. Cancer 2015, 22, R125–R134. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Solga, S.F.; Horska, A.; Bonekamp, S.; Diehl, A.M.; Brancati, F.L.; Wagenknecht, L.E.; Pi-Sunyer, F.X.; Kahn, S.E.; Clark, J.M. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010, 33, 2156–2163. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, B.; Schrauwen-Hinderling, V.B.; Jelenik, T.; Gemmink, A.; Sparks, L.M.; Havekes, B.; Bruls, Y.; Dahlmans, D.; Roden, M.; Hesselink, M.K.C.; et al. Exercise training reduces intrahepatic lipid content in people with and people without nonalcoholic fatty liver. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E165–E173. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. Dietry Guidlines for Americans 2015–2020, 8th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2015.
- De La Iglesia, R.; Lopez-Legarrea, P.; Abete, I.; Bondia-Pons, I.; Navas-Carretero, S.; Forga, L.; Martinez, J.A.; Zulet, M.A. A new dietary strategy for long-term treatment of the metabolic syndrome is compared with the American heart association (AHA) guidelines: The MEtabolic Syndrome REduction in NAvarra (RESMENA) project. Br. J. Nutr. 2014, 111, 643–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulet, M.; Bondia-Pons, I.; Abete, I.; de la Iglesia, R.; López-Legarrera, P.; Forga, L.; Navas-Carretero, S.; Martínez, J. The reduction of the metabolyc syndrome in Navarra-Spain (RESMENA-S) study: A multidisciplinary strategy based on chrononutrition and nutritional education, together with dietetic and psychological control. Nutr. Hosp. 2011, 26, 16–26. [Google Scholar] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbate, M.; Mascaró, C.M.; Montemayor, S.; Barbería-Latasa, M.; Casares, M.; Gómez, C.; Angullo-Martinez, E.; Tejada, S.; Abete, I.; Zulet, M.A.; et al. Energy Expenditure Improved Risk Factors Associated with Renal Function Loss in NAFLD and MetS Patients. Nutrients 2021, 13, 629. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, I.; Martinez-Gonzalez, M.A.; Sánchez-Tainta, A.; Corella, D.; Díaz-López, A.; Fito, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; et al. Adherence to an energy-restricted Mediterranean diet score and prevalence of cardiovascular risk factors in the PREDIMED-plus: A cross-sectional study. Rev. Española Cardiol. 2019, 72, 925–934. [Google Scholar] [CrossRef]
- Reeder, S.B.; Sirlin, C.B. Quantification of liver fat with magnetic resonance imaging. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 337–357. [Google Scholar] [CrossRef] [Green Version]
- Buckley, J.; Sim, J.; Eston, R.; Hession, R.; Fox, R. Reliability and validity of measures taken during the Chester step test to predict aerobic power and to prescribe aerobic exercise. Br. J. Sports Med. 2004, 38, 197–205. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Flohé, L.; Otting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Wu, H.; Bjornson, E.; Zhang, C.; Hakkarainen, A.; Räsänen, S.M.; Lee, S.; Mancina, R.M.; Bergentall, M.; Pietiläinen, K.H.; et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018, 27, 559–571.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeb, E.; Steffen, H.M.; Bantel, H.; Baumann, U.; Canbay, A.; Demir, M.; Drebber, U.; Geier, A.; Hampe, J.; Hellerbrand, C.; et al. [S2k Guideline non-alcoholic fatty liver disease]. Z. Gastroenterol. 2015, 53, 668–723. [Google Scholar]
- Marchesini, G.; Day, C.P.; Dufour, J.F.; Canbay, A.; Nobili, V.; Ratziu, V.; Tilg, H.; Roden, M.; Gastaldelli, A.; Yki-Jarvinen, H.; et al. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worm, N. Beyond Body Weight-Loss: Dietary Strategies Targeting Intrahepatic Fat in NAFLD. Nutrients 2020, 12, 1316. [Google Scholar] [CrossRef]
- Houttu, V.; Csader, S.; Nieuwdorp, M.; Holleboom, A.G.; Schwab, U. Dietary Interventions in Patients With Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 716783. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Bouzas, C.; Mascaró, C.M.; Casares, M.; Llompart, I.; Abete, I.; Angullo-Martinez, E.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A. Effect of Dietary and Lifestyle Interventions on the Amelioration of NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 2223. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.L.; Visioni, A.J.; Qureshi, M.M.; Bradlee, M.L.; Ellison, R.C.; D’Agostino, R. Weight loss in overweight adults and the long-term risk of hypertension: The Framingham study. Arch. Intern. Med. 2005, 165, 1298–1303. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, I.; Stampfer, M.J.; Schwarzfuchs, D.; Shai, I.; DIRECT group. Adherence and success in long-term weight loss diets: The dietary intervention randomized controlled trial (DIRECT). J. Am. Coll. Nutr. 2009, 28, 159–168. [Google Scholar] [CrossRef]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016, 36, 5–20. [Google Scholar] [CrossRef]
- Fabbrini, E.; Mohammed, B.S.; Magkos, F.; Korenblat, K.M.; Patterson, B.W.; Klein, S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 2008, 134, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; Minicis, D.S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.; Shackel, N.; Gorrell, M.; McLennan, S.; Twigg, S. Diabetes and nonalcoholic Fatty liver disease: A pathogenic duo. Endocr. Rev. 2013, 34, 84–129. [Google Scholar] [CrossRef]
- Westerbacka, J.; Cornér, A.; Tiikkainen, M.; Tamminen, M.; Vehkavaara, S.; Häkkinen, A.; Fredriksson, J.; Yki-Järvinen, H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: Implications for sex differences in markers of cardiovascular risk. Diabetologia 2004, 47, 1360–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuefeng, M.; Shousheng, L.; Zhang, J.; Mengzhen, D.; Wang, Y.; Wang, M.; Yongning, X. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 10. [Google Scholar]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.C.; Lopes, S.; Teixeira, M.; Polónia, J.; Alves, A.J.; Mesquita-Bastos, J.; Ribeiro, F. The Chester step test is a valid tool to assess cardiorespiratory fitness in adults with hypertension: Reducing the gap between clinical practice and fitness assessments. Hypertens. Res. 2019, 42, 2021–2024. [Google Scholar] [CrossRef] [PubMed]
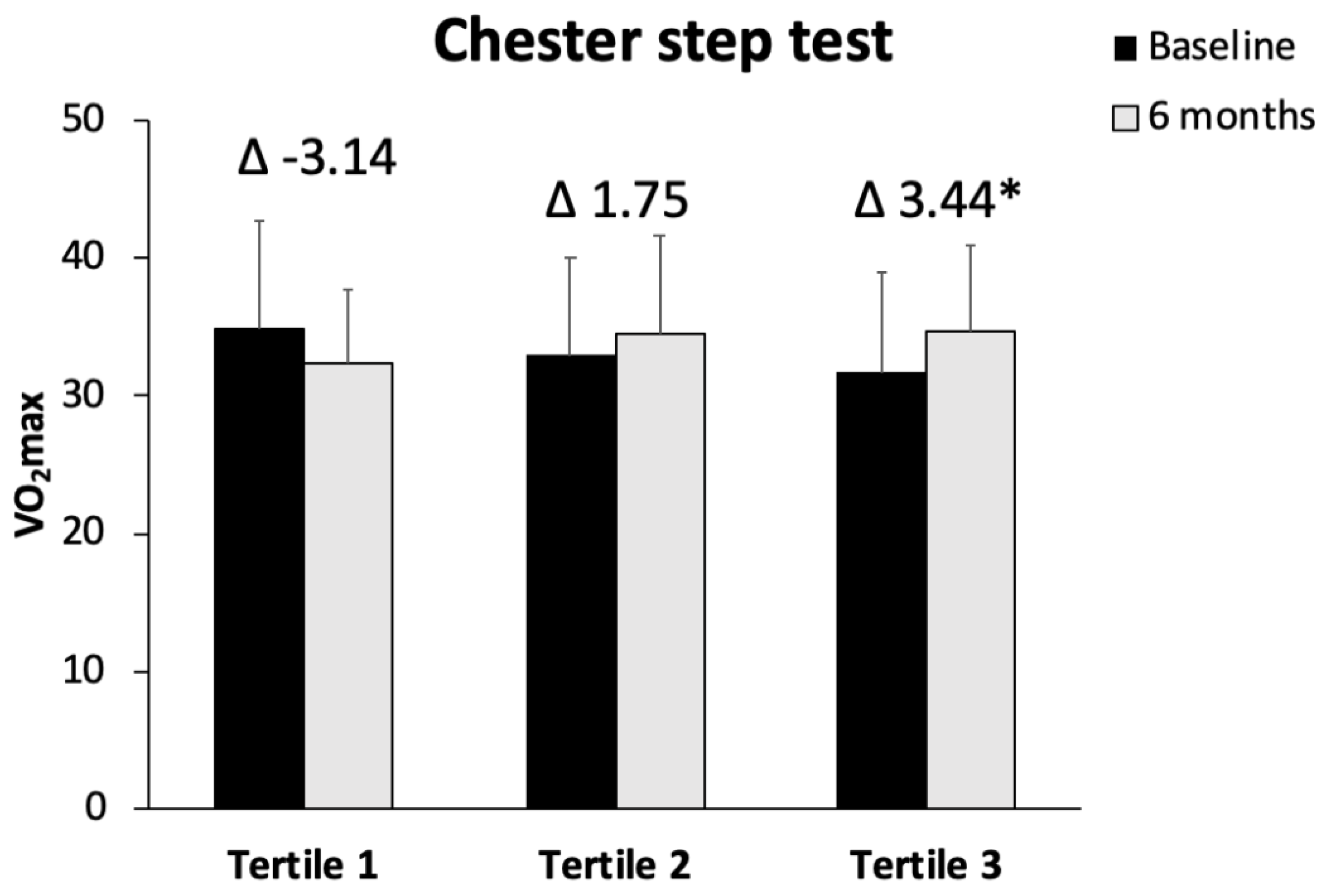
- Mascaró, C.M.; Bouzas, C.; Montemayor, S.; Casares, M.; Llompart, I.; Ugarriza, L.; Borràs, P.A.; Martínez, J.A.; Tur, J.A. Effect of a Six-Month Lifestyle Intervention on the Physical Activity and Fitness Status of Adults with NAFLD and Metabolic Syndrome. Nutrients 2022, 14, 1813. [Google Scholar] [CrossRef]
- Kwak, M.-S.; Kim, D. Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J. Intern. Med. 2018, 33, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Stine, J.G.; Soriano, C.; Schreibman, I.; Rivas, G.; Hummer, B.; Yoo, E.; Schmitz, K.; Sciamanna, C. Breaking Down Barriers to Physical Activity in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2021, 66, 3604–3611. [Google Scholar] [CrossRef]
- Świderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic. Res. 2019, 53, 841–850. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Mechanisms of oxidative damage of low density lipoprotein in human atherosclerosis. Curr. Opin. Lipidol. 1997, 8, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Ke, Y.; Wu, F.; Liu, S.; Ji, C.; Zhu, X.; Zhang, Y. Circulating Irisin Levels in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2020, 2020, 8818191. [Google Scholar] [CrossRef] [PubMed]
- Altaf, B.; Jawed, S.; Salam, R.M.T. Association of apoptotic marker cytokeratin18 with blood pressure in nonalcoholic fatty liver disease patients. J. Pak. Med. Assoc. 2020, 70, 2128–2131. [Google Scholar]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef]
- Kawanaka, M.; Nishino, K.; Nakamura, J.; Urata, N.; Oka, T.; Goto, D.; Suehiro, M.; Kawamoto, H.; Yamada, G. Correlation between serum cytokeratin-18 and the progression or regression of non-alcoholic fatty liver disease. Ann. Hepatol. 2015, 14, 837–844. [Google Scholar] [CrossRef]
- Safarian, M.; Mohammadpour, S.; Shafiee, M.; Ganji, A.; Soleimani, A.; Nematy, M.; Bahari, A. Effect of diet-induced weight loss on cytokeratin-18 levels in overweight and obese patients with liver fibrosis. Diabetes Metab. Syndr. 2019, 13, 989–994. [Google Scholar] [CrossRef]
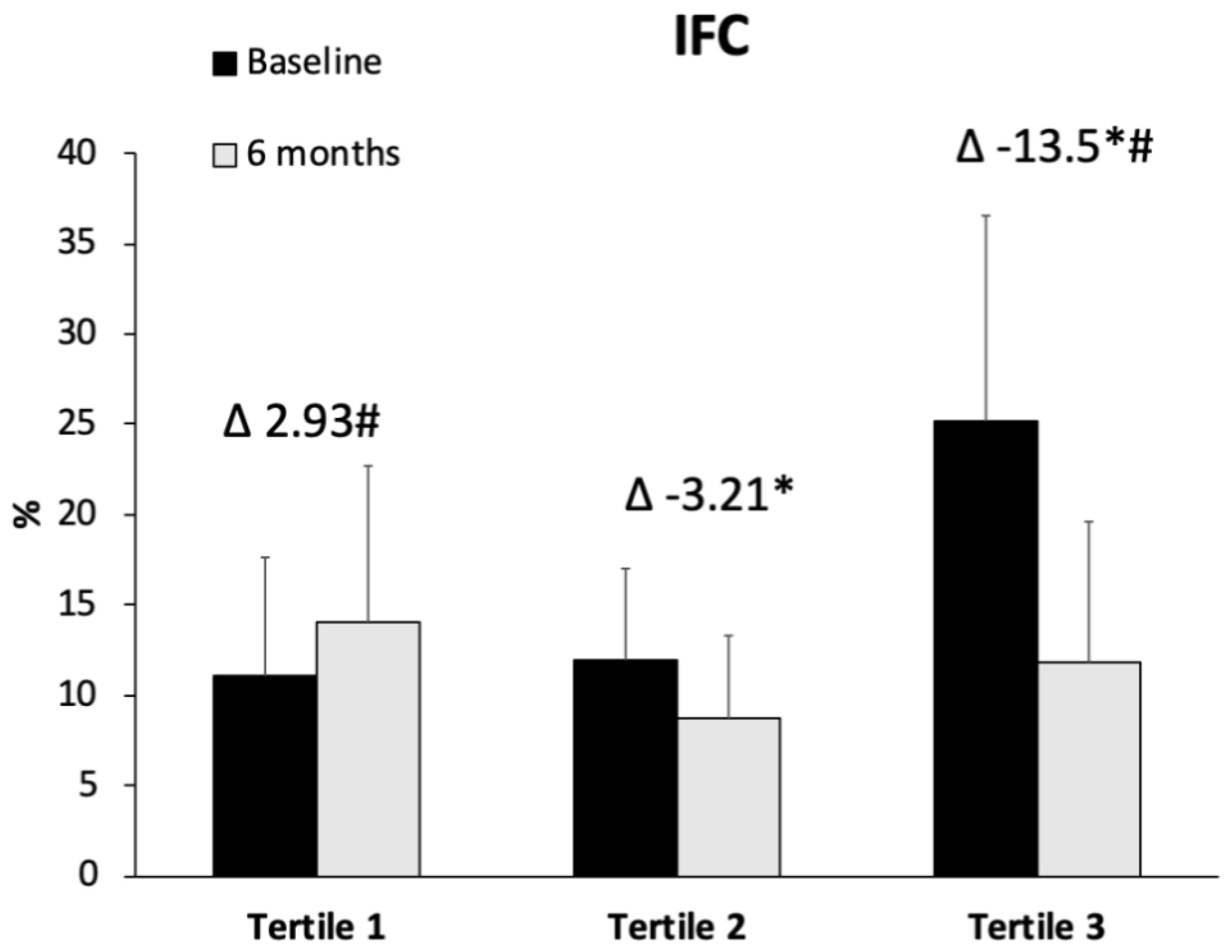

| Tertile 1 (<−0.567) n = 20 | Tertile 2 (−0.567 to −7.13) n = 20 | Tertile 3 (>−7.13) n = 20 | p-Value | ||
|---|---|---|---|---|---|
| Weight (kg) | Baseline | 91.5 ± 15.7 | 97.8 ± 16.2 | 93.9 ± 8.1 | |
| 6 months | 90.4 ± 14.9 | 92.7 ± 13.2 | 87.1 ± 8.53 | ||
| −1.06 ± 3.36 | −5.08 ± 4.83 * | −6.79 ± 5.06 * | <0.001 | ||
| BMI (kg/m2) | Baseline | 32.5 ± 3.30 | 34.4 ± 4.93 | 34.0 ± 2.70 | |
| 6 months | 32.2 ± 2.91 | 32.7 ± 4.48 | 31.5 ± 2.29 | ||
| −0.375 ± 1.17 | −1.71 ± 1.56 * | −2.51 ± 1.87 * | <0.001 | ||
| Systolic blood pressure (mmHg) | Baseline | 137.4 ± 20.5 | 134.0 ± 12.2 | 142.2 ± 16.9 | |
| 6 months | 134.0 ± 12.0 | 135.5 ± 11.1 | 132.7 ± 11.0 | ||
| −3.74 ± 12.6 | 1.56 ± 13.1 | −1.99 ± 30.4 | 0.318 | ||
| Diastolic blood pressure (mmHg) | Baseline | 80.8 ± 9.71 | 81.5 ± 7.03 | 84.3 ± 9.98 | |
| 6 months | 81.4 ± 7.55 | 82.0 ± 7.65 | 78.0 ± 9.05 | ||
| 0.031 ± 9.76 | 1.00 ± 6.83 | −0.353 ± 18.4 | 0.155 | ||
| Glucose (mg/dL) | Baseline | 109.2 ± 23.5 | 116.1 ± 41.9 | 115.1 ± 20.4 | |
| 6 months | 110.1 ± 25.7 | 112.5 ± 43.7 | 111.7 ± 41.0 | ||
| 0.850 ± 17.9 | −2.76 ± 11.0 | −3.40 ± 27.7 | 0.253 | ||
| Hb1Ac (%) | Baseline | 6.09 ± 1.08 | 6.08 ± 1.13 | 6.00 ± 0.557 | |
| 6 months | 6.05 ± 0.886 | 5.85 ± 0.880 | 5.74 ± 0.412 | ||
| −0.026 ± 0.456 | −0.229 ± 0.384 | −0.238 ± 0.447 | 0.235 | ||
| Triglycerides (mg/dL) | Baseline | 178.3 ± 81.8 | 163.0 ± 58.5 | 240.2 ± 140.6 | |
| 6 months | 232.2 ± 160.5 | 128.1 ± 34.2 | 159.4 ± 78.5 | ||
| 53.8 ± 159.7 | −46.8 ± 34.1 * | −80.8 ± 145.5 * | 0.002 | ||
| HDL cholesterol (mg/dL) | Baseline | 44.1 ± 11.9 | 42.2 ± 8.28 | 39.6 ± 6.94 | |
| 6 months | 44.7 ± 14.0 | 45.8 ± 10.9 | 39.9 ± 5.83 | ||
| 0.950 ± 6.59 | 4.24 ± 5.25 | 0.250 ± 4.51 | 0.054 | ||
| LDL cholesterol (mg/dL) | Baseline | 127.3 ± 34.5 | 125.2 ± 27.2 | 129.0 ± 28.8 | |
| 6 months | 128.5 ± 35.1 | 112.4 ± 25.8 | 121.6 ± 33.4 | ||
| 5.88 ± 33.8 | −7.38 ± 25.0 | −7.11 ± 21.3 | 0.266 | ||
| Cholesterol total (mg/dL) | Baseline | 224.0 ± 74.7 | 200.6 ± 32.1 | 214.8 ± 34.1 | |
| 6 months | 212.7 ± 38.5 | 183.6 ± 33.1 | 192.9 ± 39.2 | ||
| −6.05 ± 78.9 | −11.1 ± 26.1 | −21.9 ± 35.5 | 0.616 | ||
| Bilirubin (mg/dL) | Baseline | 0.811 ± 0.527 | 0.650 ± 0.254 | 0.700 ± 0.395 | |
| 6 months | 0.739 ± 0.379 | 0.636 ± 0.267 | 0.820 ± 0.554 | ||
| 0.024 ± 0.371 | 0.003 ± 0.223 | 0.109 ± 0.300 | 0.516 | ||
| AST (U/L) | Baseline | 23.3 ± 8.33 | 22.8 ± 5.71 | 29.4 ± 10.6 | |
| 6 months | 23.9 ± 6.40 | 20.9 ± 5.52 | 23.9 ± 7.68 | ||
| 0.550 ± 6.42 | −1.11 ± 7.83 | −3.28 ± 7.12 | 0.246 | ||
| ALT (U/L) | Baseline | 27.9 ± 10.1 | 32.4 ± 19.1 | 60.8 ± 58.7 | |
| 6 months | 28.7 ± 11.9 | 24.0 ± 9.35 | 29.8 ± 13.0 | ||
| −0.800 ± 8.46 | −8.48 ± 15.4 | −28.0 ± 50.8 * | 0.004 | ||
| GGT (U/L) | Baseline | 44.2 ± 29.2 | 32.5 ± 14.3 | 48.8 ± 25.2 | |
| 6 months | 43.8 ± 23.8 | 27.8 ± 11.3 | 44.7 ± 55.5 | ||
| −0.450 ± 19.5 | −4.68 ± 5.71 | −4.14 ± 40.4 | 0.022 | ||
| CRP (mg/dL) | Baseline | 0.498 ± 0.505 | 0.529 ± 0.509 | 0.529 ± 0.711 | |
| 6 months | 0.356 ± 0.394 | 0.435 ± 0.336 | 0.322 ± 0.272 | ||
| −0.143 ± 0.494 | −0.090 ± 0.318 | −0.220 ± 0.749 | 0.750 |
| Tertile 1 (<−0.567) n = 20 | Tertile 2 (−0.567 to −7.13) n = 20 | Tertile 3 (>−7.13) n = 20 | p-Value | ||
|---|---|---|---|---|---|
| Hematocrit (%) | Baseline | 43.7 ± 4.08 | 43.4 ± 4.11 | 44.9 ± 4.15 | |
| 6 months | 43.5 ± 3.45 | 44.3 ± 3.73 | 45.7 ± 2.76 | ||
| −0.250 ± 2.00 | 0.629 ± 1.54 | 0.780 ± 2.58 | 0.244 | ||
| Erythrocytes (106/μL) | Baseline | 4.90 ± 0.349 | 4.84 ± 0.465 | 5.07 ± 0.403 | |
| 6 months | 4.88 ± 0.329 | 4.89 ± 0.407 | 5.12 ± 0.289 | ||
| −0.022 ± 0.216 | 0.040 ± 0.216 | 0.054 ± 0.342 | 0.217 | ||
| Leukocytes (103/μL) | Baseline | 7.18 ± 2.16 | 7.68 ± 1.95 | 7.44 ± 1.50 | |
| 6 months | 6.92 ± 1.85 | 7.77 ± 1.96 | 6.94 ± 1.58 | ||
| −0.253 ± 1.25 | 0.066 ± 1.60 | −0.503 ± 0.691 | 0.533 | ||
| Platelets (103/μL) | Baseline | 228.2 ± 49.4 | 239.1 ± 44.0 | 243.7 ± 53.3 | |
| 6 months | 226.9 ± 55.0 | 242.7 ± 46.7 | 226.2 ± 47.1 | ||
| −1.30 ± 34.8 | 3.62 ± 23.7 | −17.6 ± 27.4 | 0.022 | ||
| Neutrophils (103/μL) | Baseline | 3.85 ± 1.45 | 4.37 ± 1.19 | 3.86 ± 0.953 | |
| 6 months | 3.66 ± 1.48 | 4.44 ± 1.63 | 3.76 ± 1.14 | ||
| −0.189 ± 0.843 | 0.107 ± 1.30 | −0.102 ± 0.616 | 0.914 | ||
| Lymphocytes (103/μL) | Baseline | 2.35 ± 0.672 | 2.62 ± 0.805 | 2.67 ± 0.667 | |
| 6 months | 2.35 ± 0.516 | 2.47 ± 0.695 | 2.36 ± 0.671 | ||
| 0.002 ± 0.497 | −0.151 ± 0.467 | −0.315 ± 0.278 | 0.071 | ||
| Monocytes (103/μL) | Baseline | 0.656 ± 0.266 | 0.599 ± 0.151 | 0.600 ± 0.101 | |
| 6 months | 0.613 ± 0.242 | 0.596 ± 0.159 | 0.566 ± 0.132 | ||
| −0.043 ± 0.112 | −0.003 ± 0.133 | −0.034 ± 0.097 | 0.596 | ||
| Eosinophils (103/μL) | Baseline | 0.266 ± 0.183 | 0.221 ± 0.148 | 0.244 ± 0.111 | |
| 6 months | 0.243 ± 0.139 | 0.207 ± 0.110 | 0.203 ± 0.129 | ||
| −0.023 ± 0.113 | −0.014 ± 0.067 | −0.042 ± 0.094 | 0.310 | ||
| Basophils (103/μL) | Baseline | 0.058 ± 0.026 | 0.058 ± 0.023 | 0.067 ± 0.025 | |
| 6 months | 0.059 ± 0.024 | 0.051 ± 0.024 | 0.054 ± 0.023 | ||
| 0.001 ± 0.026 | −0.006 ± 0.029 | −0.013 ± 0.033 | 0.325 |
| Tertile 1 (<−0.567) n = 20 | Tertile 2 (−0.567 to −7.13) n = 20 | Tertile 3 (>−7.13) n = 20 | p-Value | ||
|---|---|---|---|---|---|
| Enzymatic Activities | |||||
| CAT (K/L blood) | Baseline | 41.2 ± 9.41 | 48.0 ± 12.3 | 61.8 ± 16.7 | |
| 6 months | 56.8 ± 29.0 | 37.2 ± 22.6 | 36.7 ± 22.9 | ||
| 13.9 ± 30.2 | −7.84 ± 22.8 | −21.9 ± 26.2 * | 0.003 | ||
| SOD (pkat/L blood) | Baseline | 295.6 ± 60.9 | 283.4 ± 64.3 | 309.9 ± 75.6 | |
| 6 months | 311.9 ± 96.9 | 287.4 ± 65.7 | 287.8 ± 69.8 | ||
| 42.6 ± 156.3 | 9.47 ± 100.9 | −24.9 ± 125.5 | 0.361 | ||
| ELISA assays | |||||
| MPO (ng/mL) | Baseline | 4.07 ± 2.89 | 4.99 ± 2.66 | 4.16 ± 2.22 | |
| 6 months | 3.26 ± 1.06 | 3.67 ± 1.69 | 3.14 ± 1.19 | ||
| −0.874 ± 2.66 | −1.15 ± 3.32 | −1.04 ± 2.00 | 0.680 | ||
| XOD (ng/mL) | Baseline | 0.411 ± 0.122 | 0.370 ± 0.124 | 0.386 ± 0.087 | |
| 6 months | 0.460 ± 0.243 | 0.348 ± 0.161 | 0.350 ± 0.113 | ||
| 0.068 ± 0.261 | −0.017 ± 0.166 | −0.023 ± 0.106 | 0.307 | ||
| Resolvin D1 (pg/mL) | Baseline | 132.9 ± 44.2 | 135.1 ± 43.4 | 140.3 ± 33.8 | |
| 6 months | 147.5 ± 30.7 | 159.5 ± 45.5 | 162.6 ± 32.4 | ||
| 14.4 ± 36.4 | 24.0 ± 62.2 | 23.2 ± 25.8 | 0.706 | ||
| Irisin (ng/mL) | Baseline | 118.6 ± 76.3 | 102.4 ± 63.8 | 132.3 ± 72.7 | |
| 6 months | 124.7 ± 92.9 | 112.2 ± 70.9 | 92.1 ± 59.1 | ||
| 6.3 ± 76.2 | 11.6 ± 65.5 | −39.5 ± 56.4 * | 0.002 | ||
| CK-18 (U/L) | Baseline | 47.1 ± 24.6 | 71.1 ± 44.2 | 96.8 ± 55.4 | |
| 6 months | 42.4 ± 22.3 | 41.0 ± 20.7 | 54.5 ± 41.0 | ||
| −1.04 ± 21.3 | −29.6 ± 50.2 | −44.2 ± 54.0 * | 0.040 | ||
| Multiplex Assay | |||||
| Baseline | 4.25 ± 0.217 | 4.13 ± 0.259 | 4.28 ± 0.520 | ||
| IL-6 (pg/mL) | 6 months | 4.34 ± 0.377 | 4.26 ± 0.424 | 4.34 ± 0.604 | |
| 0.103 ± 0.342 | −0.015 ± 0.117 | 0.035 ± 0.289 | 0.805 | ||
| Baseline | 3.05 ± 0.543 | 3.85 ± 0.466 | 4.25 ± 1.74 | ||
| TNFα (pg/mL) | 6 months | 3.95 ± 0.441 | 3.79 ± 0.381 | 4.19 ± 1.76 | |
| −0.038 ± 0.399 | −0.209 ± 0.284 | −0.120 ± 0.338 | 0.355 | ||
| Oxidative damage | |||||
| MDA (nM) | Baseline | 1.88 ± 0.737 | 1.71 ± 0.640 | 2.01 ± 0.843 | |
| 6 months | 1.69 ± 1.41 | 1.43 ± 0.597 | 1.20 ± 0.483 | ||
| −0.111 ± 1.71 | −0.337 ± 1.01 | −0.874 ± 1.09 | 0.244 | ||
| Protein carbonyl (%) | Baseline | 100.0 ± 67.7 | 123.2 ± 65.0 | 136.8 ± 74.2 | |
| 6 months | 95.9 ± 34.3 | 88.3 ± 31.3 | 84.7 ± 18.6 | ||
| −4.1 ± 88.4 | −35.5 ± 78.4 | −50.9 ± 71.7 | 0.285 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Llompart, I.; Gámez, J.M.; Tejada, S.; Martínez, J.A.; et al. A Greater Improvement of Intrahepatic Fat Contents after 6 Months of Lifestyle Intervention Is Related to a Better Oxidative Stress and Inflammatory Status in Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 1266. https://doi.org/10.3390/antiox11071266
Monserrat-Mesquida M, Quetglas-Llabrés M, Bouzas C, Montemayor S, Mascaró CM, Casares M, Llompart I, Gámez JM, Tejada S, Martínez JA, et al. A Greater Improvement of Intrahepatic Fat Contents after 6 Months of Lifestyle Intervention Is Related to a Better Oxidative Stress and Inflammatory Status in Non-Alcoholic Fatty Liver Disease. Antioxidants. 2022; 11(7):1266. https://doi.org/10.3390/antiox11071266
Chicago/Turabian StyleMonserrat-Mesquida, Margalida, Magdalena Quetglas-Llabrés, Cristina Bouzas, Sofía Montemayor, Catalina M. Mascaró, Miguel Casares, Isabel Llompart, José M. Gámez, Silvia Tejada, J. Alfredo Martínez, and et al. 2022. "A Greater Improvement of Intrahepatic Fat Contents after 6 Months of Lifestyle Intervention Is Related to a Better Oxidative Stress and Inflammatory Status in Non-Alcoholic Fatty Liver Disease" Antioxidants 11, no. 7: 1266. https://doi.org/10.3390/antiox11071266
APA StyleMonserrat-Mesquida, M., Quetglas-Llabrés, M., Bouzas, C., Montemayor, S., Mascaró, C. M., Casares, M., Llompart, I., Gámez, J. M., Tejada, S., Martínez, J. A., Tur, J. A., & Sureda, A. (2022). A Greater Improvement of Intrahepatic Fat Contents after 6 Months of Lifestyle Intervention Is Related to a Better Oxidative Stress and Inflammatory Status in Non-Alcoholic Fatty Liver Disease. Antioxidants, 11(7), 1266. https://doi.org/10.3390/antiox11071266