Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses
Abstract
:1. Introduction
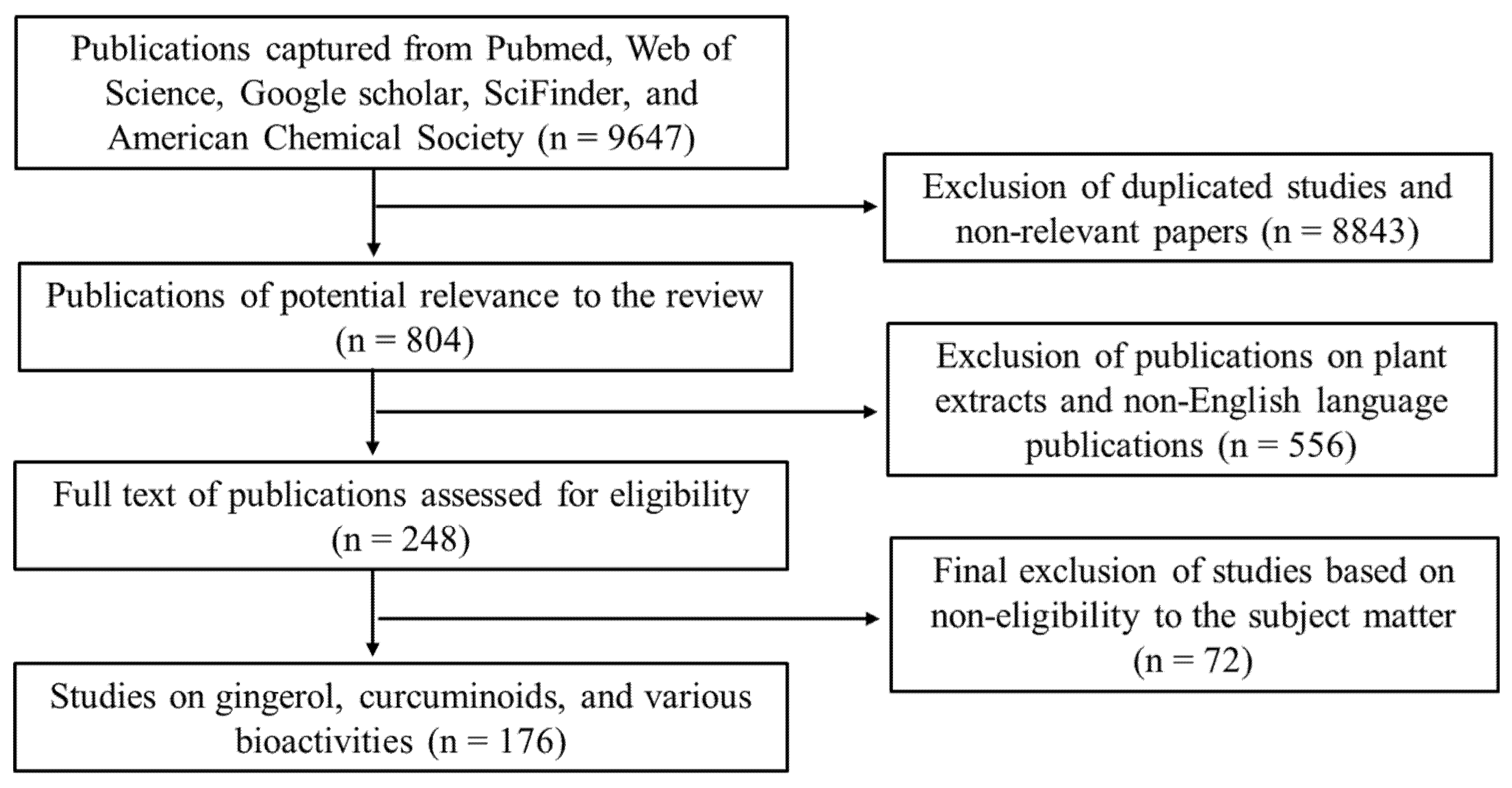
2. Methodology
3. Physicochemical Characteristics
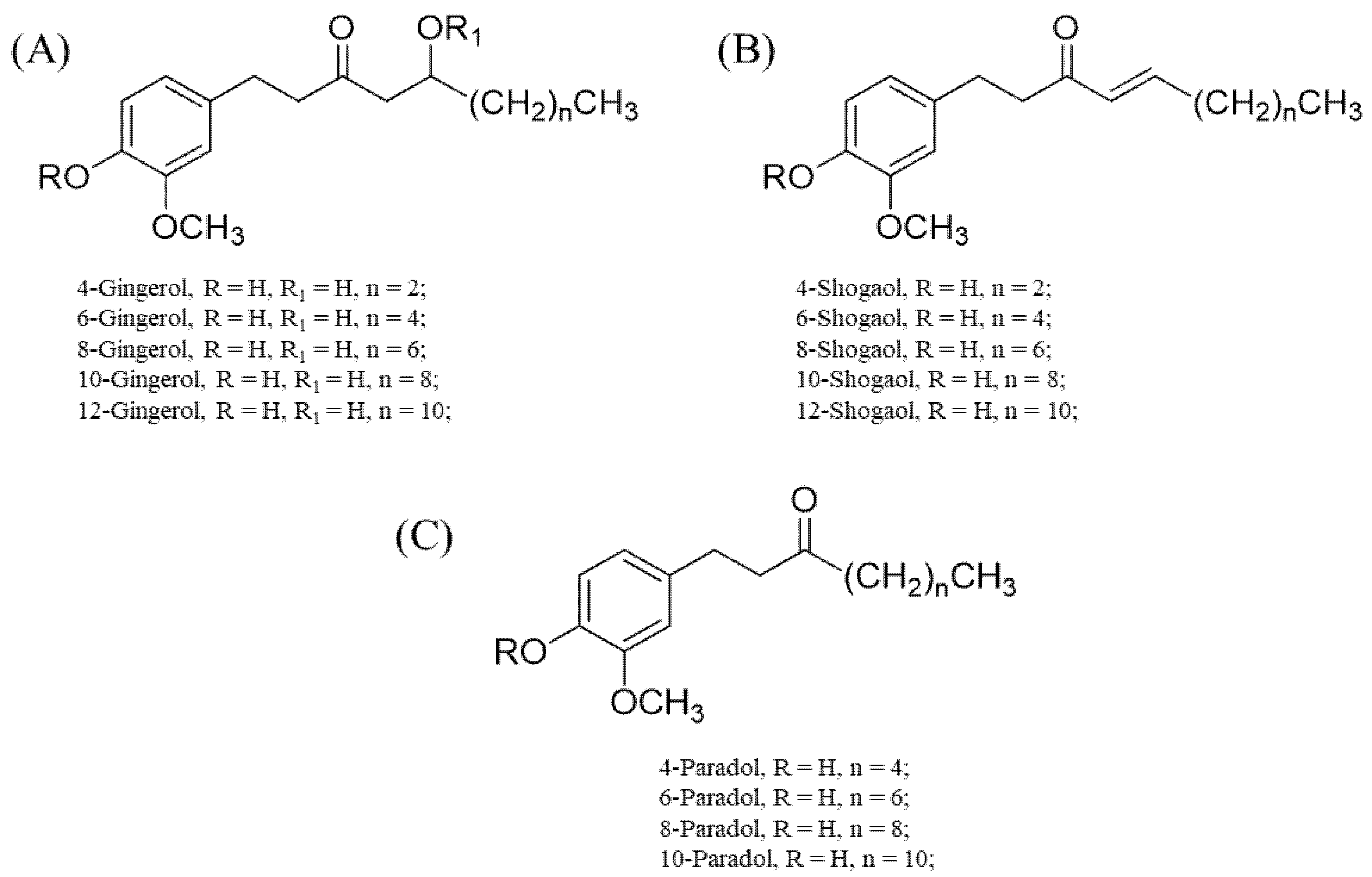
3.1. Gingerols and Their Derivatives
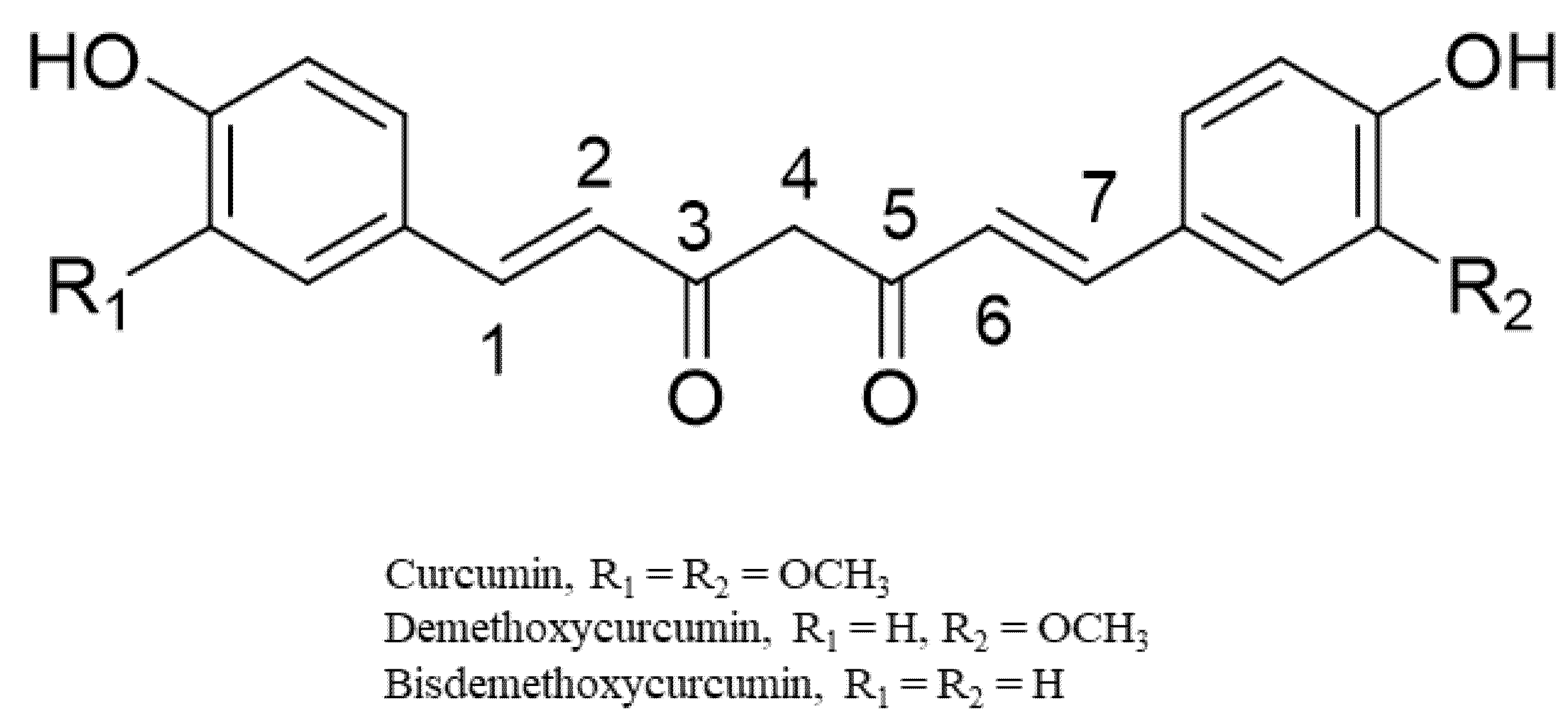
3.2. Curcuminoids
4. Notable Bioactivities
4.1. Antioxidant Activities
4.2. Anti-Inflammatory Effects
4.3. Antidiabetic Effects
4.4. Hepatoprotective Effects
4.5. Neuroprotective Effects
4.6. Anticancer Activities
4.7. Antimicrobial Properties
4.8. Safety of Compounds from Zingiberaceae
5. Enhancement of Bioactivities
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Niwa-Kawakita, M.; Ferhi, O.; Soilihi, H.; Le Bras, M.; Lallemand-Breitenbach, V.; de Thé, H. PML is a ROS sensor activating p53 upon oxidative stress. J. Exp. Med. 2017, 214, 3197–3206. [Google Scholar] [CrossRef] [Green Version]
- Achanta, G.; Huang, P. Role of p53 in sensing oxidative DNA damage in response to reactive oxygen species-generating agents. Cancer Res. 2004, 64, 6233. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, X.; Chen, Y.H.; Chen, Y.; Jiang, W.; Zheng, H.; Huang, F.Q.; Liu, B.; Zhou, W.; Qi, L.W.; et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics 2021, 11, 1703–1720. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Sekar, M.; Chitranshi, N.; Malviya, R.; et al. Comprehensive review of methodology to detect reactive oxygen species (ROS) in mammalian species and establish its relationship with antioxidants and cancer. Antioxidants 2021, 10, 128. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [Green Version]
- Que, X.; Hung, M.Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Alsamri, H.; Athamneh, K.; Pintus, G.; Eid, A.H.; Iratni, R. Pharmacological and antioxidant activities of Rhus coriaria L. (Sumac). Antioxidants 2021, 10, 73. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Tomei, F.; Sancini, A.; Morabito, G.; Serafini, M. Effect of plant foods and beverages on plasma non-enzymatic antioxidant capacity in human subjects: A meta-analysis. Br. J. Nutr. 2013, 109, 1544–1556. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Daliu, P.; Narciso, V.; Tenore, G.C.; Novellino, E. Colon bioaccessibility and antioxidant activity of white, green and black tea polyphenols extract after in vitro simulated gastrointestinal digestion. Nutrients 2018, 10, 1711. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.P.; Mitscher, L.A.; Menon, S.R.; Pillai, C.A.; Shankel, D.M. Antimutagenic/antioxidant activity of green tea components and related compounds. J. Environ. Pathol. Toxicol. Oncol. 1999, 18, 147–158. [Google Scholar]
- Gulati, K.; Pankaj, V.; Nishanti, R.; Arunabha, R. Chapter 7—Role of nutraceuticals in respiratory and allied diseases. In Nutraceuticals, 2nd ed.; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Academic Press: London, UK, 2021; pp. 101–115. [Google Scholar]
- Taylor, T.N.; Taylor, E.L.; Krings, M. Chapter 22—Flowering Plants. In Paleobotany, 2nd ed.; Taylor, T.N., Taylor, E.L., Krings, M., Eds.; Academic Press: London, UK, 2009; pp. 873–997. [Google Scholar]
- Barbosa, G.B.; Jayasinghe, N.S.; Natera, S.H.A.; Inutan, E.D.; Peteros, N.P.; Roessner, U. From common to rare Zingiberaceae plants—A metabolomics study using GC-MS. Phytochemistry 2017, 140, 141–150. [Google Scholar] [CrossRef]
- Kumar, K.M.; Asish, G.R.; Sabu, M.; Balachandran, I. Significance of gingers (Zingiberaceae) in Indian system of medicine—Ayurveda: An overview. Anc. Sci. Life. 2013, 32, 253–261. [Google Scholar] [CrossRef]
- Degot, P.; Huber, V.; Hofmann, E.; Hahn, M.; Touraud, D.; Kunz, W. Solubilization and extraction of curcumin from Curcuma Longa using green, sustainable, and food-approved surfactant-free microemulsions. Food Chem. 2021, 336, 127660. [Google Scholar] [CrossRef]
- Li, X.; Ao, M.; Zhang, C.; Fan, S.; Chen, Z.; Yu, L. Zingiberis Rhizoma Recens: A review of its rraditional uses, phytochemistry, pharmacology, and toxicology. Evid.-Based Complement. Alternat. Med. 2021, 2021, 6668990. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Basri, A.M.; Taha, H.; Ahmad, N. A review on the pharmacological activities and phytochemicals of Alpinia officinarum (Galangal) extracts derived from bioassay-guided fractionation and isolation. Pharmacogn. Rev. 2017, 11, 43–56. [Google Scholar] [PubMed] [Green Version]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Ther. 2018, 182, 56–69. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Llano, S.; Gómez, S.; Londoño, J.; Restrepo, A. Antioxidant activity of curcuminoids. Phys. Chem. Chem. Phys. 2019, 21, 3752–3760. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Reddy, A.C.; Lokesh, B.R. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell Biochem. 1992, 111, 117–124. [Google Scholar] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Reddy, A.C.; Lokesh, B.R. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol. Cell Biochem. 1994, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Umar, S.; Ashafaq, M.; Akhtar, M.; Iqbal, Z.; Samim, M.; Ahmad, F.J. A comparative study of PNIPAM nanoparticles of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and their effects on oxidative stress markers in experimental stroke. Protoplasma 2013, 250, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Sreejayan; Rao, M.N.; Devasagayam, T.P.; Singh, B.B. Diminution of singlet oxygen-induced DNA damage by curcumin and related antioxidants. Mutat. Res. 1994, 311, 249–255. [Google Scholar] [CrossRef]
- Park, S.A.; Park, I.H.; Cho, J.S.; Moon, Y.M.; Lee, S.H.; Kim, T.H.; Lee, S.H.; Lee, H.M. Effect of [6]-gingerol on myofibroblast differentiation in transforming growth factor beta 1-induced nasal polyp-derived fibroblasts. Am. J. Rhinol. Allergy 2012, 26, 97–103. [Google Scholar] [CrossRef]
- Huang, H.C.; Chiu, S.H.; Chang, T.M. Inhibitory effect of [6]-gingerol on melanogenesis in B16F10 melanoma cells and a possible mechanism of action. Biosci. Biotechnol. Biochem. 2011, 75, 1067–1072. [Google Scholar] [CrossRef] [Green Version]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.K.; Hu, L.L.; Zhang, Y.X.; Xu, Y.L.; Liu, X.Y.; He, P.K.; Jia, Y.H. 6-Gingerol ameliorates sepsis-induced liver injury through the Nrf2 pathway. Int. Immunopharmacol. 2020, 80, 106196. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Sideras, P.; Apostolou, E.; Stavropoulos, A.; Sountoulidis, A.; Gavriil, A.; Apostolidou, A.; Andreakos, E. Activin, neutrophils, and inflammation: Just coincidence? Semin. Immunopathol. 2013, 35, 481–499. [Google Scholar] [CrossRef]
- Bengmark, S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: A shield against acute and chronic diseases. J. Parenter. Enter. Nutr. 2006, 30, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Meka, B.; Ravada, S.R.; Muthyala, M.K.K.; Kurre, P.N.; Golakoti, T. Synthesis, in vitro and in silico evaluation of diaryl heptanones as potential 5LOX enzyme inhibitors. Bioorg. Chem. 2018, 80, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Hatairaktham, S.; Masaratana, P.; Hantaweepant, C.; Srisawat, C.; Sirivatanauksorn, V.; Siritanaratkul, N.; Panichkul, N.; Kalpravidh, R.W. Curcuminoids supplementation ameliorates iron overload, oxidative stress, hypercoagulability, and inflammation in non-transfusion-dependent β-thalassemia/Hb E patients. Ann. Hematol. 2021, 100, 891–901. [Google Scholar] [CrossRef]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 253–262. [Google Scholar] [CrossRef]
- Hong, W.; Zhi, F.X.; Kun, T.H.; Hua, F.J.; Ling, L.H.; Fang, F.; Wen, C.; Jie, W.; Yang, L.C. 6-Gingerol attenuates ventilator-induced lung injury via anti-inflammation and antioxidative stress by modulating the PPARγ/NF-κBsignalling pathway in rats. Int. Immunopharmacol. 2021, 92, 107367. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, N.; Gao, Y.F.; Sun, L.L.; Zhang, J.G. Therapeutic effects of 6-gingerol, 8-gingerol, and 10-gingerol on dextran sulfate sodium-induced acute ulcerative colitis in rats. Phytother. Res. 2017, 31, 1427–1432. [Google Scholar] [CrossRef]
- Bernard, M.; Furlong, S.J.; Coombs, M.R.P.; Hoskin, D.W. Differential inhibition of T lymphocyte proliferation and cytokine synthesis by [6]-gingerol, [8]-gingerol, and [10]-gingerol. Phytother. Res. 2015, 29, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Babiker, A.Y.; Almogbel, M.A.; Khan, A.A.; Rahmani, A.H. 6-gingerol, a bioactive compound of ginger attenuates renal damage in Streptozotocin-induced diabetic rats by regulating the oxidative stress and inflammation. Pharmaceutics 2021, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.K.; Tsai, Y.H.; Korinek, M.; Hung, P.H.; El-Shazly, M.; Cheng, Y.B.; Wu, Y.C.; Hsieh, T.J.; Chang, F.R. 6-paradol and 6-shogaol, the pungent compounds of ginger, promote glucose utilization in adipocytes and myotubes, and 6-paradol reduces blood glucose in high-fat diet-fed mice. Int. J. Mol. Sci. 2017, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.K.; Ryoo, Z.Y.; Ha, J.J.; Oh, D.Y.; Kim, M.O.; Kim, S.H. Beneficial effects of 6-shogaol on hyperglycemia, islet morphology and apoptosis in some tissues of streptozotocin-induced diabetic mice. Diabetol. Metab. Syndr. 2019, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017, 226, 79–88. [Google Scholar] [CrossRef]
- Samad, M.B.; Mohsin, M.N.A.B.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Lepr(db/db) type 2 diabetic mice. BMC Complement. Altern. Med. 2017, 17, 395. [Google Scholar]
- Li, X.H.; McGrath, K.C.Y.; Tran, V.H.; Li, Y.M.; Duke, C.C.; Roufogalis, B.D.; Heather, A.K. Attenuation of proinflammatory responses by S- [6]-gingerol via inhibition of ROS/NF-Kappa B/COX2 activation in HuH7 cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 146142. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Wu, M.; Lu, Y.; Xian, T.; Wang, Y.; Huang, B.; Zeng, G.; Huang, Q. Protective effects of 6-Gingerol on vascular endothelial cell injury induced by high glucose via activation of PI3K-AKT-eNOS pathway in human umbilical vein endothelial cells. Biomed. Pharmacother. 2017, 93, 788–795. [Google Scholar] [CrossRef]
- Lee, J.O.; Kim, N.; Lee, H.J.; Moon, J.W.; Lee, S.K.; Kim, S.J.; Kim, J.K.; Park, S.H.; Kim, H.S. [6]-gingerol affects glucose metabolism by dual regulation via the AMPKα2-mediated AS160-Rab5 pathway and AMPK-mediated insulin sensitizing effects. J. Cell Biochem. 2015, 116, 1401–1410. [Google Scholar] [CrossRef]
- Ho, S.C.; Chang, Y.H. Comparison of inhibitory capacities of 6-, 8- and 10-gingerols/shogaols on the canonical NLRP3 inflammasome-mediated IL-1β secretion. Molecules 2018, 23, 466. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Khalili, N.; Namazi, S.; Reiner, Z.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complementary Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Mokgalaboni, K.; Ntamo, Y.; Ziqubu, K.; Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Chellan, N.; Tiano, L.; Dludla, P.V. Curcumin supplementation improves biomarkers of oxidative stress and inflammation in conditions of obesity, type 2 diabetes and NAFLD: Updating the status of clinical evidence. Food Funct. 2021, 12, 12235–12249. [Google Scholar] [CrossRef]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef]
- Yao, Q.; Ke, Z.Q.; Guo, S.; Yang, X.S.; Zhang, F.X.; Liu, X.F.; Chen, X.; Chen, H.G.; Ke, H.Y.; Liu, C. Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J. Mol. Cell Cardiol. 2018, 124, 26–34. [Google Scholar] [CrossRef]
- Tu, Q.; Li, Y.; Jin, J.; Jiang, X.; Ren, Y.; He, Q. Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm. Biol. 2019, 57, 778–786. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Curcuminoids plus piperine modulate adipokines in type 2 diabetes mellitus. Curr. Clin. Pharmacol. 2017, 12, 253–258. [Google Scholar] [CrossRef]
- ALTamimi, J.Z.; AlFaris, N.A.; Al-Farga, A.M.; Alshammari, G.M.; BinMowyna, M.N.; Yahya, M.A. Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p (66) Shc axis and activation of FOXO-3a. J. Nutr. Biochem. 2021, 87, 108515. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, M.S.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Gu, Y.; Yan, N.; Li, Y.; Sun, L.; Li, B. Curcumin functions as an anti-inflammatory and antioxidant agent on arsenic-induced hepatic and kidney injury by inhibiting MAPKs/NF-κB and activating Nrf2 pathways. Environ. Toxicol. 2021, 36, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Xiao, Y.; Wang, Y.; Wan, Y.; Li, X.; Li, Q.; Tang, X.; Cai, D.; Ran, B.; et al. Curcumin ameliorates mercuric chloride-induced liver injury via modulating cytochrome P450 signaling and Nrf2/HO-1 pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, L.; Zhang, L.; Ying, Z.; Su, W.; Wang, T. Curcumin attenuates D-galactosamine/lipopolysaccharide-induced liver injury and mitochondrial dysfunction in mice. J. Nutr. 2014, 144, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vipin, A.V.; Raksha, R.K.; Kurrey, N.K.; Anu Appaiah, K.A.; Venkateswaran, G. Protective effects of phenolics rich extract of ginger against Aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed Pharmacother. 2017, 91, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.A.; Khan, A.A.; Anwar, S.A.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-gingerol, a major ingredient of ginger attenuates diethylnitrosamine-induced liver injury in rats through the modulation of oxidative stress and anti-inflammatory activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Vipin, A.V.; Raksha, K.R.; Kurrey, N.K.; Anu, A.K.A.; Venkateswaran, G. Protective effects of phenolics rich extract of ginger against Aflatoxin B (1)-induced oxidative stress and hepatotoxicity. Biomed. Pharmacother. 2017, 91, 415–424. [Google Scholar]
- Guo, X.; Qiu, J.; Qian, Y. 6-shogaol mitigates sepsis-associated hepatic injury through transcriptional regulation. Nutrients 2021, 13, 3427. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.G.; Yang, W.; Xu, P.; Xiao, Y.L.; Zhang, H.T. 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed. Pharmacother. 2018, 107, 1523–1529. [Google Scholar] [CrossRef]
- Kang, C.; Kang, M.; Han, Y.; Zhang, T.; Quan, W.; Gao, J. 6-Gingerols (6G) reduces hypoxia-induced PC-12 cells apoptosis and autophagy through regulation of miR-103/BNIP3. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1653–1661. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.K.; Moon, F.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Liu, B.G.; Mo, X.N.; Zou, M.; Mei, X.P.; Chen, W.; Huang, G.D.; Wu, L. Gingerol ameliorates neuronal damage induced by hypoxia-reoxygenation via the miR-210/brain-derived neurotrophic factor axis. Kaohsiung J. Med. Sci. 2022, 38, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, S.; Zhang, Z.; Gu, Y.; Xia, S.; Bao, X.Y.; Cao, X.; Xu, C. 6-Gingerol attenuates microglia-mediated neuroinflammation and ischemic brain injuries through Akt-mTOR-STAT3 signaling pathway. Eur. J. Pharmacol. 2020, 883, 173294. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Yang, C.; Tan, J.; Zhao, J.; Jiang, N.; Zhao, Y. 6-Gingerol protects against cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome and apoptosis via TRPV1/FAF1 complex dissociation-mediated autophagy. Int. Immunopharmacol. 2021, 100, 108146. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, G.H.; Kim, C.Y.; Jang, J.H. [6]-gingerol attenuates β-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2011, 49, 1261–1269. [Google Scholar] [CrossRef]
- Lapchak, P.A. Neuroprotective and neurotrophic curcuminoids to treat stroke: A translational perspective. Expert Opin. Investig. Drugs 2011, 20, 13–22. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, J.Y.; Han, Y. Curcuminoids in neurodegenerative diseases. Recent Pat. CNS Drug Discov. 2012, 7, 184–204. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, Z.; Qiu, D.; Gu, Q.; Lei, Q.; Mao, L. The inhibitory effects of different curcuminoids on β-amyloid protein, β-amyloid precursor protein and β-site amyloid precursor protein cleaving enzyme 1 in swAPP HEK293 cells. Neurosci. Lett. 2010, 485, 83–88. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, J.S.; Hou, S.X.; Shi, X.X.; Qin, J.; Zhang, T.S.; Wang, X.J. Neuroprotective effects of curcumin alleviate lumbar intervertebral disc degeneration through regulating the expression of iNOS, COX-2, TGF-β1/2, MMP-9 and BDNF in a rat model. Mol. Med. Rep. 2017, 16, 6864–6869. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Sindona, C.; Bramanti, P.; Mazzon, E. A state of the art of antioxidant properties of curcuminoids in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 3168. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Saberi-Karimian, M.; Valizadeh, O.; Behnam, B.; Saadat, A.; Jamialahmadi, T.; Majeed, M.; Sahebkar, A. Effects of curcuminoids on systemic inflammation and quality of life in patients with colorectal cancer undergoing chemotherapy: A randomized controlled trial. Adv. Exp. Med. Biol. 2021, 1328, 1–9. [Google Scholar]
- Mohd Yusof, Y.A. Gingerol and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 177–207. [Google Scholar]
- Konmun, J.; Danwilai, K.; Ngamphaiboon, N.; Sripanidkulchai, B.; Sookprasert, A.; Subongkot, S. A phase II randomized double-blind placebo-controlled study of 6-gingerol as an anti-emetic in solid tumor patients receiving moderately to highly emetogenic chemotherapy. Med. Oncol. 2017, 34, 69. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar]
- Skwarczynski, M.; Bashiri, S.; Yuan, Y.; Ziora, Z.M.; Nabil, O.; Masuda, K.; Khongkow, M.; Rimsueb, N.; Cabral, H.; Ruktanonchai, U.; et al. Antimicrobial activity enhancers: Towards smart delivery of antimicrobial agents. Antibiotics 2022, 11, 412. [Google Scholar] [CrossRef]
- Varaprasad, K.; Yallapu, M.M.; Núñez, D.; Oyarzún, P.; López, M.; Jayaramudud, T.; Karthikeyan, C. Generation of engineered core-shell antibiotic nanoparticles. RSC Adv. 2019, 9, 8326–8332. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, G.; Kaviyil, J.E.; Paul, W.; Sasi, R.; Joseph, R. Gallium–curcumin nanoparticle conjugates as an antibacterial agent against pseudomonas aeruginosa: Synthesis and characterization. ACS Omega 2022, 7, 6795–6809. [Google Scholar] [CrossRef] [PubMed]
- Azari, B.; Moghadam, S.Z.; Zarrinfar, H.; Tasbandi, A.; Jamialahmadi, T.; Sahebkar, A. Antifungal activity of curcuminoids and difluorinated curcumin against clinical isolates of Candida species. Adv. Exp. Med. Biol. 2021, 1328, 123–129. [Google Scholar]
- Zarrinfar, H.; Behnam, M.; Hatamipour, M.; Sahebkar, A. Antifungal activities of curcuminoids and difluorinated curcumin against clinical dermatophyte isolates. Adv. Exp. Med. Biol. 2021, 1308, 101–107. [Google Scholar] [PubMed]
- Nagoshi, C.; Shiota, S.; Kuroda, T.; Hatano, T.; Yoshida, T.; Kariyama, R.; Tsuchiya, T. Synergistic effect of [10]-gingerol and aminoglycosides against vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2006, 29, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskar, A.; Kumari, A.; Singh, M.; Kumar, S.; Kumar, S.; Dabla, A.; Chaturvedi, S.; Yadav, V.; Chattopadhyay, D.; Dwivedi, V.P. [6]-Gingerol exhibits potent anti-mycobacterial and immunomodulatory activity against tuberculosis. Int. Immunopharmacol. 2020, 87, 106809. [Google Scholar] [CrossRef]
- Ham, S.Y.; Kim, H.S.; Jo, M.J.; Lee, J.H.; Byun, Y.; Ko, G.J.; Park, H.D. Combined treatment of 6-gingerol analog and tobramycin for inhibiting pseudomonas aeruginosa infections. Microbiol. Spectr. 2021, 9, e0019221. [Google Scholar] [CrossRef]
- Hayati, R.F.; Better, C.D.; Denis, D.; Komarudin, A.G.; Bowolaksono, A.; Yohan, B.; Sasmono, R.T. [6]-gingerol inhibits chikungunya virus infection by suppressing viral replication. Biomed Res. Int. 2021, 2021, 6623400. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Choi, P.; Ham, J.; Park, J.G.; Lee, J. Antibiofilm and antivirulence activities of 6-gingerol and 6-shogaol against candida albicans due to hyphal inhibition. Front. Cell. Infect. Microbiol. 2018, 8, 299. [Google Scholar] [CrossRef]
- Anderson, M.S.; Bluestone, J.A. The NOD mouse: A model of immune dysregulation. Annu. Rev. Immunol. 2005, 23, 447–485. [Google Scholar] [CrossRef]
- Alolga, R.N.; Nuer-Allornuvor, G.F.; Kuugbee, E.D.; Yin, X.; Ma, G. Ginsenoside Rg1 and the control of inflammation implications for the therapy of type 2 diabetes: A review of scientific findings and call for further research. Pharmacol. Res. 2020, 152, 104630. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Kim, J.W.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014, 157, 1339–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Iturbe, E.; Arbones-Mainar, J.M.; Moreno-Aliaga, M.J.; Lostao, M.P. GLUT12 and adipose tissue: Expression, regulation and its relation with obesity in mice. Acta Physiol. (Oxf.) 2019, 226, 13283. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diab. Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Vanisree, A.J.; Sudha, N. Curcumin combats against cigarette smoke and ethanol-induced lipid alterations in rat lung and liver. Mol. Cell Biochem. 2006, 288, 115–123. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.W.; Lee, G.H.; Choi, M.K.; Jung, H.W.; Kim, Y.J.; Kwon, H.J.; Chae, H.J. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complement. Altern. Med. 2016, 16, 316. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Hou, Y.; Zhou, W.; Keerthiga, R.; Fu, A. Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes. Bioeng. Transl. Med. 2020, 30, 10209. [Google Scholar] [CrossRef]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav. Immun. 2021, 91, 740–755. [Google Scholar] [CrossRef]
- Bader, V.; Winklhofer, K.F. Mitochondria at the interface between neurodegeneration and neuroinflammation. Semin. Cell Dev. Biol. 2020, 99, 163–171. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Munjal, A. Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol. 2020, 119, 199–245. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Jung, M.Y.; Lee, M.K.; Park, H.J.; Oh, E.B.; Shin, J.Y.; Park, J.S.; Jung, S.Y.; Oh, J.H.; Choi, D.S. Heat-induced conversion of gingerols to shogaols in ginger as affected by heat type (dry or moist heat), sample type (fresh or dried), temperature and time. Food Sci. Biotechnol. 2017, 27, 687–693. [Google Scholar] [CrossRef]
- Sang, S.; Hong, J.; Wu, H.; Liu, J.; Yang, C.S.; Pan, M.H.; Badmaev, V.; Ho, C.T. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J. Agric. Food Chem. 2009, 57, 10645–10650. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Scientific report of EFSA—Compendium of botanicals reported tocontain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA J. 2012, 10, 2663. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing curcumin oral bioavailability through nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Behroozeh, A.; Tabrizi, M.M.; Kazemi, S.M.; Choupani, E.; Kabiri, N.; Ilbeigi, D.; Nasab, A.H.; Khiyavi, A.A.; Kurdi, A.S. Evaluation the anti-cancer effect of PEGylated nano-niosomal gingerol, on breast cancer cell lines (T47D), in-vitro. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 645–648. [Google Scholar]
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2018, 122, 214–229. [Google Scholar] [CrossRef]
- Manatunga, D.C.; de Silva, R.M.; de Silva, K.M.N.; de Silva, N.; Bhandari, S.; Yap, Y.K.; Costha, N.P. pH responsive controlled release of anti-cancer hydrophobic drugs from sodium alginate and hydroxyapatite bi-coated iron oxide nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 29–38. [Google Scholar] [CrossRef]
- Zanesco-Fontes, I.; Silva, A.C.L.; da Silva, P.B.; Duarte, J.L.; Di Filippo, L.D.; Chorilli, M.; Cominetti, M.R.; Martin, A.C.B.M. [10]-gingerol-loaded nanoemulsion and its biological effects on triple-negative breast cancer cells. AAPS Pharmscitech 2021, 22, 157. [Google Scholar] [CrossRef]
- Wilson, J.M. Effect of ginger tea on the fetal development of Sprague-Dawley rats. Reprod. Toxicol. 2000, 14, 507–512. [Google Scholar]
- Nag, A.; Paul, S.; Banerjee, R.; Kundu, R. In silico study of some selective phytochemicals against a hypothetical SARS-CoV-2 spike RBD using molecular docking tools. Comput. Biol. Med. 2021, 137, 104818. [Google Scholar] [CrossRef]
- Halder, P.; Pal, U.; Paladhi, P.; Dutta, S.; Paul, P.; Pal, S.; Das, D.; Ganguly, A.; Dutta, I.; Mandal, S.; et al. Evaluation of potency of the selected bioactive molecules from Indian medicinal plants with M(Pro) of SARS-CoV-2 through in silico analysis. J. Ayurveda Integr. Med. 2022, 13, 100449. [Google Scholar] [CrossRef] [PubMed]
- Oso, B.J.; Adeoye, A.O.; Olaoye, I.F. Pharmacoinformatics and hypothetical studies on allicin, curcumin, and gingerol as potential candidates against COVID-19-associated proteases. J. Biomol. Struct. Dyn. 2022, 40, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kumar Verma, A.; Kumar, V.; Singh, S.; Goswami, B.C.; Camps, I.; Sekar, A.; Yoon, S.; Lee, K.W. Repurposing potential of Ayurvedic medicinal plants derived active principles against SARS-CoV-2 associated target proteins revealed by molecular docking, molecular dynamics and MM-PBSA studies. Biomed. Pharmacother. 2021, 137, 111356. [Google Scholar] [CrossRef] [PubMed]
- Hassaniazad, M.; Eftekhar, E.; Inchehsablagh, B.R.; Kamali, H.; Tousi, A.; Jaafari, M.Z.; Rafat, M.; Fathalipour, M.; Nikoofal-Sahlabadi, S.; Gouklani, H.; et al. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother. Res. 2021, 35, 6417–6427. [Google Scholar] [CrossRef]
- Ahmadi, R.; Salari, S.; Sharifi, M.D.; Reihani, H.; Rostamiani, M.B.; Behmadi, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, M.D.; Jaafari, M.R.; et al. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial. Food Sci. Nutr. 2021, 9, 4068–4075. [Google Scholar] [CrossRef]
| Representative Plant | Product | Compounds | Reference |
|---|---|---|---|
| Curcuma longa L. | Turmeric (rhizome) | CUR, DMC, and BMDC | [20] |
| Zingiber officinale Roscoe | Ginger (rhizome) | 6-gingerol and 4-shogaol | [21] |
| Elettaria cardamomum (L.) Maton. | Cardamom (fruit) | 1,8-cineole and catechin | [22] |
| Alpinia galanga (L.) Willd. | Galangal (rhizome) | 3,5,7-trihydroxyflavone (galangin) | [23] |
| Bioactivity | Compounds | Mechanism of Actions | References |
|---|---|---|---|
| Antioxidant | Curcuminoids | Inhibition of lipid peroxidation; ROS scavenging; antioxidant pathway activation | [31,32,33,34,35,36,37,38,42] |
| Gingerols | ROS scavenging; inhibition of NO production; significant reductions in iNOS levels; antioxidant pathway activation | [39,40,41,43] | |
| Anti-inflammatory | Curcuminoids | Inhibition of NO synthetase induction; inhibition of 5-lipoxygenase; NO scavenging | [44,45,46,47,48,49,50] |
| Gingerols | Regulation of oxidative stress; inhibition of the PPARγ/NF-κB signaling pathway; inhibition of T-lymphocyte proliferation and cytokine synthesis | [41,51,52,53] | |
| Antidiabetic | Gingerols | Regulation of oxidative stress and inhibition of inflammation; promotion of glucose utilization; reduction in hyperglycemia; regulation of glucose metabolism and insulin sensitivity | [54,55,56,57,58,59,60,61,62] |
| Curcuminoids | Reduction in insulin resistance and blood lipid levels; alleviation of oxidative stress and inflammation; modulation of innate immune system; modulation of adipokines | [63,64,65,66,67,68,69,70,71,72] | |
| Hepatoprotection | Curcuminoids | Inhibition of inflammation; lowering lipid peroxidation; enhancing the internal antioxidant defense system | [73,74,75,76] |
| Gingerols | Inhibition of inflammation; modulation of oxidative stress; regulation of lipid metabolism | [43,77,78,79,80] | |
| Neuroprotection | Gingerols | Modulation of neuroinflammation; inhibition of NLRP3 inflammasome activation and apoptosis; fortification of the cellular antioxidant defense system | [81,82,83,84,85,86,87] |
| Curcuminoids | Attenuation of oxidative stress and the actions of inflammatory cytokines; prevention of β-amyloid accumulation and/or aggregation and oligomer-dependent Aβ toxicity; attenuation of α-synuclein aggregation | [88,89,90,91,92,93,94] | |
| Anticancer | Curcuminoids Gingerols | Modulation of various signaling pathways related to inflammation and cancer | [95,96,97] [98,99,100,101] |
| Antimicrobial | Curcuminoids | Antibacterial activity; antiviral activity; antifungal activity; enhance the inhibitory effect of existing antimicrobial agents through synergism | [100,101,102,103,104,105] |
| Gingerols | Antibacterial activity; antiviral activity; antifungal activity; synergistic antimicrobial activity | [106,107,108,109,110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alolga, R.N.; Wang, F.; Zhang, X.; Li, J.; Tran, L.-S.P.; Yin, X. Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants 2022, 11, 1281. https://doi.org/10.3390/antiox11071281
Alolga RN, Wang F, Zhang X, Li J, Tran L-SP, Yin X. Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants. 2022; 11(7):1281. https://doi.org/10.3390/antiox11071281
Chicago/Turabian StyleAlolga, Raphael N., Feizuo Wang, Xinyao Zhang, Jia Li, Lam-Son Phan Tran, and Xiaojian Yin. 2022. "Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses" Antioxidants 11, no. 7: 1281. https://doi.org/10.3390/antiox11071281
APA StyleAlolga, R. N., Wang, F., Zhang, X., Li, J., Tran, L. -S. P., & Yin, X. (2022). Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants, 11(7), 1281. https://doi.org/10.3390/antiox11071281









