Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Serum Paraments
2.3. Fatty Acid Profile Analysis of Fish Oil and Serum
2.4. Histological Analyses of the Liver
2.5. Assay of Enzyme Activity in Liver
2.6. Relative mRNA Expression in Liver
2.7. Western Blotting
2.8. Statistical Analyses
3. Results
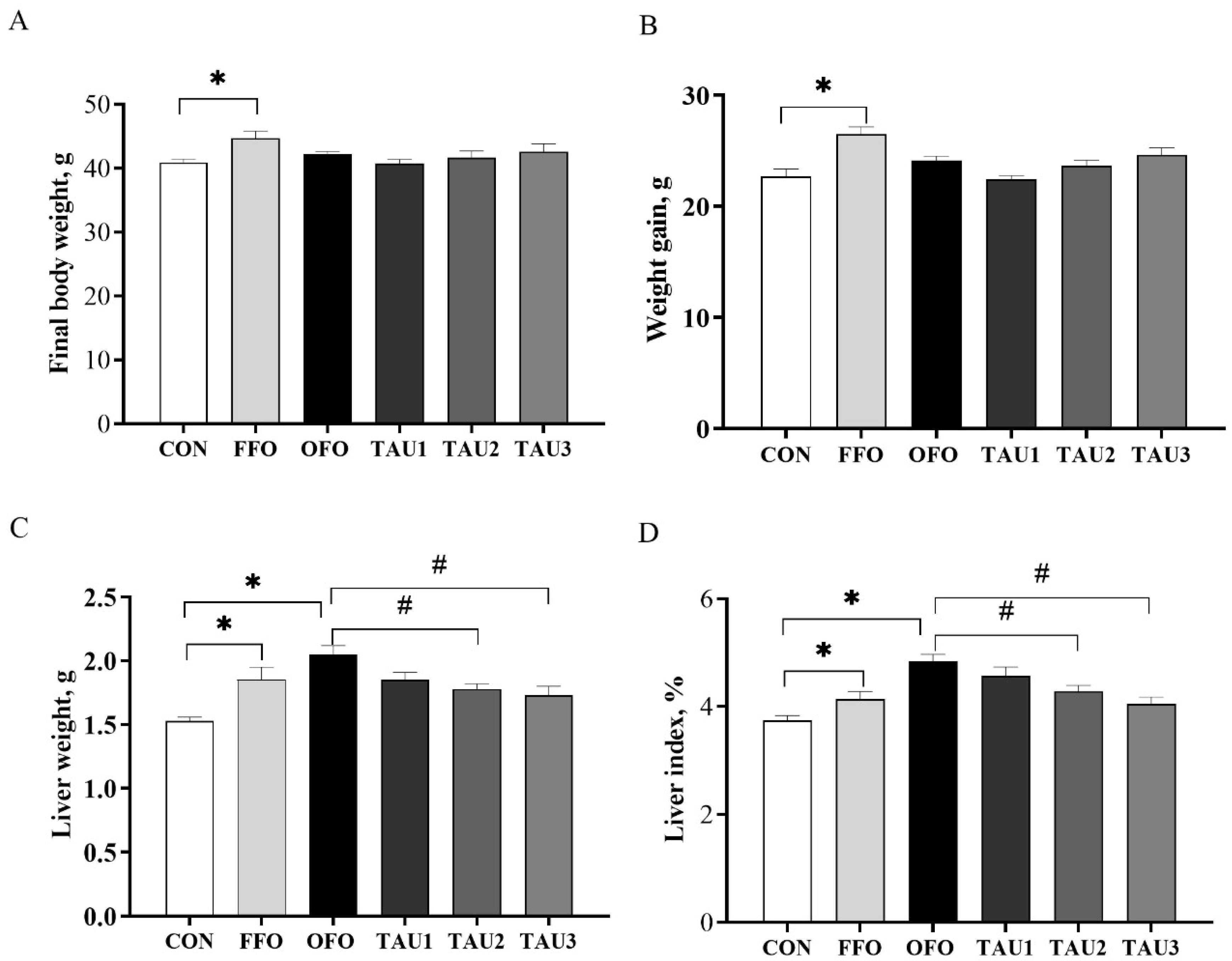
3.1. Taurine Decreased the Liver Index in OFO-Treated Mice
3.2. Taurine Decreased Liver Injury in OFO-Treated Mice
3.3. Taurine Decreased Oxidative Stress and Liver Injury in OFO-Treated Mice
3.4. Taurine Altered Serum Lipid Parameters in OFO-Treated Mice
3.5. Taurine Changed Serum Fatty Acid Profile in OFO-Treated Mice
3.6. Taurine Improved Lipid Metabolism in Liver in OFO-Treated Mice
3.7. Taurine Regulated Autophagy Flux in OFO-Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, W.; Li, S.; Li, J.; Wang, J.; Zhang, R.; Zhou, Y.; Yin, Q.; Zheng, Y.; Wang, F.; Xia, Y.; et al. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1459790. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Fard, S.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of marine omega-3 supplements and human health. Biomed. Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef] [PubMed]
- Gueraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Albert, B.B.; Derraik, J.G.; Cameron-Smith, D.; Hofman, P.L.; Tumanov, S.; Villas-Boas, S.G.; Garg, M.L.; Cutfield, W.S. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci. Rep. 2015, 5, 7928. [Google Scholar] [CrossRef]
- Opperman, M.; Benade, S. Analysis of the omega-3 fatty acid content of South African fish oil supplements: A follow-up study. Cardiovasc. J. Afr. 2013, 24, 297–302. [Google Scholar] [CrossRef]
- Ritter, J.C.; Budge, S.M.; Jovica, F. Quality analysis of commercial fish oil preparations. J. Sci. Food Agric. 2013, 93, 1935–1939. [Google Scholar] [CrossRef]
- Bellanti, F.; Villani, R.; Facciorusso, A.; Vendemiale, G.; Serviddio, G. Lipid oxidation products in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2017, 111, 173–185. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Ruart, M.; Chavarria, L.; Camprecios, G.; Suarez-Herrera, N.; Montironi, C.; Guixe-Muntet, S.; Bosch, J.; Friedman, S.L.; Garcia-Pagan, J.C.; Hernandez-Gea, V. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J. Hepatol. 2019, 70, 458–469. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.J.; Dong, L.; Liu, Y.F.; Xu, N.; Ma, W.; Weng, S.Q.; Janssen, H.L.A.; Wu, S.D. Role of Autophagy in Chronic Liver Inflammation and Fibrosis. Curr. Protein Pept. Sci. 2019, 20, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Li, F.; Zhang, L.; Duan, Y.; Guo, Q.; Wang, W.; He, S.; Li, J.; Yin, Y. Taurine is Involved in Energy Metabolism in Muscles, Adipose Tissue, and the Liver. Mol. Nutr. Food Res. 2019, 63, e1800536. [Google Scholar] [CrossRef]
- Chen, W.; Guo, J.; Zhang, Y.; Zhang, J. The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 2016, 7, 1849–1863. [Google Scholar] [CrossRef]
- Wen, C.; Li, F.; Duan, Y.; Guo, Q.; Wang, W.; Zhang, L.; Li, J.; He, S.; Chen, W.; Yin, Y. Dietary taurine regulates free amino acid profiles and taurine metabolism in piglets with diquat-induced oxidative stress. J. Funct. Foods 2019, 62, 103569. [Google Scholar] [CrossRef]
- Yang, L.; Qiu, T.; Yao, X.; Jiang, L.; Wei, S.; Pei, P.; Wang, Z.; Bai, J.; Liu, X.; Yang, G.; et al. Taurine protects against arsenic trioxide-induced insulin resistance via ROS-Autophagy pathway in skeletal muscle. Int. J. Biochem. Cell Biol. 2019, 112, 50–60. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, J.; Yao, X.; Jiang, L.; Wu, W.; Yang, L.; Gao, N.; Qiu, T.; Yang, G.; Hidru, T.H. Taurine rescues the arsenic-induced injury in the pancreas of rat offsprings and in the INS-1 cells. Biomed. Pharmacother. 2019, 109, 815–822. [Google Scholar] [CrossRef]
- Tsuboyama-Kasaoka, N.; Shozawa, C.; Sano, K.; Kamei, Y.; Kasaoka, S.; Hosokawa, Y.; Ezaki, O. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 2006, 147, 3276–3284. [Google Scholar] [CrossRef]
- Lin, S.; Hirai, S.; Yamaguchi, Y.; Goto, T.; Takahashi, N.; Tani, F.; Mutoh, C.; Sakurai, T.; Murakami, S.; Yu, R. Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol. Nutr. Food Res. 2013, 57, 2155–2165. [Google Scholar] [CrossRef]
- Hortwitz, W. AOAC official method 965.33, Peroxide value of oils and fats. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersberg, MD, USA, 2002. [Google Scholar]
- Li, F.; Duan, Y.; Li, Y.; Tang, Y.; Geng, M.; Oladele, O.A.; Kim, S.W.; Yin, Y. Effects of dietary n-6:n-3 PUFA ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Brit. J. Nutr. 2015, 113, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish oil supplementation and insulin sensitivity: A systematic review and meta-analysis. Lipids Health Dis. 2017, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Khaza’ai, H.; Abed, Y.; Rahmat, A.; Ismail, P.; Ranneh, Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology 2015, 23, 79–89. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Wang, G.; Yang, Y.; Xiong, Z.; Lv, F.; Zhou, W.; Ai, L. Lactic Acid Bacteria with Antioxidant Activities Alleviating Oxidized Oil Induced Hepatic Injury in Mice. Front. Microbiol. 2018, 9, 2684. [Google Scholar] [CrossRef]
- Schuster, S.; Johnson, C.D.; Hennebelle, M.; Holtmann, T.; Taha, A.Y.; Kirpich, I.A.; Eguchi, A.; Ramsden, C.E.; Papouchado, B.G.; McClain, C.J.; et al. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice. J. Lipid Res. 2018, 59, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Ono, A.; Kawasaki, A.; Takenaga, T.; Ito, T. Taurine attenuates the development of hepatic steatosis through the inhibition of oxidative stress in a model of nonalcoholic fatty liver disease in vivo and in vitro. Amino Acids 2018, 50, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, C.; Kerr, B.; Weber, T.; Johnston, L.J.; Shurson, G.C. Influence of thermally oxidized vegetable oils and animal fats on growth performance, liver gene expression, and liver and serum cholesterol and triglycerides in young pigs. J. Anim. Sci. 2014, 92, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Shaw, H.M.; Chao, P.M. Impairment of glucose metabolism in mice induced by dietary oxidized frying oil is different from that induced by conjugated linoleic acid. Nutrition 2008, 24, 744–752. [Google Scholar] [CrossRef]
- Lu, T.; Harper, A.F.; Zhao, J.; Estienne, M.J.; Dalloul, R.A. Supplementing antioxidants to pigs fed diets high in oxidants: I. Effects on growth performance, liver function, and oxidative status. J. Anim. Sci. 2014, 92, 5455–5463. [Google Scholar] [CrossRef]
- Dong, X.; Lei, W.; Zhu, X.; Han, D.; Yang, Y.; Xie, S. Effects of dietary oxidized fish oil on growth performance and skin colour of Chinese longsnout catfish (Leiocassis longirostris Günther). Aquacult. Nutr. 2011, 17, e861–e868. [Google Scholar] [CrossRef]
- Bower, G.; Toma, T.; Harling, L.; Jiao, L.R.; Efthimiou, E.; Darzi, A.; Athanasiou, T.; Ashrafian, H. Bariatric Surgery and Nonalcoholic Fatty Liver Disease: A Systematic Review of Liver Biochemistry and Histology. Obes. Surg. 2015, 25, 2280–2289. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Hour, J.L.; Cheng, H.M. Effect of taurine on toxicity of oxidized fish oil in rats. Food Chem. Toxicol. 2000, 38, 585–591. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, X.; Sun, D.; Li, J.; Wang, Y.; Cao, P.; Liu, Y. Effects of Polar Compounds Generated from the Deep-Frying Process of Palm Oil on Lipid Metabolism and Glucose Tolerance in Kunming Mice. J. Agric. Food Chem. 2017, 65, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Xie, S.; Huo, Y.; Guo, T.; Fang, H.; Zhang, Y.; Liu, Y.; Tian, L.; Niu, J. Effects of dietary oxidized fish oil on growth performance, antioxidant defense system, apoptosis and mitochondrial function of juvenile largemouth bass (Micropterus salmoides). Aquaculture 2019, 500, 347–358. [Google Scholar] [CrossRef]
- Awney, H.A. The effects of Bifidobacteria on the lipid profile and oxidative stress biomarkers of male rats fed thermally oxidized soybean oil. Biomarkers 2011, 16, 445–452. [Google Scholar] [CrossRef]
- Kode, A.; Rajagopalan, R.; Penumathsa, S.V.; Menon, V.P. Influence of a thiazole derivative on ethanol and thermally oxidized sunflower oil-induced oxidative stress. Fundam. Clin. Pharmacol. 2004, 18, 565–571. [Google Scholar] [CrossRef]
- Kang, Y.J.; Choi, M.J. Liver Antioxidant Enzyme Activities Increase After Taurine in Ovariectomized Rats. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 1071–1080. [Google Scholar] [CrossRef]
- Uzunhisarcikli, M.; Aslanturk, A. Hepatoprotective effects of curcumin and taurine against bisphenol A-induced liver injury in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 37242–37253. [Google Scholar] [CrossRef]
- Schuller-Levis, G.B.; Park, E. Taurine and its chloramine: Modulators of immunity. Neurochem. Res. 2004, 29, 117–126. [Google Scholar] [CrossRef]
- Yu, J.; Kim, A.K. Effect of taurine on antioxidant enzyme system in B16F10 melanoma cells. In Taurine 7; Springer: New York, NY, USA, 2009; pp. 491–499. [Google Scholar]
- Ince, S.; Arslan-Acaroz, D.; Demirel, H.H.; Varol, N.; Ozyurek, H.A.; Zemheri, F.; Kucukkurt, I. Taurine alleviates malathion induced lipid peroxidation, oxidative stress, and proinflammatory cytokine gene expressions in rats. Biomed. Pharmacother. 2017, 96, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Hiraishi, A.; Touyama, M.; Sakamoto, K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophs. Res. Commun. 2008, 375, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Keller, U.; Brandsch, C. Effects of a dietary oxidized fat on cholesterol in plasma and lipoproteins and the susceptibility of low-density lipoproteins to lipid peroxidation in guinea pigs fed diets with different concentrations of vitamins E and C. Int. J. Vitam. Nutr. Res. 2004, 74, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Aruna, K.; Rukkumani, R.; Varma, P.S.; Menon, V.P. Therapeutic role of Cuminum cyminum on ethanol and thermally oxidized sunflower oil induced toxicity. Phytother. Res. 2005, 19, 416–421. [Google Scholar] [CrossRef]
- Brandsch, C.; Eder, K. Effects of peroxidation products in thermoxidised dietary oil in female rats during rearing, pregnancy and lactation on their reproductive performance and the antioxidative status of their offspring. Br. J. Nutr. 2004, 92, 267–275. [Google Scholar] [CrossRef]
- Ammouche, A.; Rouaki, F.; Bitam, A.; Bellal, M.M. Effect of ingestion of thermally oxidized sunflower oil on the fatty acid composition and antioxidant enzymes of rat liver and brain in development. Ann. Nutr. Metab. 2002, 46, 268–275. [Google Scholar] [CrossRef]
- Hochgraf, E.; Mokady, S.; Cogan, U. Dietary oxidized linoleic acid modifies lipid composition of rat liver microsomes and increases their fluidity. J. Nutr. 1997, 127, 681–686. [Google Scholar] [CrossRef]
- Shafaeizadeh, S.; Jamalian, J.; Owji, A.A.; Azadbakht, L.; Ramezani, R.; Karbalaei, N.; Rajaeifard, A.; Tabatabai, N. The effect of consuming oxidized oil supplemented with fiber on lipid profiles in rat model. J. Res. Med. Sci. 2011, 16, 1541–1549. [Google Scholar]
- Lim, H.Y.; Thiam, C.H.; Yeo, K.P.; Bisoendial, R.; Hii, C.S.; McGrath, K.C.; Tan, K.W.; Heather, A.; Alexander, J.S.J.; Angeli, V. Lymphatic Vessels Are Essential for the Removal of Cholesterol from Peripheral Tissues by SR-BI-Mediated Transport of HDL. Cell Metab. 2013, 17, 671–684. [Google Scholar] [CrossRef]
- Valentini, K.J.; Pickens, C.A.; Wiesinger, J.A.; Fenton, J.I. The effect of fish oil supplementation on brain DHA and EPA content and fatty acid profile in mice. Int. J. Food Sci. Nutr. 2018, 69, 705–717. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, L.; Duan, Y.; Wang, W.; Huang, R.; Li, F.; Miglior, F. Changes in carcass traits, meat quality, muscle fiber characteristics, and liver function of finishing pigs fed high level of fish oil. Can. J. Anim. Sci. 2021, 101, 342–352. [Google Scholar] [CrossRef]
- Lu, T.; Harper, A.F.; Dibner, J.J.; Scheffler, J.M.; Corl, B.A.; Estienne, M.J.; Zhao, J.; Dalloul, R.A. Supplementing antioxidants to pigs fed diets high in oxidants: II. Effects on carcass characteristics, meat quality, and fatty acid profile. J. Anim. Sci. 2014, 92, 5464–5475. [Google Scholar] [CrossRef] [PubMed]
- Mikami, N.; Hosokawa, M.; Miyashita, K. Dietary combination of fish oil and taurine decreases fat accumulation and ameliorates blood glucose levels in type 2 diabetic/obese KK-A(y) mice. J. Food Sci. 2012, 77, H114–H120. [Google Scholar] [CrossRef] [PubMed]
- Elvevoll, E.O.; Eilertsen, K.E.; Brox, J.; Dragnes, B.T.; Falkenberg, P.; Olsen, J.O.; Kirkhus, B.; Lamglait, A.; Osterud, B. Seafood diets: Hypolipidemic and antiatherogenic effects of taurine and n-3 fatty acids. Atherosclerosis 2008, 200, 396–402. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Lodhi, I.J.; Yin, L.; Malapaka, R.R.; Xu, H.E.; Turk, J.; Semenkovich, C.F. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell 2009, 138, 476–488. [Google Scholar] [CrossRef]
- Veiga, F.M.S.; Graus-Nunes, F.; Rachid, T.L.; Barreto, A.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Anti-obesogenic effects of WY14643 (PPAR-alpha agonist): Hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice. Biochimie 2017, 140, 106–116. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Dietary taurine supplementation decreases fat synthesis by suppressing the liver X receptor alpha pathway and alleviates lipid accumulation in the liver of chronic heat-stressed broilers. J. Sci. Food Agric. 2019, 99, 5631–5637. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in nonalcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Chou, C.H.; Chiu, C.H.; Yang, K.T.; Lin, Y.L.; Weng, W.L.; Chen, Y.C. Preventive effects of taurine on development of hepatic steatosis induced by a high-fat/cholesterol dietary habit. J. Agric. Food Chem. 2011, 59, 450–457. [Google Scholar] [CrossRef]
- Xiao, H.B.; Liang, L.; Luo, Z.-F.; Sun, Z.-L. Paeoniflorin regulates GALNT2-ANGPTL3-LPL pathway to attenuate dyslipidemia in mice. Eur. J. Pharmacol. 2018, 836, 122–128. [Google Scholar] [CrossRef]
- Li, M.; Reynolds, C.M.; Sloboda, D.M.; Gray, C.; Vickers, M.H. Effects of taurine supplementation on hepatic markers of inflammation and lipid metabolism in mothers and offspring in the setting of maternal obesity. PLoS ONE 2013, 8, e76961. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.J.; Feng, K.; He, C.; Li, P.; Hu, Y.J.; Su, H.; Wan, J.B. Dietary alpha-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci. Rep. 2016, 6, 26826. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Buttner, S.; Schmidt, A.; van de Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K.; et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 2011, 17, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.N.; Ables, G.P.; Otlivanchik, O.A.; Schoiswohl, G.; Zechner, R.; Blaner, W.S.; Goldberg, I.J.; Schwabe, R.F.; Chua, S.C., Jr.; Huang, L.S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 2008, 283, 13087–13099. [Google Scholar] [CrossRef] [PubMed]
- Dihingia, A.; Bordoloi, J.; Dutta, P.; Kalita, J.; Manna, P. Hexane-Isopropanolic Extract of Tungrymbai, a North-East Indian fermented soybean food prevents hepatic steatosis via regulating AMPK-mediated SREBP/FAS/ACC/HMGCR and PPARalpha/CPT1A/UCP2 pathways. Sci. Rep. 2018, 8, 10021. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, A.; Mayoral, R.; Agra, N.; Valdecantos, M.P.; Pardo, V.; Miquilena-Colina, M.E.; Vargas-Castrillon, J.; Lo Iacono, O.; Corazzari, M.; Fimia, G.M.; et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014, 5, e1179. [Google Scholar] [CrossRef]
- Bai, J.; Yao, X.; Jiang, L.; Qiu, T.; Liu, S.; Qi, B.; Zheng, Y.; Kong, Y.; Yang, G.; Chen, M.; et al. Taurine protects against As2O3-induced autophagy in pancreas of rat offsprings through Nrf2/Trx pathway. Biochimie 2016, 123, 1–6. [Google Scholar] [CrossRef]
- Copolovici, D.; Bungau, S.; Boscencu, R.; Tit, D.M.; Copolovici, L.J.R.C. The fatty acids composition and antioxidant activity of walnut cold press oil. Rev. Chim. 2017, 68, 507–509. [Google Scholar] [CrossRef]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Csakvari, A.C.; Lupitu, A.; Bungău, S.; Gîtea, M.A.; Gîtea, D.; Tit, D.M.; Copolovici, L.; Nemeth, S.; Copolovici, D. Fatty Acids Profile and Antioxidant Activity of Almond Oils Obtained from Six Romanian Varieties. Farmacia 2019, 67, 882–887. [Google Scholar] [CrossRef]
- Mendez, L.; Medina, I. Polyphenols and Fish Oils for Improving Metabolic Health: A Revision of the Recent Evidence for Their Combined Nutraceutical Effects. Molecules 2021, 26, 2438. [Google Scholar] [CrossRef] [PubMed]




| Items | Fresh Fish Oil | Oxidized Fish Oil |
|---|---|---|
| C14:0 | 9.46 | 10.24 |
| C16:0 | 24.71 | 28.77 |
| C16:1 | 10.88 | 9.40 |
| C17:0 | 1.38 | 1.90 |
| C18:0 | 4.91 | 5.58 |
| C18:1n9t | 0.19 | 0.21 |
| C18:1n9c | 16.90 | 15.34 |
| C18:2n6c | 3.23 | 2.86 |
| C20:0 | 0.95 | 1.02 |
| C18:3n6 | 0.29 | 0.21 |
| C20:1 | 3.10 | 4.63 |
| C18:3n3 | 1.97 | 2.23 |
| C20:3n6 | 0.20 | 0.14 |
| C20:4n6 | 1.44 | 1.19 |
| C22:6n3 | 20.39 | 16.28 |
| Gene | Sequence | Size (bp) | GeneBank No. |
|---|---|---|---|
| SOD2 | TTCTGGACAAACCTGAGCCCTAA GAACCTTGGACTCCCACAGACAC | 134 | NM_013671.3 |
| GPX1 | AGGAGAATGGCAAGAATGAAGAGA GGAAGGTAAAGAGCGGGTGAG | 135 | NM_001329528.1 |
| FAS | TGGTGAATTGTCTCCGAAAAGA CACGTTCATCACGAGGTCATG | 149 | AF127033 |
| PPARα | ATCCCATCACTCTCTCTGTG AACTACCTGCTCAGGACTCA | 161 | NM_011144.6 |
| LPL | CTGCTGGCGTAGCAGGAAGT GCTGGAAAGTGCCTCCATTG | 231 | NM_008509.2 |
| ATGL | ATTTATCCCGGTGTACTGTG GGGACACTGTGATGGTATTC | 119 | XM_021167897.2 |
| HSL | GTGAATGAGATGGCGAGGGT GTGCCCTCACAGCAGGAATA | 101 | NM_010719.5 |
| CPT-1 | AGCACACCAGGCAGTAGCTT AGGATGCCATTCTTGATTCG | 144 | NM_009948 |
| β-actin | TCTTTTCCAGCCTTCCTTCTTG GAGGTCTTTACGGATGTCAACG | 100 | NM_007393 |
| Items | CON | FFO | OFO | TAU1 | TAU2 | TAU3 |
|---|---|---|---|---|---|---|
| Serum | ||||||
| TAG, mmol/L | 1.49 ± 0.07 | 1.05 ± 0.08 * | 1.46 ± 0.10 | 1.23 ± 0.10 | 1.25 ± 0.08 | 1.15 ± 0.07 # |
| CHOL, mmol/L | 3.24 ± 0.10 | 2.76 ± 0.11 * | 4.03 ± 0.26 * | 3.55 ± 0.11 # | 3.75 ± 0.14 | 3.25 ± 0.12 # |
| LDL-C, mmol/L | 0.33 ± 0.01 | 0.23 ± 0.01 * | 0.41 ± 0.02 * | 0.38 ± 0.03 | 0.35 ± 0.03 | 0.27 ± 0.02 # |
| HDL-C, mmol/L | 2.77 ± 0.08 | 2.68 ± 0.15 | 3.00 ± 0.13 | 3.16 ± 0.10 | 3.18 ± 0.18 | 3.16 ± 0.15 |
| CHOL/HDL-C | 1.17 ± 0.01 | 1.03 ± 0.03 | 1.34 ± 0.10 * | 1.12 ± 0.01 # | 1.18 ± 0.02 # | 1.03 ± 0.04 # |
| HDL-C/LDL-C | 8.29 ± 0.43 | 11.70 ± 0.56 * | 7.28 ± 0.35 * | 8.38 ± 0.43 | 9.16 ± 0.46 # | 11.72 ± 0.44 # |
| Liver | ||||||
| TAG, mmol/g protein | 37.73 ± 1.99 | 38.47 ± 1.87 | 48.65 ± 1.80 * | 41.57 ± 3.63 | 41.70 ± 2.80 | 34.95 ± 2.47 |
| Items | CON | FFO | OFO | TAU1 | TAU2 | TAU3 |
|---|---|---|---|---|---|---|
| C14:0 | 0.62 ± 0.01 | 0.52 ± 0.03 | 0.47 ± 0.01 | 0.54 ± 0.01 | 0.51 ± 0.01 | 0.43 ± 0.01 |
| C16:0 | 24.99 ± 0.03 | 25.26 ± 1.20 | 29.47 ± 0.41 * | 27.92 ± 1.18 | 28.09 ± 0.44 | 28.18 ± 0.79 |
| C16:1 | 1.48 ± 0.06 | 1.16 ± 0.17 * | 1.09 ± 0.05 * | 1.00 ± 0.06 | 0.98 ± 0.03 | 0.99 ± 0.05 |
| C17:0 | 0.67 ± 0.01 | 0.74 ± 0.15 | 0.76 ± 0.05 | 0.93 ± 0.06 | 0.86 ± 0.05 | 0.89 ± 0.02 |
| C18:0 | 11.11 ± 0.02 | 7.00 ± 0.61 | 11.45 ± 0.89 | 12.64 ± 0.63 | 12.31 ± 0.42 | 12.75 ± 0.45 |
| C18:1n9c | 8.72 ± 0.10 | 7.91 ± 0.46 | 8.97 ± 0.31 | 8.05 ± 0.16 # | 8.60 ± 0.01 | 9.20 ± 0.26 |
| C18:2n6c | 19.56 ± 0.33 | 13.99 ± 1.33 * | 19.05 ± 0.51 | 17.58 ± 0.63 | 17.06 ± 0.03 # | 16.05 ± 0.41 # |
| C18:3n3 | 0.43 ± 0.03 | 0.67 ± 0.00 * | 0.48 ± 0.00 | 0.30 ± 0.15 | 0.41 ± 0.00 | 0.41 ± 0.01 |
| C20:3n6 | 1.56 ± 0.07 | 2.34 ± 0.11 * | 1.76 ± 0.08 | 2.01 ± 0.21 | 2.04 ± 0.04 | 2.30 ± 0.06 # |
| C20:4n6 | 6.87 ± 0.10 | 4.66 ± 0.35 * | 6.16 ± 0.40 | 4.86 ± 0.61 | 5.04 ± 0.29 | 4.40 ± 0.25 |
| C24:0 | 8.33 ± 0.27 | 8.98 ± 0.36 | 5.46 ± 0.31 * | 5.18 ± 0.41 | 4.75 ± 0.03 | 4.19 ± 0.33 # |
| C22:6n3 | 15.65 ± 0.18 | 26.11 ± 0.99 * | 16.50 ± 0.23 | 18.98 ± 0.39 # | 19.33 ± 0.14 # | 20.17 ± 0.17 # |
| SFA 2 | 45.73 ± 0.34 | 42.48 ± 0.77 * | 47.62 ± 1.03 | 47.20 ± 0.66 | 46.53 ± 0.07 | 46.46 ± 0.53 |
| UFA 3 | 54.27 ± 0.34 | 56.61 ± 0.35 * | 53.00 ± 0.96 | 52.77 ± 0.63 | 53.47 ± 0.07 | 53.51 ± 0.51 |
| PUFA 4 | 44.07 ± 0.29 | 47.54 ± 0.59 * | 42.95 ± 0.60 | 43.73 ± 0.67 | 43.89 ± 0.09 | 43.32 ± 0.71 |
| n-3 5 | 17.64 ± 0.14 | 28.89 ± 1.21 * | 21.24 ± 0.30 | 21.29 ± 0.40 | 21.79 ± 0.17 | 22.87 ± 0.15 |
| n-6 6 | 27.98 ± 0.50 | 20.99 ± 0.89 * | 25.97 ± 0.83 | 24.45 ± 0.34 # | 24.14 ± 0.23 # | 22.74 ± 0.53 # |
| SFA/UFA | 0.84 ± 0.01 | 0.75 ± 0.02 * | 0.90 ± 0.03 | 0.89 ± 0.02 | 0.87 ± 0.00 | 0.87 ± 0.02 |
| n-6/n-3 | 1.59 ± 0.04 | 0.73 ± 0.06 * | 1.27 ± 0.06 * | 1.15 ± 0.01 # | 1.11 ± 0.02 # | 1.00 ± 0.02 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Zhang, L.; Yin, Y.; Gong, S.; Yang, Y.; Chen, S.; Han, M.; Duan, Y. Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice. Antioxidants 2022, 11, 1391. https://doi.org/10.3390/antiox11071391
Guo Q, Zhang L, Yin Y, Gong S, Yang Y, Chen S, Han M, Duan Y. Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice. Antioxidants. 2022; 11(7):1391. https://doi.org/10.3390/antiox11071391
Chicago/Turabian StyleGuo, Qiuping, Lingyu Zhang, Yunju Yin, Saiming Gong, Yuhuan Yang, Sisi Chen, Mengmeng Han, and Yehui Duan. 2022. "Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice" Antioxidants 11, no. 7: 1391. https://doi.org/10.3390/antiox11071391
APA StyleGuo, Q., Zhang, L., Yin, Y., Gong, S., Yang, Y., Chen, S., Han, M., & Duan, Y. (2022). Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice. Antioxidants, 11(7), 1391. https://doi.org/10.3390/antiox11071391






