Butein Ameliorates Oxidative Stress in H9c2 Cardiomyoblasts through Activation of the NRF2 Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Kits
2.2. Cell Culture and Treatment with Butein and H2O2
2.3. Cell Viability Assay
2.4. Hoechst 33342 Staining
2.5. Analysis of Intracellular ROS Production
2.6. MitoSOX Red Staining
2.7. Immunostaining of Nrf2 Protein
2.8. Western Blot Analysis
2.9. Nuclear Fractionation of H9c2 Cardiomyocytes
2.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.11. Statistical Analysis
3. Results
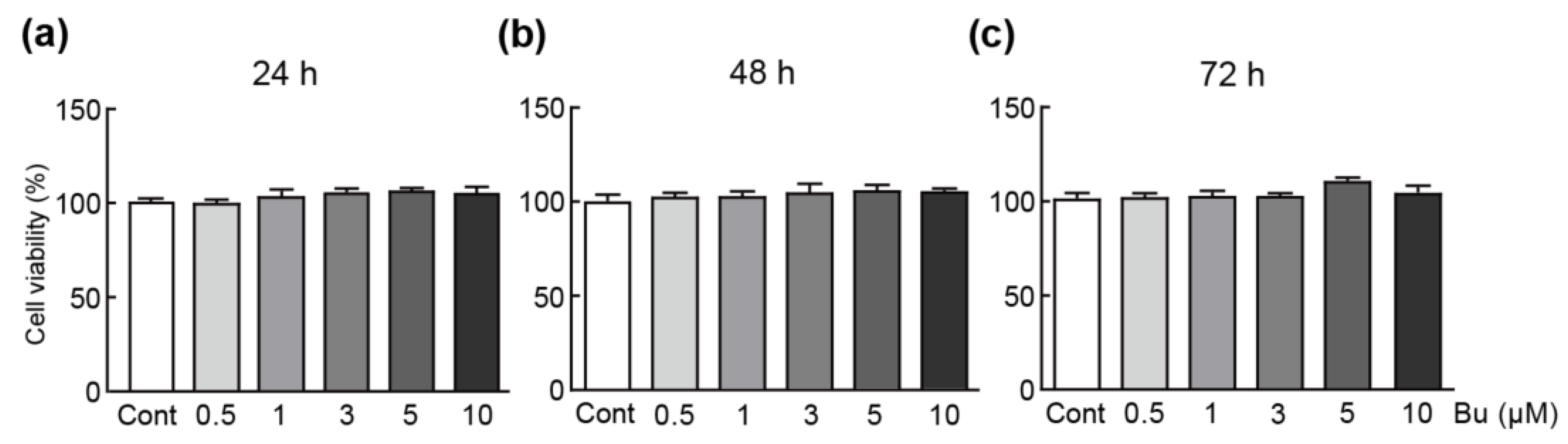
3.1. Butein Has No Cytotoxicity on H9c2 Cardiomyoblasts
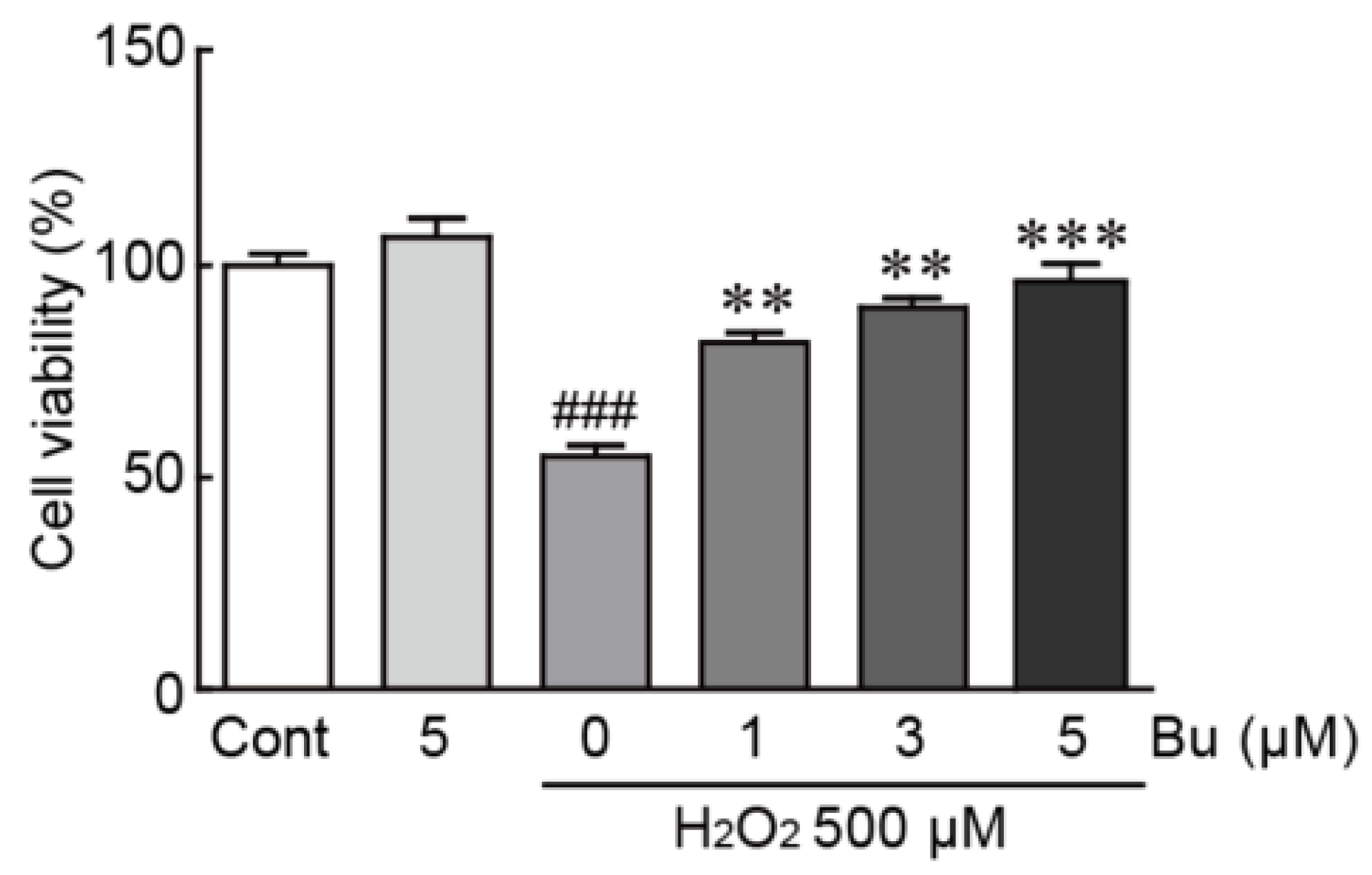
3.2. Butein Protects H9c2 Cardiomyoblasts from H2O2-Induced Oxidative Stress
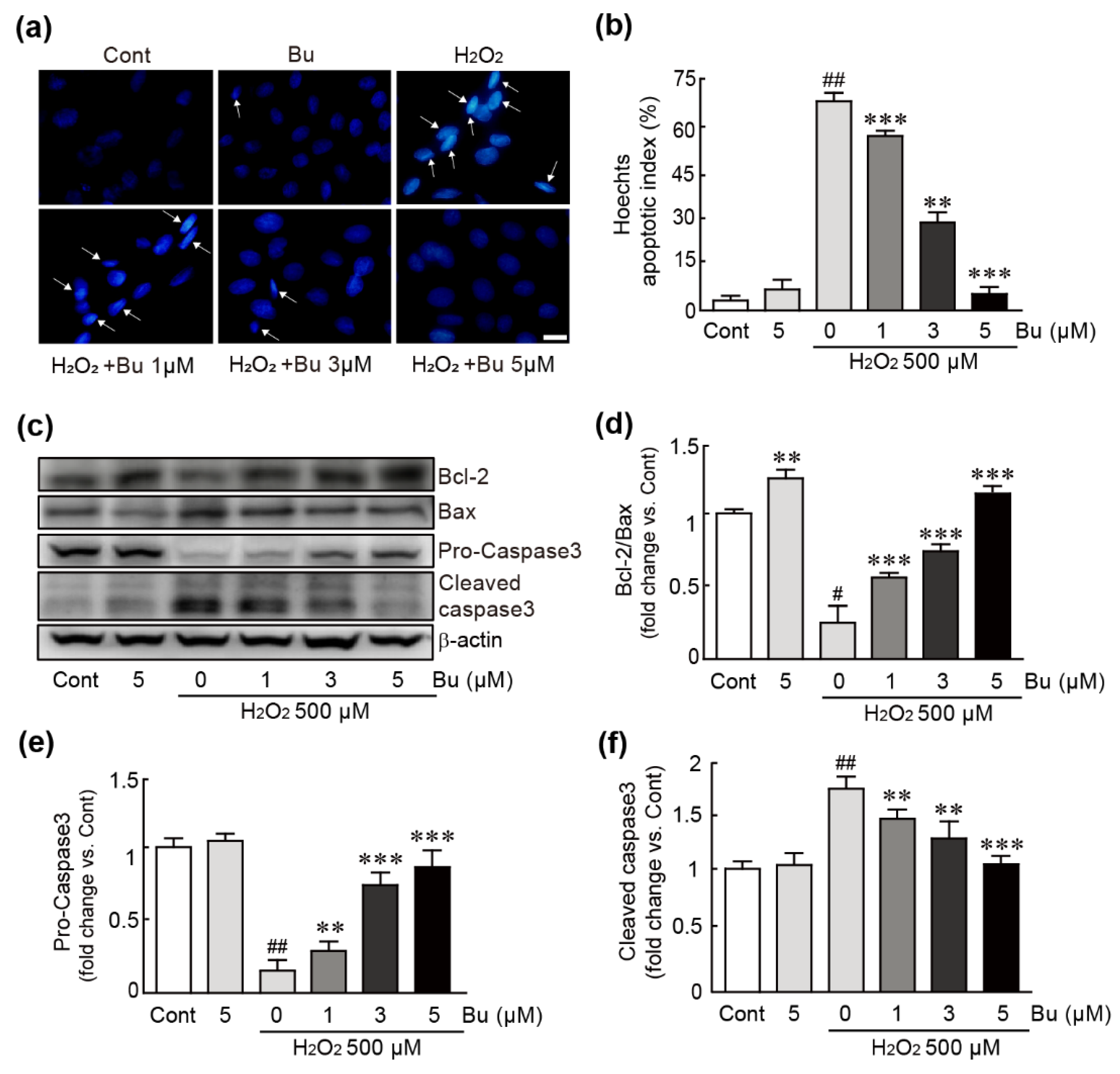
3.3. Butein Ameliorates Oxidative-Stress-Induced Apoptotic Cell Death in H9c2 Cardiomyoblasts
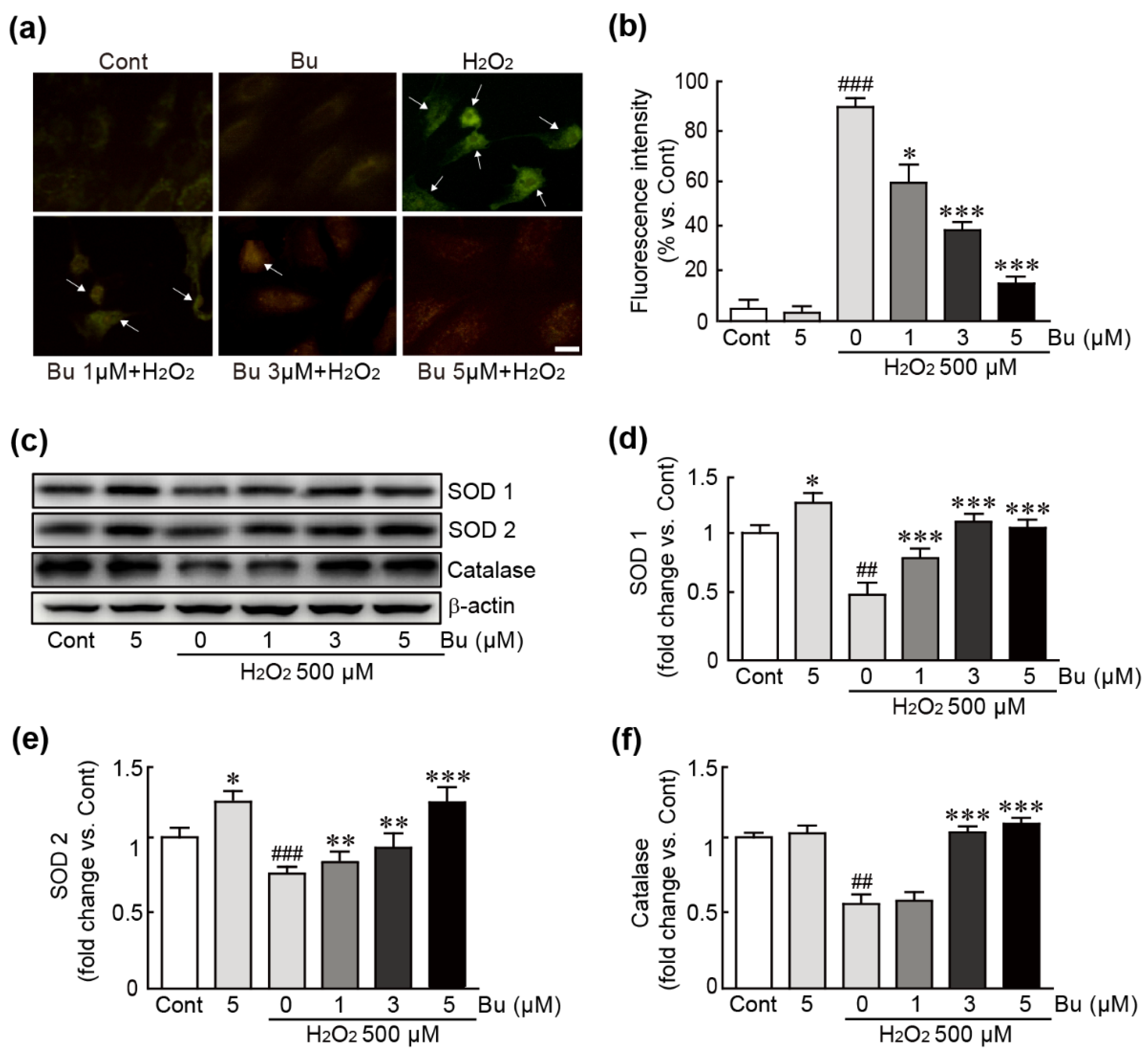
3.4. Butein Attenuates Oxidative Stress in H9c2 Cardiomyoblasts
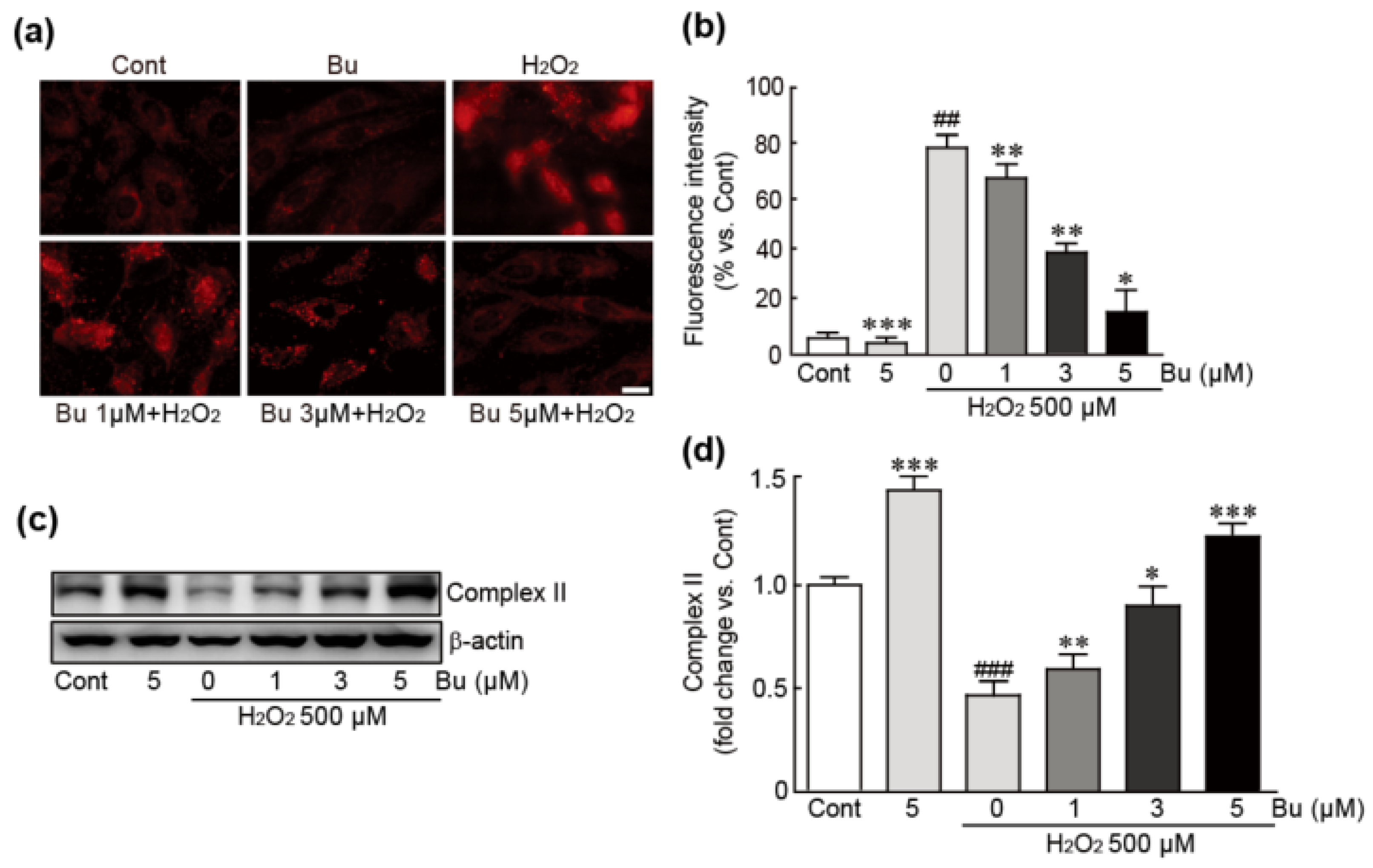
3.5. Butein Attenuates Oxidative-Stress-Induced Mitochondrial Superoxide Production and Dysfunction in H9c2 Cardiomyoblasts
3.6. Butein Inhibits Oxidative-Stress-Induced ER Stress in H9c2 Cardiomyoblasts
3.7. Butein Activates the Nrf2-Involved Signaling Pathway in H9c2 Cardiomyoblasts under Oxidative Stress Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gas-trointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Hare, J.M.; Stamler, J.S. NO/redox disequilibrium in the failing heart and cardiovascular system. J. Clin. Investig. 2005, 115, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, S.; Venditti, P.; De Leo, T. Tissue protection against oxidative stress. Experientia 1996, 52, 786–794. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef] [Green Version]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, G.S.; Sohn, D.H. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by butein in RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2004, 323, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int. Immunopharmacol 2011, 11, 295–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.-J.; Kuo, S.-C.; Chan, S.-C.; Ko, F.-N.; Teng, C.-M. Antioxidant properties of butein isolated from Dalbergia odorifera. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1998, 1392, 291–299. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, W.-C.; Kim, K.-S.; Park, J.-W.; Lee, S.H.; Yoon, S.-W. Shrinkage of Gastric Cancer in an elderly patient who received Rhus verniciflua Stokes extract. J. Altern. Complement. Med. 2010, 16, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, S.; Nihro, Y.; Ueda, H.; Miki, T.; Matsumoto, H.; Satoh, T. Protective effects of hydroxychalcones on free radi-cal-induced cell damage. Biol. Pharm. Bull. 1994, 17, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szuster-Ciesielska, A.; Mizerska-Dudka, M.; Daniluk, J.; Kandefer-Szerszen, M. Butein inhibits ethanol-induced activation of liver stellate cells through TGF-beta, NFkappaB, p38, and JNK signaling pathways and inhibition of oxidative stress. J. Gas-troenterol. 2013, 48, 222–237. [Google Scholar]
- Yang, D.K.; Kim, S.-J. Cucurbitacin I Protects H9c2 Cardiomyoblasts against H2O2-Induced Oxidative Stress via Protection of Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2018, 2018, 3016382. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Bethesda: Rockville, MD, USA, 2004. [Google Scholar]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, 778–781. [Google Scholar] [CrossRef]
- Yun, U.J.; Yang, D.K. Sinapic Acid Inhibits Cardiac Hypertrophy via Activation of Mitochondrial Sirt3/SOD2 Signaling in Neonatal Rat Cardiomyocytes. Antioxidants 2020, 9, 1163. [Google Scholar] [CrossRef]
- Cheung, K.G.; Cole, L.K.; Xiang, B.; Chen, K.; Ma, X.; Myal, Y.; Hatch, G.M.; Tong, Q.; Dolinsky, V.W. Sirtuin-3 (SIRT3) Protein Attenuates Doxorubicin-induced Oxidative Stress and Improves Mitochondrial Respiration in H9c2 Cardiomyocytes. J. Biol. Chem. 2015, 290, 10981–10993. [Google Scholar] [CrossRef] [Green Version]
- Ransy, C.; Vaz, C.; Lombes, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell. Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharm. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Buraparuangsang, P.; Worachartcheewan, A.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules 2008, 13, 904–921. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.-G.; Woo, S.-M.; Ko, S.-G. Butein suppresses breast cancer growth by reducing a production of intracellular reactive oxygen species. J. Exp. Clin. Cancer Res. 2014, 33, 51. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.C.; Chen, C.; Chung, C.H.; Wang, P.C.; Wu, N.L.; Cheng, J.K.; Lai, Y.W.; Sun, H.L.; Peng, C.Y.; Tang, C.H.; et al. Inhibitory effects of butein on cancer metastasis and bioenergetic modulation. J. Agric. Food Chem. 2014, 62, 9109–9117. [Google Scholar] [CrossRef]
- Hayashi, K.; Nagamatsu, T.; Honda, S.; Suzuki, Y. Butein (3,4,2’,4’-tetrahydroxychalcone) ameliorates experimental anti-glomerular basement membrane antibody-associated glomerulonephritis (3). Eur. J. Pharm. 1996, 316, 297–306. [Google Scholar] [CrossRef]
- Song, N.-J.; Chang, S.-H.; Kim, S.; Panic, V.; Jang, B.-H.; Yun, U.J.; Choi, J.H.; Li, Z.; Park, K.-M.; Yoon, J.-H.; et al. PI3Ka-Akt1-mediated Prdm4 induction in adipose tissue increases energy expenditure, inhibits weight gain, and improves insulin resistance in diet-induced obese mice. Cell Death Dis. 2018, 9, 876. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, D.S.; Rajeswari, V.D. PPAR-Gamma as putative gene target involved in Butein mediated anti-diabetic effect. Mol. Biol. Rep. 2020, 47, 5273–5283. [Google Scholar] [CrossRef]
- Kang, D.G.; Kim, Y.C.; Sohn, E.J.; Lee, Y.M.; Lee, A.S.; Yin, M.H.; Lee, H.S. Hypotensive effect of butein via the inhibition of angiotensin converting enzyme. Biol. Pharm. Bull. 2003, 26, 1345–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Pan, Q. Butein Inhibits Oxidative Stress Injury in Rats with Chronic Heart Failure via ERK/Nrf2 Signaling. Cardiovasc. Ther. 2022, 2022, 8684014. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Ward, J.F.; Evans, J.W.; Limoli, C.L.; Calabro-Jones, P.M. Radiation and hydrogen peroxide induced free radical damage to DNA. Br. J. Cancer. Suppl. 1987, 8, 105–112. [Google Scholar] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Bayeva, M.; Gheorghiade, M.; Ardehali, H. Mitochondria as a therapeutic target in heart failure. J. Am. Coll. Cardiol. 2013, 61, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Binder, P.; Fang, Q.; Wang, Z.; Xiao, W.; Liu, W.; Wang, X. Endoplasmic reticulum stress in the heart: Insights into mechanisms and drug targets. Br. J. Pharmacol. 2018, 175, 1293–1304. [Google Scholar] [CrossRef] [Green Version]
- Ellgaard, L.; Helenius, A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003, 4, 181–191. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, F.V.; Jiménez-González, S.; Delgado-Valero, B.; Jurado-López, R.; Genty, M.; Romero-Miranda, A.; Rodríguez, C.; Nieto, M.L.; Martínez-Martínez, E.; Cachofeiro, V. The Interplay of Mitochondrial Oxidative Stress and Endoplasmic Reticulum Stress in Cardiovascular Fibrosis in Obese Rats. Antioxidants 2021, 10, 1274. [Google Scholar] [CrossRef]
- Ruan, Y.; Zeng, J.; Jin, Q.; Chu, M.; Ji, K.; Wang, Z.; Li, L. Endoplasmic reticulum stress serves an important role in cardiac ischemia/reperfusion injury (Review). Exp. Ther. Med. 2020, 20, 268. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for cardiac protection: Pharmacological options against oxidative stress. Trends Pharmacol. Sci. 2021, 42, 729–744. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, W.; Zhang, Z.; Zheng, Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid. Med. Cell. Longev. 2014, 2014, 260429. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ichikawa, T.; Villacorta, L.; Janicki, J.S.; Brower, G.L.; Yamamoto, M.; Cui, T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arter. Thromb. Vasc. Biol. 2009, 29, 1843–1850. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungalag, T.; Park, K.W.; Yang, D.K. Butein Ameliorates Oxidative Stress in H9c2 Cardiomyoblasts through Activation of the NRF2 Signaling Pathway. Antioxidants 2022, 11, 1430. https://doi.org/10.3390/antiox11081430
Tungalag T, Park KW, Yang DK. Butein Ameliorates Oxidative Stress in H9c2 Cardiomyoblasts through Activation of the NRF2 Signaling Pathway. Antioxidants. 2022; 11(8):1430. https://doi.org/10.3390/antiox11081430
Chicago/Turabian StyleTungalag, Tsendsuren, Kye Won Park, and Dong Kwon Yang. 2022. "Butein Ameliorates Oxidative Stress in H9c2 Cardiomyoblasts through Activation of the NRF2 Signaling Pathway" Antioxidants 11, no. 8: 1430. https://doi.org/10.3390/antiox11081430





