Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Collection and Extraction
2.3. Compositional Analysis of Crude Extract
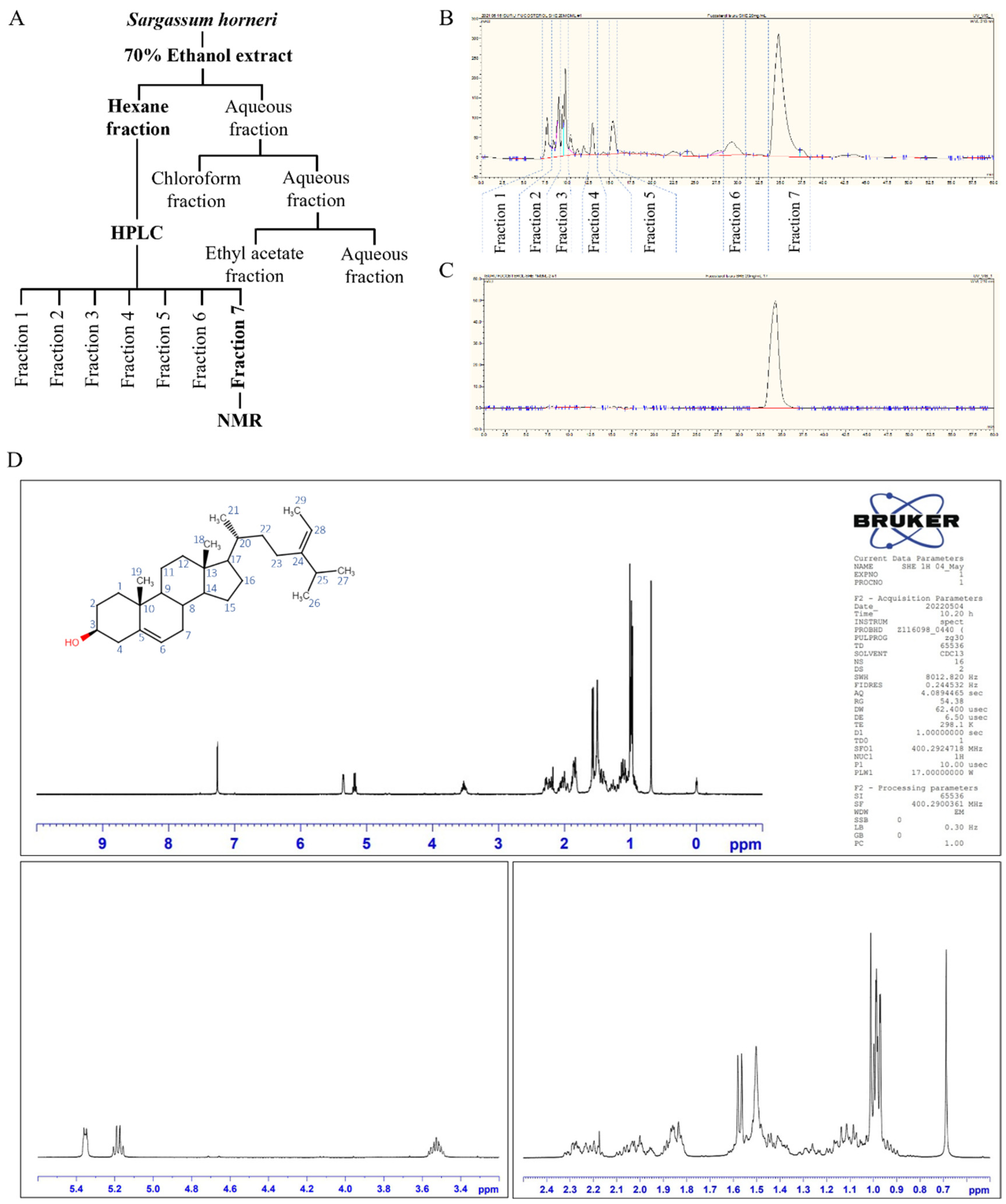
2.4. High-Performance Liquid Chromatography (HPLC) Analysis and Structural Identification of Compounds
2.5. Cell Culture
2.6. Cell Viability and ROS Production Analysis
2.7. Western Blot Analysis
2.8. Immunofluorescence Analysis
2.9. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.10. Statistical Analysis
3. Results
3.1. Extraction of S. horneri, Isolation of Fucosterol by HPLC, and Structural Elucidation
3.2. Effect of Fucosterol on Cell Viability and Intracellular ROS Production
3.3. Fucosterol Regulated the Nrf2/HO-1 Signaling
3.4. Fucosterol Downregulated Inflammatory Mediators, MMP and Tissue Inhibitors of Metalloproteinases (TIMP)
3.5. Fucosterol Regulated the NF-κB and MAPK Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernando, I.S.; Jayawardena, T.U.; Sanjeewa, K.A.; Wang, L.; Jeon, Y.-J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-S.; Xiang, X.-W.; Jin, H.-X.; Guo, X.-Y.; Liu, L.-J.; Huang, Y.-N.; OuYang, X.-K.; Qu, Y.-L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264. 7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, A.I.; Hassan, A.A.; Larsen, S.J.; Gomez-Rangel, V.; Elbatreek, M.; Kleikers, P.W.; Guney, E.; Egea, J.; López, M.G.; Baumbach, J. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 7129–7136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasaki, M.; Kawagoe, C.; Ito, A.; Kumon, H.; Narayan, B.; Hosokawa, M.; Miyashita, K. Spatial and seasonal variations in the biofunctional lipid substances (fucoxanthin and fucosterol) of the laboratory-grown edible Japanese seaweed (Sargassum horneri Turner) cultured in the open sea. Saudi J. Biol. Sci. 2017, 24, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Kalsait, R.P.; Khedekar, P.B.; Saoji, A.N.; Bhusari, K.P. Isolation of phytosterols and antihyperlipidemic activity of Lagenaria siceraria. Arch. Pharmacal Res. 2011, 34, 1599–1604. [Google Scholar] [CrossRef]
- Juárez-Portilla, C.; Olivares-Bañuelos, T.; Molina-Jiménez, T.; Sánchez-Salcedo, J.A.; Del Moral, D.I.; Meza-Menchaca, T.; Flores-Muñoz, M.; López-Franco, Ó.; Roldán-Roldán, G.; Ortega, A. Seaweeds-derived compounds modulating effects on signal transduction pathways: A systematic review. Phytomedicine 2019, 63, 153016. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.; Pagès, A.K.; Seca, A.M.; Pinto, D.C. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in vivo studies and clinical trials. Mar. Drugs 2019, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Mo, W.; Wang, C.; Li, J.; Chen, K.; Xia, Y.; Li, S.; Xu, L.; Lu, X.; Wang, W.; Guo, C. Fucosterol protects against concanavalin A-induced acute liver injury: Focus on P38 MAPK/NF-κB pathway activity. Gastroenterol. Res. Pract. 2018, 2018, 2824139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, I.S.; Jayawardena, T.U.; Kim, H.-S.; Lee, W.W.; Vaas, A.; De Silva, H.; Abayaweera, G.; Nanayakkara, C.; Abeytunga, D.; Lee, D.-S. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Jayawardena, T.U.; Kim, H.-S.; Vaas, A.; De Silva, H.; Nanayakkara, C.; Abeytunga, D.; Lee, W.; Ahn, G.; Lee, D.-S. A keratinocyte and integrated fibroblast culture model for studying particulate matter-induced skin lesions and therapeutic intervention of fucosterol. Life Sci. 2019, 233, 116714. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Lee, H.-G.; Nagahawatta, D.P.; Yang, H.-W.; Kang, M.-C.; Jeon, Y.-J. Particulate Matter-Induced Inflammation/Oxidative Stress in Macrophages: Fucosterol from Padina boryana as a Potent Protector, Activated via NF-κB/MAPK Pathways and Nrf2/HO-1 Involvement. Mar. Drugs 2020, 18, 628. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Han, E.J.; Oh, G.-W.; Jung, W.-K.; Ahn, G. Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Han, E.-J.; Fernando, I.P.S.; Kim, H.-S.; Lee, D.-S.; Kim, A.; Je, J.-G.; Seo, M.-J.; Jee, Y.-H.; Jeon, Y.-J.; Kim, S.-Y.; et al. (–)-Loliolide Isolated from Sargassum horneri Suppressed Oxidative Stress and Inflammation by Activating Nrf2/HO-1 Signaling in IFN-γ/TNF-α-Stimulated HaCaT Keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.M.; Song, H.Y.; Lee, S.J.; Seo, W.Y.; Sin, D.H.; Goh, A.R.; Kang, Y.-H.; Kang, I.-J.; Won, M.-H.; Yi, J.-S. Suppression of thymus-and activation-regulated chemokine (TARC/CCL17) production by 1, 2, 3, 4, 6-penta-O-galloyl-β-d-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 2009, 387, 115–120. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264. 7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S. Upregulation of heme oxygenase-1 expression by dehydrodiconiferyl alcohol (DHCA) through the AMPK–Nrf2 dependent pathway. Toxicol. Appl. Pharmacol. 2014, 281, 87–100. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Kim, H.-S.; Han, E.-J.; Kim, M.-J.; Seo, M.-J.; Ahn, G. Effects of (–)-Loliolide against Fine Dust Preconditioned Keratinocyte Media-Induced Dermal Fibroblast Inflammation. Antioxidants 2021, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Priyan Shanura Fernando, I.; Kim, K.-N.; Kim, D.; Jeon, Y.-J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Kirindage, K.G.I.S.; Fernando, I.P.S.; Jayasinghe, A.M.K.; Han, E.-J.; Dias, M.K.H.M.; Kang, K.-P.; Moon, S.-I.; Shin, T.-S.; Ma, A.; Ahn, G. Moringa oleifera Hot Water Extract Protects Vero Cells from Hydrogen Peroxide-Induced Oxidative Stress by Regulating Mitochondria-Mediated Apoptotic Pathway and Nrf2/HO-1 Signaling. Foods 2022, 11, 420. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Dasu, M.R.; Barrow, R.E.; Spies, M.; Herndon, D.N. Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns 2003, 29, 527–531. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.S.; Lee, W.W.; Sanjeewa, K.A.; Kim, H.-S.; Lee, D.-S.; Jeon, Y.-J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, T.; Muthulakshmi, V.; Sachdanandam, P. Salubrious effect of Semecarpus anacardium against lipid peroxidative changes in adjuvant arthritis studied in rats. Mol. Cell Biochem. 1997, 175, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, Y.-W.; Liu, Q.; Liu, L.-P.; Luo, F.-L.; Zhou, H.-C.; Isoda, H.; Ohkohchi, N.; Li, Y.-M. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. React. Oxyg. Species (ROS) Living Cells 2018, 8, 69–87. [Google Scholar]
- Sanjeewa, K.A.; Lee, W.; Jeon, Y.-J. Nutrients and bioactive potentials of edible green and red seaweed in Korea. Fish. Aquat. Sci. 2018, 21, 19. [Google Scholar] [CrossRef]
- Xia, M.; Liu, C.; Gao, L.; Lu, Y. One-step preparative separation of phytosterols from edible brown seaweed Sargassum horneri by high-speed countercurrent chromatography. Mar. Drugs 2019, 17, 691. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.; Lee, H.; Kim, N.; Jo, H.G.; Woo, E.-R.; Lee, K.; Han, Y.S.; Park, S.R.; Ahn, G.; Cheong, S.H. The anti-oxidative and anti-neuroinflammatory effects of sargassum horneri by heme oxygenase-1 induction in BV2 and HT22 cells. Antioxidants 2021, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.-S.; Shin, J.-S.; Choi, H.-E.; Cho, Y.-W.; Bang, M.-H.; Baek, N.-I.; Lee, K.-T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-κB and p38 mitogen-activated protein kinase in RAW264. 7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.M.; Lee, S.; Hong, S.; Lee, S.; Jung, K.; Kang, K.S. Protective Effect of Polymethoxyflavones Isolated from Kaempferia parviflora against TNF-α-Induced Human Dermal Fibroblast Damage. Antioxidants 2021, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Yoon, C.-S.; Kim, K.-W.; Lee, H.; Kim, N.; Woo, E.-R.; Kim, Y.-C.; Kang, D.G.; Lee, H.S.; Oh, H. Neuroprotective and anti-inflammatory effects of Kuwanon C from Cudrania tricuspidata are mediated by heme oxygenase-1 in HT22 hippocampal cells, RAW264. 7 macrophage, and BV2 microglia. Int. J. Mol. Sci. 2020, 21, 4839. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Russo, B.; Brembilla, N.C.; Chizzolini, C. Interplay between keratinocytes and fibroblasts: A systematic review providing a new angle for understanding skin fibrotic disorders. Front. Immunol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef] [PubMed]
- Youn, U.J.; Nam, K.-W.; Kim, H.-S.; Choi, G.; Jeong, W.S.; Lee, M.Y.; Chae, S. 3-Deoxysappanchalcone inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 expression in human keratinocytes through activated protein-1 inhibition and nuclear factor-kappa B DNA binding activity. Biol. Pharm. Bull. 2011, 34, 890–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.H.; Joo, Y.H.; Karadeniz, F.; Ko, J.; Kong, C.-S. Syringaresinol inhibits UVA-induced MMP-1 expression by suppression of MAPK/AP-1 signaling in HaCaT keratinocytes and human dermal fibroblasts. Int. J. Mol. Sci. 2020, 21, 3981. [Google Scholar] [CrossRef]




| SHE | Composition % |
|---|---|
| Yield | 8.12 ± 0.26 |
| Carbohydrates | 3.92 ± 0.19 |
| Protein | 1.09 ± 0.07 |
| Total polyphenols | 14.82 ± 0.68 |
| No. | 1H-NMR Value |
|---|---|
| 1 | 1.81 (1H, m), 1.10 (1H, m) |
| 2 | 1.52 (1H, m), 1.38 (1H, m) |
| 3 | 3.51 (1H, m) |
| 4 | 2.17 (1H, m), 2.07 (1H, m) |
| 6 | 5.34 (1H, d) |
| 7 | 1.89 (1H, m), 1.60 (1H, m) |
| 8 | 1.43 (1H, m) |
| 9 | 0.91 (1H, m) |
| 11 | 1.50 (1H, m), 1.43 (1H, m) |
| 12 | 1.99 (1H, m, 1.16 (1H, m) |
| 14 | 1.01 (1H, m) |
| 15 | 1.58 (1H, m), 1.07 (1H, m) |
| 16 | 1.82 (1H, m), 1.26 (1H, m) |
| 17 | 1.16 (1H, m) |
| 18 | 0.69 (3H, s) |
| 19 | 0.99 (3H, s) |
| 20 | 1.40 (1H, m) |
| 21 | 1.00 (3H, d) |
| 22 | 1.41 (1H, m), 1.09 (1H, m) |
| 23 | 2.03 (1H, m), 1.90 (1H, m) |
| 25 | 2.20 (1H, m) |
| 26 | 0.97 (3H, s) |
| 27 | 0.97 (3H, s) |
| 28 | 5.17 (1H, dd) |
| 29 | 1.56 (3H, s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Jee, Y.; Kim, H.-J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways. Antioxidants 2022, 11, 1429. https://doi.org/10.3390/antiox11081429
Kirindage KGIS, Jayasinghe AMK, Han E-J, Jee Y, Kim H-J, Do SG, Fernando IPS, Ahn G. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways. Antioxidants. 2022; 11(8):1429. https://doi.org/10.3390/antiox11081429
Chicago/Turabian StyleKirindage, Kirinde Gedara Isuru Sandanuwan, Arachchige Maheshika Kumari Jayasinghe, Eui-Jeong Han, Youngheun Jee, Hyun-Jin Kim, Sun Gil Do, Ilekuttige Priyan Shanura Fernando, and Ginnae Ahn. 2022. "Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways" Antioxidants 11, no. 8: 1429. https://doi.org/10.3390/antiox11081429
APA StyleKirindage, K. G. I. S., Jayasinghe, A. M. K., Han, E.-J., Jee, Y., Kim, H.-J., Do, S. G., Fernando, I. P. S., & Ahn, G. (2022). Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways. Antioxidants, 11(8), 1429. https://doi.org/10.3390/antiox11081429







