An Electrochemical Determination of the Total Reducing Capacity of Wheat, Spelt, and Rye Breads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Rye, Wheat, and Spelt Breads Baking Methods
2.3. Sample Preparation for Measurement of the Total Reducing Capacity by Cyclic Voltammetry
2.4. Cyclic Voltammetry Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. The Overall Reducing Capacity of White and Dark Flours and Breads Provided by Cyclic Voltammetry
3.2. The Total Reducing Capacity (TRC) Values of White and Dark Flours and Breads Based on the Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewettinck, K.; van Bockstaele, F.; Kühne, B.; van de Walle, D.; Courtens, T.M.; Gellynck, X. Nutritional value of bread: Influence of processing, food interaction and consumer perception. J. Cereal Sci. 2008, 48, 243–257. [Google Scholar] [CrossRef]
- Michalska, A.; Ceglińska, A.; Amarowicz, R.; Piskuła, M.K.; Szarawa-Nowak, D.; Zieliński, H. The antioxidant contents and antioxidative properties of traditional rye breads. J. Agric. Food Chem. 2007, 55, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Ceglińska, A.; Michalska, A. Bioactive compounds in spelt bread. Eur. Food Res. Technol. 2008, 226, 537–544. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta. 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Zieliński, H.; Zielińska, D.; Kostyra, H. Antioxidant capacity of a new crispy type food products determined by updated analytical strategies. Food Chem. 2012, 130, 1098–1104. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Zielińska, D.; Szawara-Nowak, D.; Zieliński, H. Comparison of spectrophotometric and electrochemical methods for the evaluation of antioxidant capacity of buckwheat products after hydrothermal treatment. J. Agric. Food Chem. 2007, 55, 6124–6131. [Google Scholar] [CrossRef]
- Yang, B.; Kotani, A.; Arai, K.; Kusu, F. Estimation of the antioxidant activities of flavonoids from their oxidation potentials. Anal. Sci. 2001, 17, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Martinez, S.; Valek, L.; Prtović, Z.; Metikos-Huković, M.; Pijac, J. Catechin antioxidant action at various pH studied by cyclic voltammetry and PM3 semi-empirical calculations. J. Electroanal. Chem. 2005, 584, 92–99. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Simon, B.; Cortina, M.; Campas, M.; Calas-Blanchard, C. Electrochemical biosensors as a tool for antioxidant capacity assessment. Sens. Actuators B 2008, 129, 459–466. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Voronova, O.A.; Dorozhko, V. Study of antioxidant properties of flavonoids by voltammetry. J. Solid State Electrochem. 2012, 16, 2435–2440. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Głód, B.; Kiersztyn, I.; Piszcz, P. Total antioxidant potential assay with cyclic voltammetry and/or differential pulse voltammetry measurements. J. Electroanal. Chem. 2014, 719, 24–29. [Google Scholar] [CrossRef]
- Przygodzka, M.; Piskula, M.K.; Kukurová, K.; Ciesarová, Z.; Bednarikova, A.; Zieliński, H. Factors Influencing Acrylamide Formation in Rye, Wheat and Spelt Breads. J. Cereal Sci. 2015, 65, 96–102. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Enache, T.A.; de Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Katina, K.; Arendt, E.; Liukkonen, K.-H.; Autio, K.; Flander, L.; Poutanen, K. Potential of sourdough for healthier cereal products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Liukkonen, K.-H.; Katina, K.; Wilhelmsson, A.; Myllymaki, O.; Lampi, A.-M.; Kariluoto, S.; Piironen, V.; Heinonen, S.-M.; Nurmi, T.; Adlercreutz, H.; et al. Process-inducted changes on bioactive compounds in whole grain rye. Proc. Nutr. Soc. 2003, 62, 117–122. [Google Scholar] [CrossRef]
- Li, W.; Bollecker, S.S.; Schofield, J.D. Glutathione and related thiol compounds. I. Glutathione and related thiol compounds in flour. J. Cereal Sci. 2004, 39, 205–212. [Google Scholar]
- Michalska, A.; Amigo-Benavent, M.; Zieliński, H.; del Castillo, M.D. Effect of baking on the formation of MRPs contributing to the overall antioxidant activity of rye bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Szawara-Nowak, D.; Bączek, N.; Zieliński, H. Antioxidant Capacity and Bioaccessibility of Buckwheat-Enhanced Wheat Bread Phenolics. J. Food Sci. Technol. 2016, 53, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szawara-Nowak, D.; Zielińska, D.; Zieliński, H.; Wronkowska, M. Antioxidant properties of dark wheat bread with added exogenous buckwheat flours addition. In Superfood and Functional Food—An Overview of Their Processing and Utilization; Waisundara, V., Shiomi, N., Eds.; IntechOpen: London, UK, 2017; Chapter 13; pp. 273–289. ISBN 978-953-51-2920-2. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, D. The Bioaccessible reducing capacity of buckwheat-enhanced wheat breads estimated by electrochemical method. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-920-1. [Google Scholar] [CrossRef] [Green Version]
- Błaszczak, W.; Zielińska, D.; Zieliński, H.; Szawara-Nowak, D.; Fornal, J. Antioxidant properties and rutin content of high pressure-treated raw and roasted buckwheat groats. Food Bioprocess Technol. 2013, 6, 92–100. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Zieliński, H.; Wiczkowski, W.; Zielińska, D.; Martínez-Villaluenga, C. High pressure-assisted enzymatic release of peptides and phenolics increase angiotensin converting enzyme I inhibitory and antioxidant activities of pinto bean hydrolysates. J. Agric. Food Chem. 2016, 64, 1730–1740. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Honke, J.; Giménez-Bastida, J.A. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
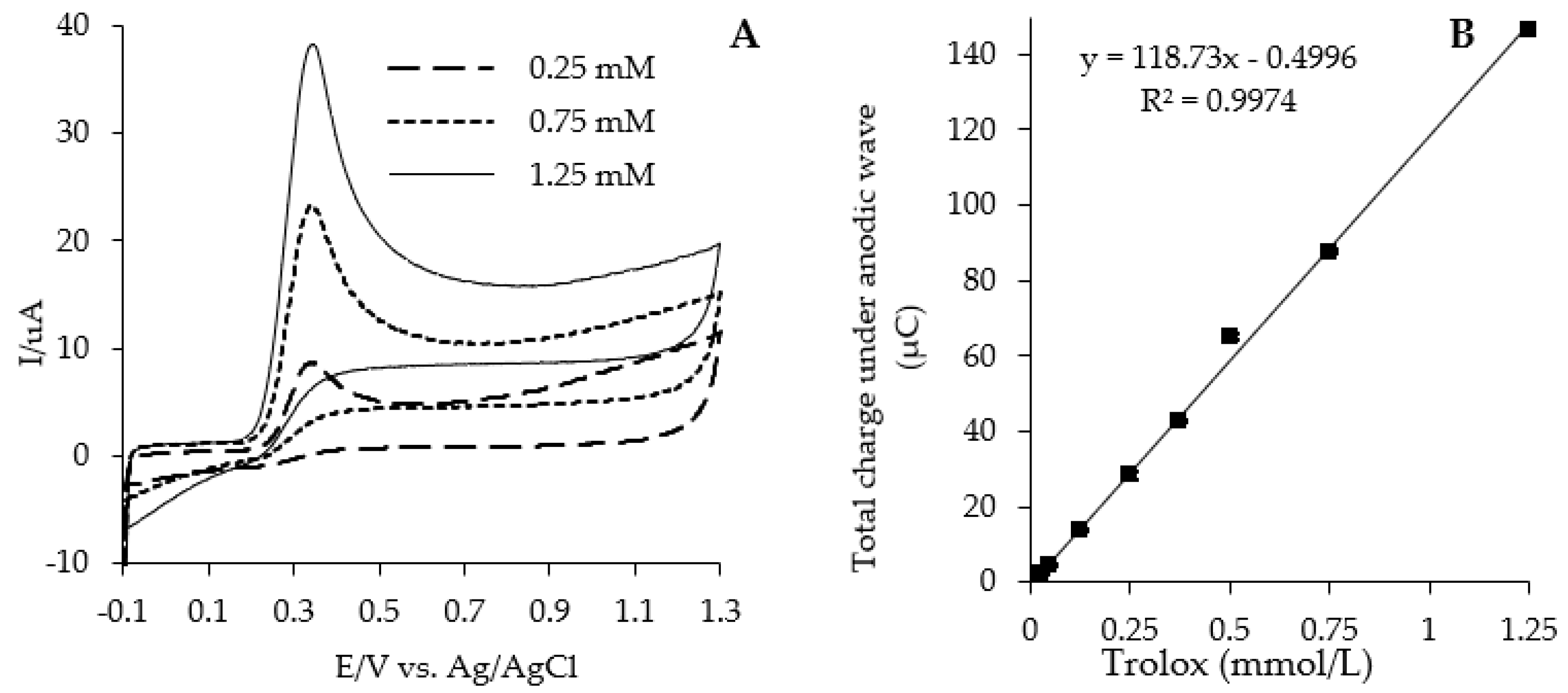
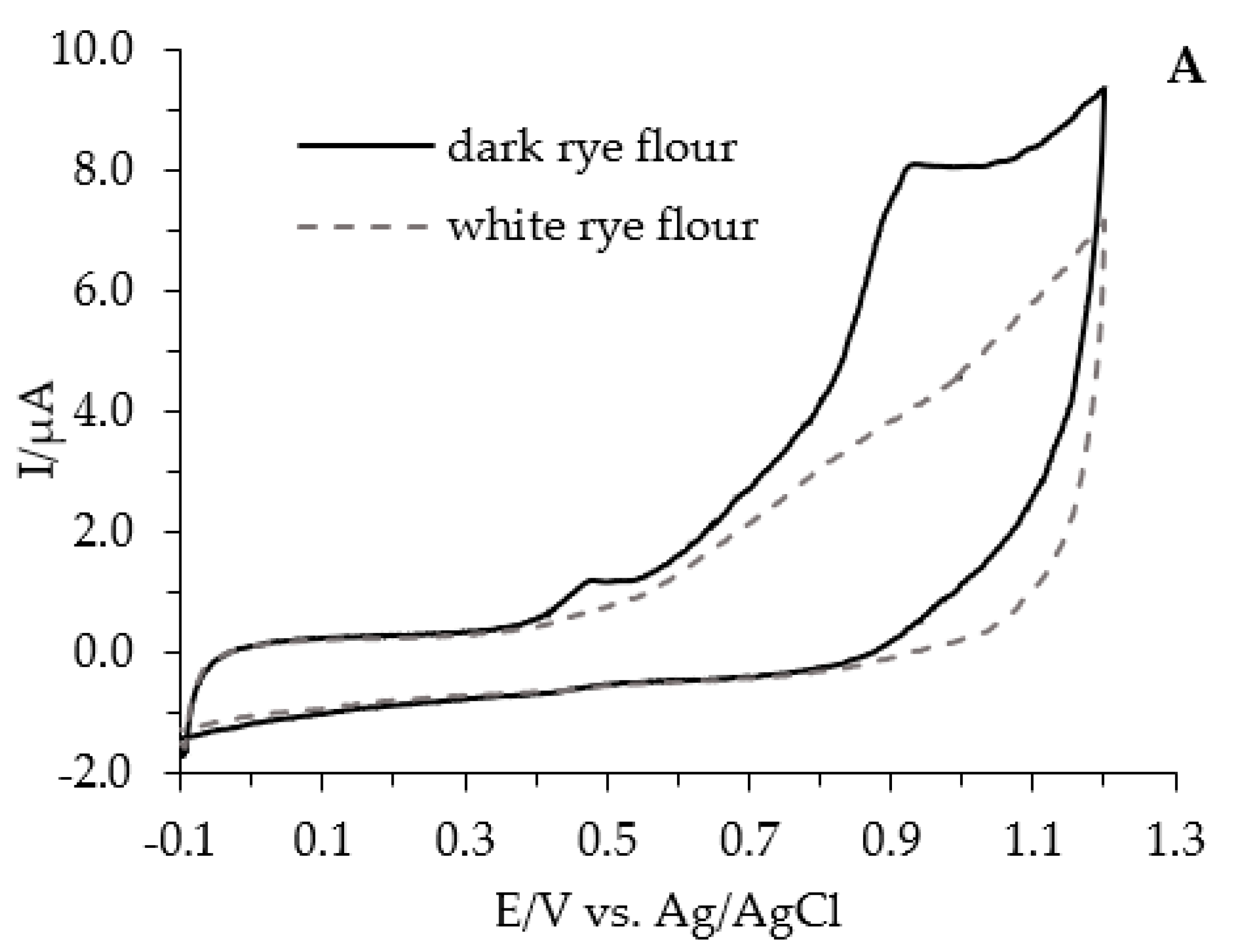
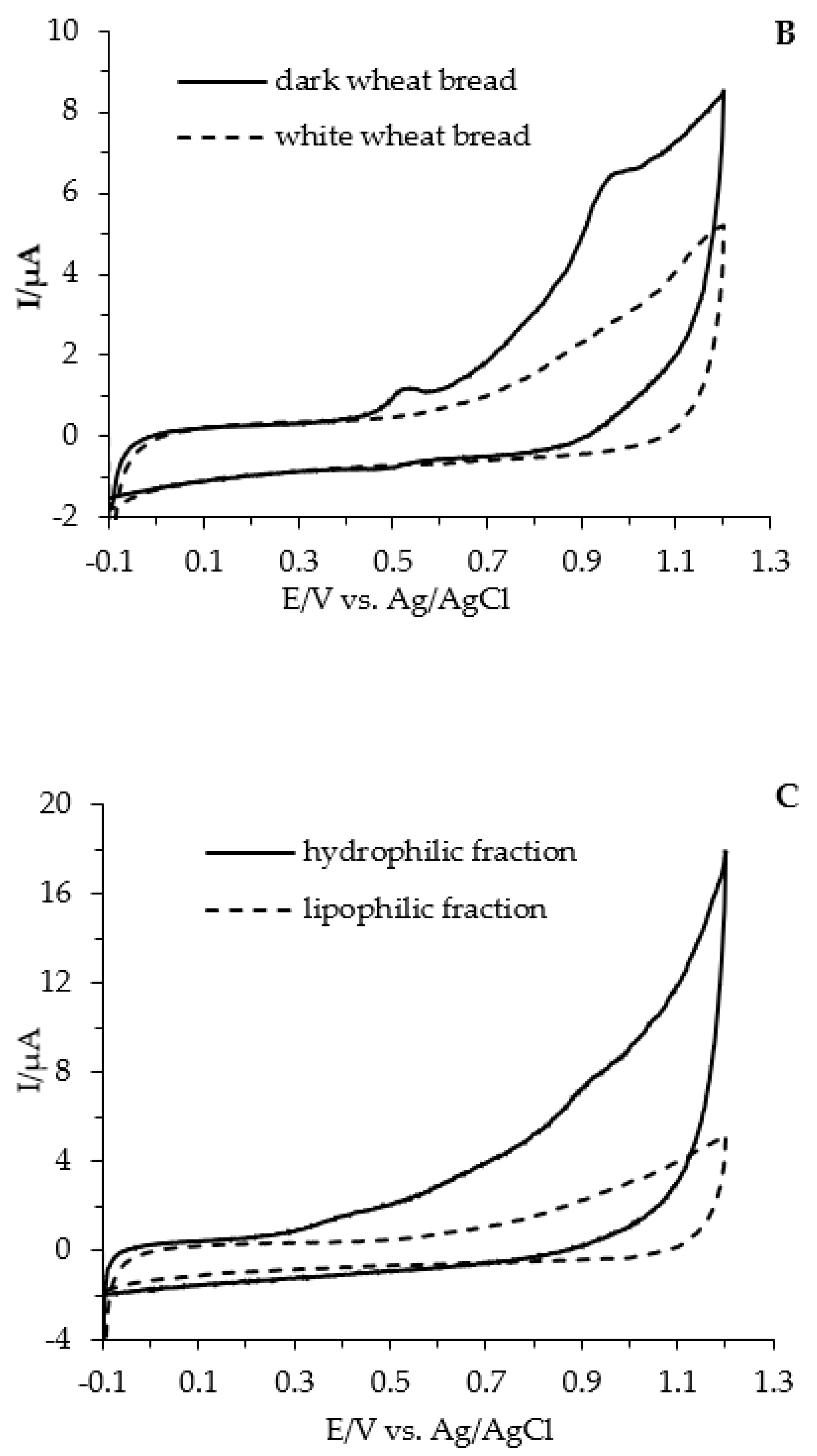

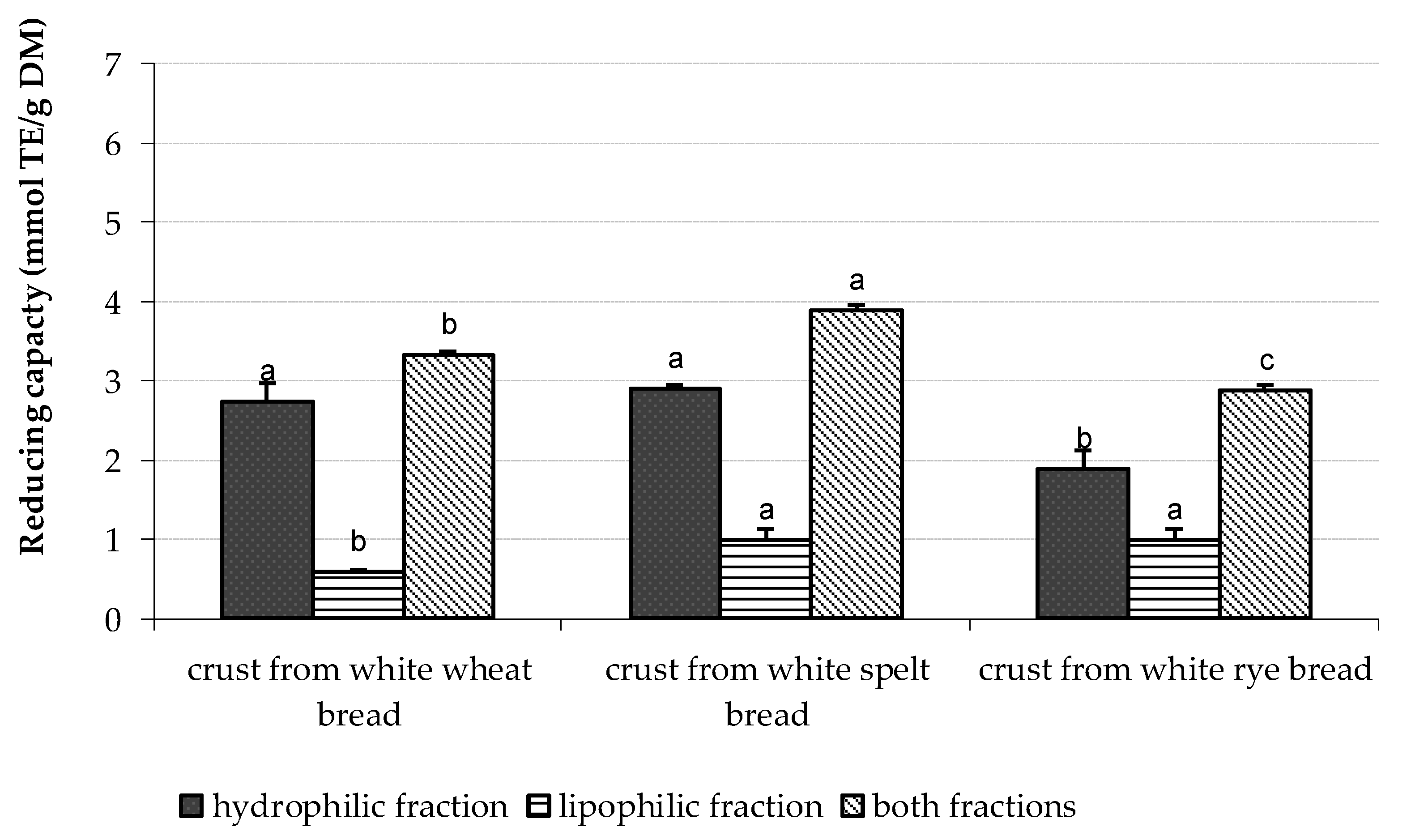

| Type of Bread | Baking Conditions | Flour | Dough | Bread Slice | Bread Crumb | Bread Crust |
|---|---|---|---|---|---|---|
| White wheat bread | 200 °C/35 min 240 °C/30 min | 0.75 ± 0.15 A | 1.16 ± 0.13 A | 1.44 ± 0.06 b,A 1.18 ± 0.12 a,A | 1.13 ± 0.07 a,A 1.15 ± 0.13 a,A | 1.40 ± 0.17 a,A 2.74 ± 0.23 b,A |
| Dark wheat bread | 200 °C/35 min 240 °C/30 min | 1.68 ± 0.30 B | 2.32 ± 0.09 B | 2.36 ± 0.02 a,B 2.44 ± 0.11 a,B | 2.44 ± 0.19 a,B 2.30 ± 0.01 a,B | 4.17 ± 0.26 a,B 3.54 ± 0.34 a,B |
| White spelt bread | 200 °C/35 min 240 °C/30 min | 0.83 ± 0.06 A | 1.01 ± 0.25 A | 1.04 ± 0.12 a,A 1.15 ± 0.08 a,A | 1.14 ± 0.17 a,A 1.04 ± 0.18 a,A | 1.57 ± 0.09 a,A 2.90 ± 0.04 b,A |
| Dark spelt bread | 200 °C/35 min 240 °C/30 min | 1.94 ± 0.23 B | 1.99 ± 0.28 B | 2.37 ± 0.34 a,B 2.10 ± 0.06 a,B | 2.26 ± 0.02 b,B 2.03 ± 0.09 a,B | 2.81 ± 0.16 a,B 3.24 ± 0.29 a,B |
| White rye bread | 200 °C/35 min 240 °C/30 min | 0.89 ± 0.07 A | 1.25 ± 0.15 A | 1.51 ± 0.03 b,A 1.28 ± 0.01 a,A | 1.55 ± 0.06 b,A 1.33 ± 0.01 a,A | 1.71 ± 0.01 a,A 1.89 ± 0.04 b,A |
| Dark rye bread | 200 °C/35 min 240 °C/30 min | 2.16 ± 0.09 B | 2.87 ± 0.02 B | 2.66 ± 0.10 a,B 2.99 ± 0.19 a,B | 3.04 ± 0.17 b,B 2.64 ± 0.02 a,B | 3.35 ± 0.11 a,B 3.24 ± 0.13 a,B |
| Type of Bread | Baking Conditions | Flour | Dough | Bread Slice | Bread Crumb | Bread Crust |
|---|---|---|---|---|---|---|
| White wheat bread | 200 °C/35 min 240 °C/30 min | 0.38 ± 0.14 A | 0.73 ± 0.06 A | 0.52 ± 0.05 a,A 0.63 ± 0.04 b,A | 0.72 ± 0.05 b,A 0.50 ± 0.03 a,A | 0.87 ± 0.13 b,A 0.59 ± 0.06 a,A |
| Dark wheat bread | 200 °C/35 min 240 °C/30 min | 2.05 ± 0.19 B | 2.52 ± 0.14 B | 2.02 ± 0.11 a,B 1.96 ± 0.10 a,B | 2.21 ± 0.09 a,B 2.17 ± 0.12 a,B | 1.85 ± 0.08 a,B 1.99 ± 0.12 a,B |
| White spelt bread | 200 °C/35 min 240 °C/30 min | 0.68 ± 0.13 A | 1.02 ± 0.02 A | 0.80 ± 0.02 a,A 0.83 ± 0.03 a,A | 0.87 ± 0.07 a,A 0.86 ± 0.09 a,A | 0.78 ± 0.01 a,A 0.99 ± 0.15 a,A |
| Dark spelt bread | 200 °C/35 min 240 °C/30 min | 2.25 ± 0.28 B | 2.84 ± 0.05 B | 2.39 ± 0.07 a,B 2.37 ± 0.20 a,B | 2.29 ± 0.07 a,B 2.32 ± 0.09 a,B | 1.85 ± 0.11 a,B 2.04 ± 0.10 a,B |
| White rye bread | 200 °C/35 min 240 °C/30 min | 0.98 ± 0.07 A | 1.36 ± 0.11 A | 1.31 ± 0.01 b,A 0.98 ± 0.02 a,A | 1.19 ± 0.06 b,A 1.10 ± 0.01 a,A | 0.97 ± 0.05 a,A 0.99 ± 0.07 a,A |
| Dark rye bread | 200 °C/35 min 240 °C/30 min | 2.66 ± 0.15 B | 3.51 ± 0.06 B | 3.07 ± 0.01 a,B 2.95 ± 0.05 a,B | 3.13 ± 0.11 a,B 3.05 ± 0.05 a,B | 2.88 ± 0.11 a,B 2.93 ± 0.09 a,B |
| Type of Bread | Baking Conditions | Fluor | Dough | Bread Slice | Bread Crumb | Bread Crust |
|---|---|---|---|---|---|---|
| White wheat bread | 200 °C/35 min 240 °C/30 min | 1.13 ± 0.29 A | 1.88 ± 0.19 A | 1.96 ± 0.11 a,A 1.81 ± 0.16 a,A | 1.85 ± 0.12 a,A 1.65 ± 0.16 a,A | 2.27 ± 0.30 a,A 3.33 ± 0.29 b,B |
| Dark wheat bread | 200 °C/35 min 240 °C/30 min | 3.73 ± 0.49 B | 4.84 ± 0.23 B | 4.37 ± 0.13 a,A 4.41 ± 0.21 a,A | 4.65 ± 0.28 a,A 4.47 ± 0.13 a,A | 6.02 ± 0.34 b,A 5.53 ± 0.46 a,A |
| White spelt bread | 200 °C/35 min 240 °C/30 min | 1.51 ± 0.19 A | 2.03 ± 0.38 A | 1.83 ± 0.14 a,A 1.98 ± 0.11 a,A | 2.01 ± 0.24 a,A 1.91 ± 0.27 a,A | 2.36 ± 0.10 b,A 3.89 ± 0.19 b,B |
| Dark spelt bread | 200 °C/35 min 240 °C/30 min | 4.20 ± 0.51 B | 4.84 ± 0.33 B | 4.76 ± 0.38 a,A 4.47 ± 0.26 a,A | 4.56 ± 0.09 a,B 4.35 ± 0.18 a,A | 4.66 ± 0.27 a,A 5.27 ± 0.39 b,B |
| White rye bread | 200 °C/35 min 240 °C/30 min | 1.87 ± 0.14 A | 2.62 ± 0.26 A | 2.82 ± 0.04 a,A 2.26 ± 0.03 a,B | 2.74 ± 0.07 a,B 2.43 ± 0.02 a,A | 2.69 ± 0.06 a,A 2.88 ± 0.11 a,A |
| Dark rye bread | 200 °C/35 min 240 °C/30 min | 4.83 ± 0.24 B | 6.39 ± 0.08 B | 5.72 ± 0.11 a,A 5.94 ± 0.24 a,A | 6.17 ± 0.28 b,B 5.69 ± 0.07 a,A | 6.23 ± 0.22 b,A 6.17 ± 0.22 a,A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Zieliński, H.; Piskuła, M.K. An Electrochemical Determination of the Total Reducing Capacity of Wheat, Spelt, and Rye Breads. Antioxidants 2022, 11, 1438. https://doi.org/10.3390/antiox11081438
Zielińska D, Zieliński H, Piskuła MK. An Electrochemical Determination of the Total Reducing Capacity of Wheat, Spelt, and Rye Breads. Antioxidants. 2022; 11(8):1438. https://doi.org/10.3390/antiox11081438
Chicago/Turabian StyleZielińska, Danuta, Henryk Zieliński, and Mariusz Konrad Piskuła. 2022. "An Electrochemical Determination of the Total Reducing Capacity of Wheat, Spelt, and Rye Breads" Antioxidants 11, no. 8: 1438. https://doi.org/10.3390/antiox11081438







