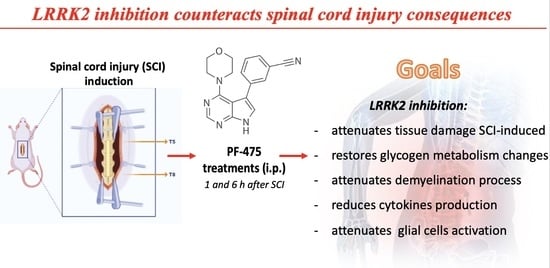
LRRK2 Inhibition by PF06447475 Antagonist Modulates Early Neuronal Damage after Spinal Cord Trauma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. SCI Procedure
2.4. Experimental Groups
- -
- Sham + vehicle: mice were subjected to laminectomy, but the aneurysm clip was not applied; these mice were administered with saline + DMSO intraperitoneally, 1 and 6 h after laminectomy;
- -
- SCI + vehicle: mice were subjected to SCI plus intraperitoneal administration of saline + DMSO;
- -
- SCI + PF-475 2.5 mg/kg: mice were subjected to SCI plus intraperitoneal administration of PF-475 at the dose of 2.5 mg/kg 1 and 6 h after SCI;
- -
- SCI + PF-475 5 mg/kg: mice were subjected to SCI plus intraperitoneal administration of PF-475 at the dose of 5 mg/kg 1 and 6 h after SCI);
- -
- SCI+PF-475 10 mg/kg: mice were subjected to SCI plus intraperitoneal administration of PF-475 at the dose of 10 mg/kg 1 and 6 h after SCI;
2.5. Basso Mouse Scale Score
2.6. Histological Evaluation
2.7. Luxol Fast Blue (LFB) Staining
2.8. Periodic Acid Schiff (PAS) Staining
2.9. Enzyme-Linked Immunosorbent Assay (ELISA Kit)
2.10. Western Blot Analysis of p-LRRK2, LRRK2, IL-6, IL-10, IL-1β, IL-13, TNF-α, GYS, and p-GSK3-α/β
2.11. Immunofluorescence Analysis of Glial Cells
2.12. Measurements of Reactive Oxygen Species (ROS)
2.13. Malondialdehyde (MDA) Assay
2.14. Statistical Analysis
3. Results
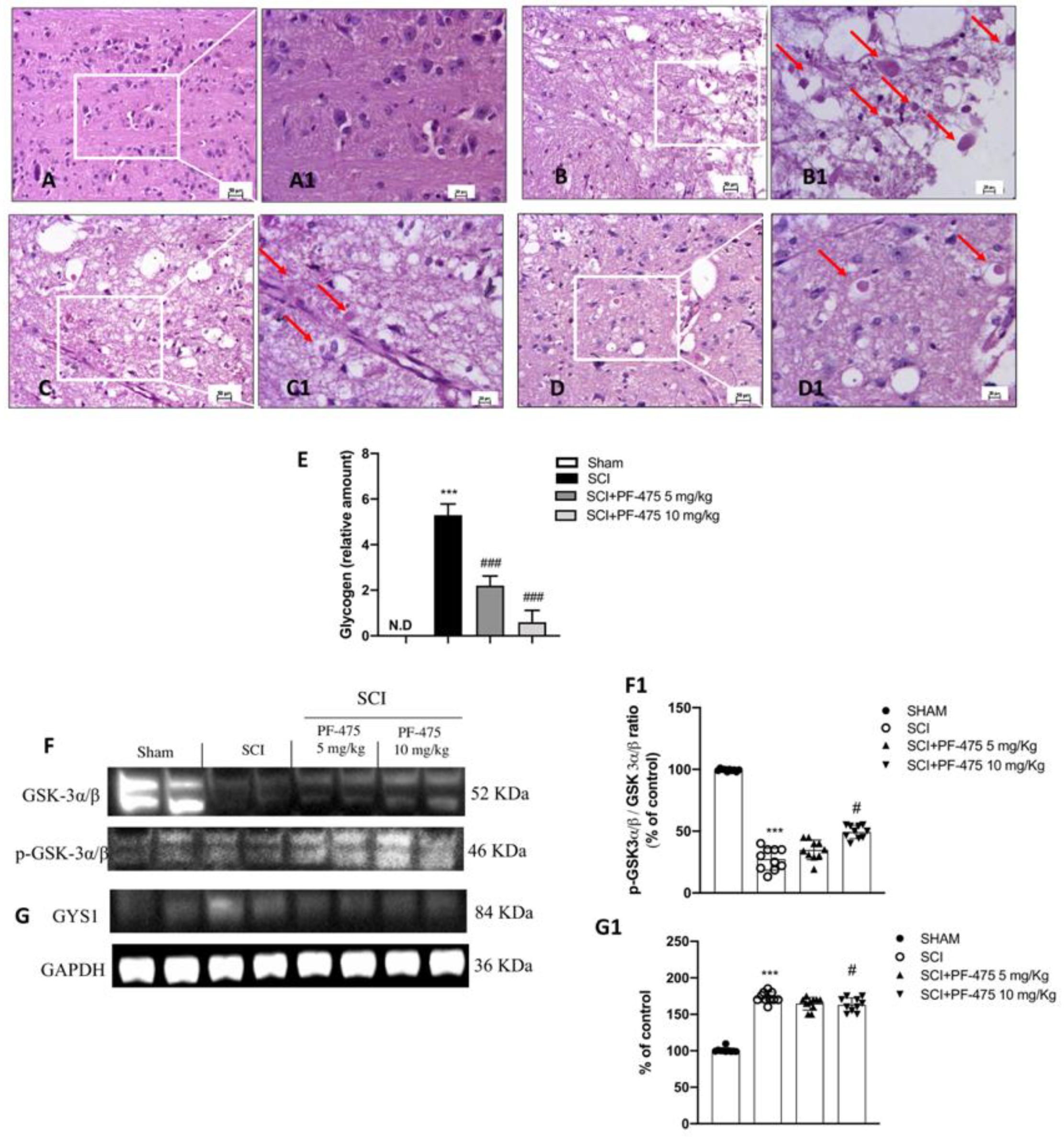
3.1. Treatment with PF-475 Antagonist Attenuates SCI-Induced Tissue Damage and Restores Neuronal Metabolic Homeostasis by Reducing Glycogen Accumulation
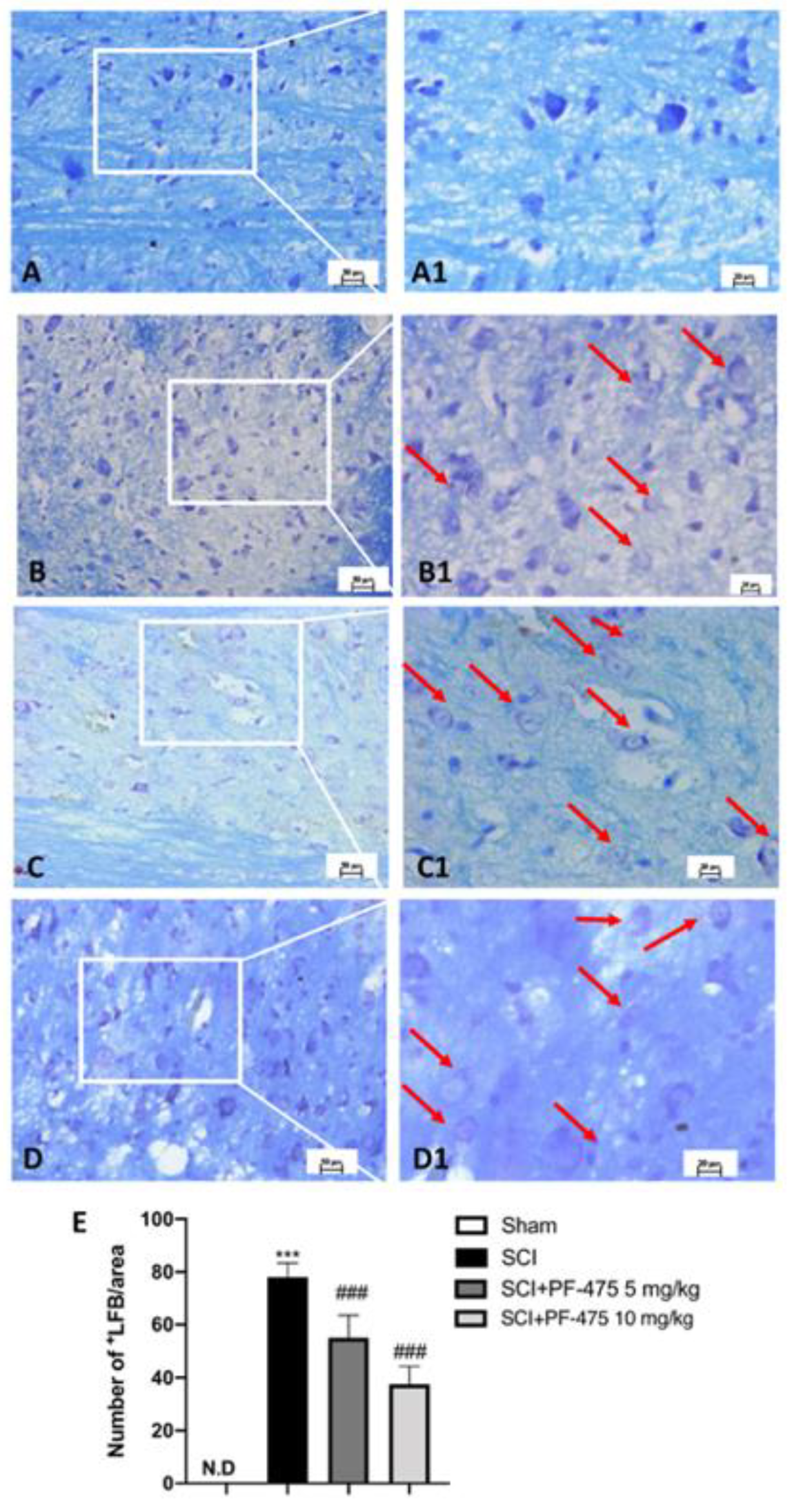
3.2. LRRK2 Inhibition by PF-475 Treatment Prevents SCI-Induced Demyelination Process
3.3. PF-475 Treatments Attenuated Glial Cells Activation and Correlated SCI-Induced Oxidative Stress
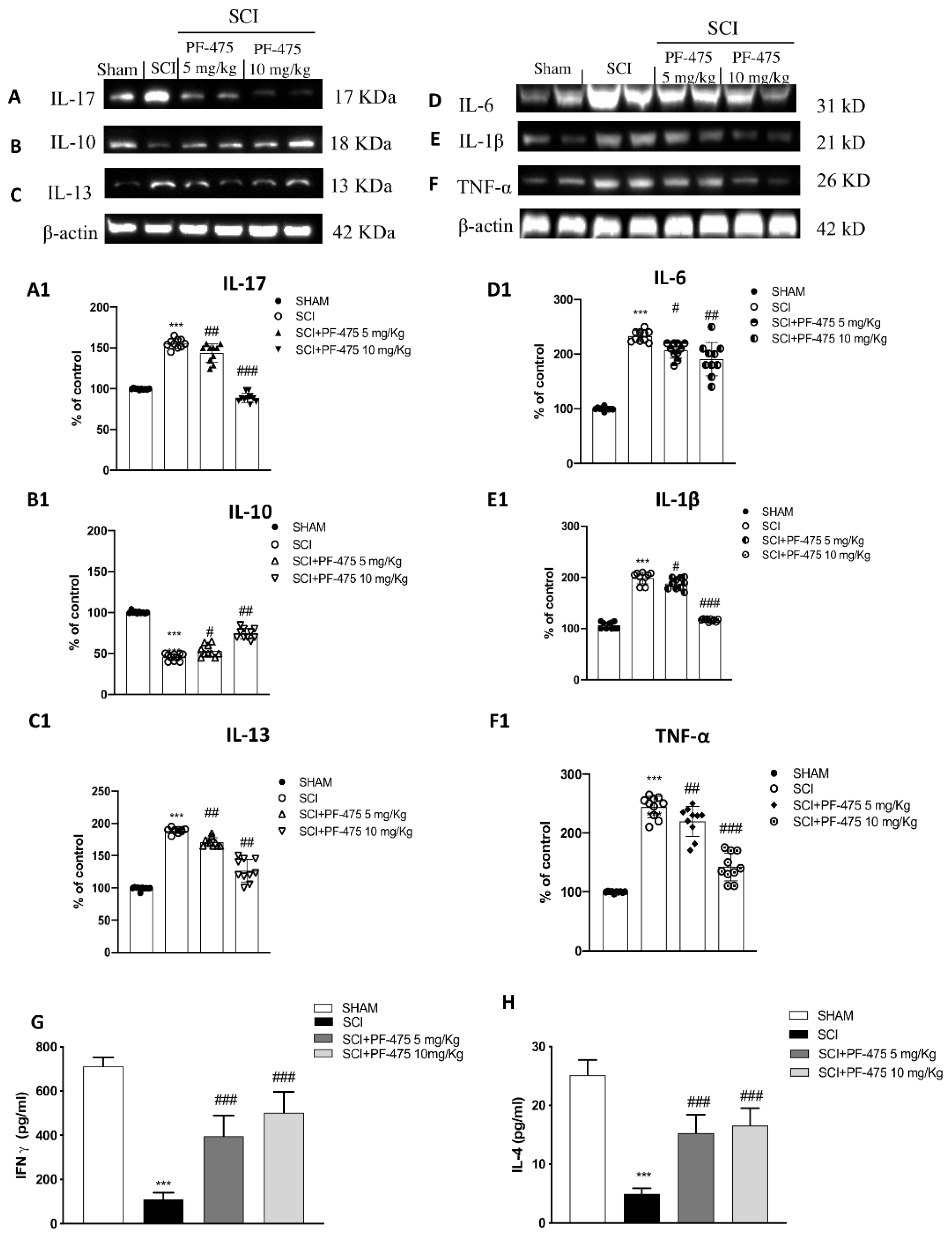
3.4. Modulation of Inflammatory Cytokines by LRRK2 Inhibition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Q.; Xie, Y.; Ordaz, J.D.; Huh, A.J.; Huang, N.; Wu, W.; Liu, N.; Chamberlain, K.A.; Sheng, Z.H.; Xu, X.M. Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab. 2020, 31, 623–641.e8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic spinal injury: Global epidemiology and worldwide volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Campolo, M.; Casili, G.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Sodium butyrate exerts neuroprotective effects in spinal cord injury. Mol. Neurobiol. 2019, 56, 3937–3947. [Google Scholar] [CrossRef] [PubMed]
- Steger, M.; Tonelli, F.; Ito, G.; Davies, P.; Trost, M.; Vetter, M.; Wachter, S.; Lorentzen, E.; Duddy, G.; Wilson, S.; et al. Phosphoproteomics reveals that Parkinson’s disease kinase lrrk2 regulates a subset of rab gtpases. Elife 2016, 5, e12813. [Google Scholar] [CrossRef] [Green Version]
- Kozina, E.; Sadasivan, S.; Jiao, Y.; Dou, Y.; Ma, Z.; Tan, H.; Kodali, K.; Shaw, T.; Peng, J.; Smeyne, R.J. Mutant lrrk2 mediates peripheral and central immune responses leading to neurodegeneration in vivo. Brain 2018, 141, 1753–1769. [Google Scholar] [CrossRef]
- Madureira, M.; Connor-Robson, N.; Wade-Martins, R. Lrrk2: Autophagy and lysosomal activity. Front. Neurosci. 2020, 14, 498. [Google Scholar] [CrossRef]
- Moehle, M.S.; Webber, P.J.; Tse, T.; Sukar, N.; Standaert, D.G.; DeSilva, T.M.; Cowell, R.M.; West, A.B. Lrrk2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 2012, 32, 1602–1611. [Google Scholar] [CrossRef]
- Van der Perren, A.; Cabezudo, D.; Gelders, G.; Ramos, J.M.P.; van den Haute, C.; Baekelandt, V.; Lobbestael, E. Lrrk2 ablation attenuates alphalpha-synuclein-induced neuroinflammation without affecting neurodegeneration or neuropathology in vivo. Neurotherapeutics 2021, 18, 949–961. [Google Scholar] [CrossRef]
- Lewis, P.A.; Manzoni, C. Lrrk2 and human disease: A complicated question or a question of complexes? Sci. Signal. 2012, 5, pe2. [Google Scholar] [CrossRef]
- Tolosa, E.; Vila, M.; Klein, C.; Rascol, O. Lrrk2 in Parkinson disease: Challenges of clinical trials. Nat. Rev. Neurol. 2020, 16, 97–107. [Google Scholar] [CrossRef]
- MacLeod, D.; Dowman, J.; Hammond, R.; Leete, T.; Inoue, K.; Abeliovich, A. The familial parkinsonism gene lrrk2 regulates neurite process morphology. Neuron 2006, 52, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, J.L.; Kormos, B.L.; Hayward, M.M.; Coffman, K.J.; Jasti, J.; Kurumbail, R.G.; Wager, T.T.; Verhoest, P.R.; Noell, G.S.; Chen, Y.; et al. Discovery and preclinical profiling of 3-[4-(morpholin-4-yl)-7h-pyrrolo[2,3-d]pyrimidin-5-yl]benzonitrile (pf-06447475), a highly potent, selective, brain penetrant, and in vivo active lrrk2 kinase inhibitor. J. Med. Chem. 2015, 58, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Protective effect of sodium propionate in abeta1-42 -induced neurotoxicity and spinal cord trauma. Neuropharmacology 2020, 166, 107977. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Ni, H.; Gao, F.; Dang, B.; Li, D.; Gao, R.; Chen, G. Lrrk2 contributes to secondary brain injury through a p38/drosha signaling pathway after traumatic brain injury in rats. Front. Cell. Neurosci. 2018, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bespalov, A.; Wicke, K.; Castagne, V. Blinding and randomization. Handb. Exp. Pharmacol. 2020, 257, 81–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ritzel, R.M.; Khan, N.; Cao, T.; He, J.; Lei, Z.; Matyas, J.J.; Sabirzhanov, B.; Liu, S.; Li, H.; et al. Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice. Theranostics 2020, 10, 11376–11403. [Google Scholar] [CrossRef]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. The anti-inflammatory and antioxidant effects of sodium propionate. Int. J. Mol. Sci. 2020, 21, 30226. [Google Scholar] [CrossRef]
- Campolo, M.; Filippone, A.; Biondo, C.; Mancuso, G.; Casili, G.; Lanza, M.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Tlr7/8 in the pathogenesis of Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 9384. [Google Scholar] [CrossRef]
- Filippone, A.; Casili, G.; Ardizzone, A.; Lanza, M.; Mannino, D.; Paterniti, I.; Esposito, E.; Campolo, M. Inhibition of prolyl oligopeptidase prevents consequences of reperfusion following intestinal ischemia. Biomedicines 2021, 9, 1354. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Zheng, B.; Tian, N. Sinomenine attenuates traumatic spinal cord injury by suppressing oxidative stress and inflammation via nrf2 pathway. Neurochem. Res. 2019, 44, 763–775. [Google Scholar] [CrossRef]
- Huong, N.T.; Matsumoto, K.; Kasai, R.; Yamasaki, K.; Watanabe, H. In vitro antioxidant activity of vietnamese ginseng saponin and its components. Biol. Pharm. Bull. 1998, 21, 978–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves, A.R.; G-Fernandes, M.; Santos, D.; Januario, C.; Cardoso, S.M. The upshot of lrrk2 inhibition to Parkinson’s disease paradigm. Mol. Neurobiol. 2015, 52, 1804–1820. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Matsas, R. From demyelination to remyelination: The road toward therapies for spinal cord injury. Glia 2015, 63, 1101–1125. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Du, L.; Zhang, L.; Zhang, Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via nrf2/ho-1 pathway. Life Sci. 2019, 217, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Xu, C.; Lin, J.; Hu, H.; Zhang, C.; Mei, X. Regulation of inflammatory cytokines for spinal cord injury recovery. Histol. Histopathol. 2021, 36, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Makela, J.; Koivuniemi, R.; Korhonen, L.; Lindholm, D. Interferon-gamma produced by microglia and the neuropeptide pacap have opposite effects on the viability of neural progenitor cells. PLoS ONE 2010, 5, e11091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, R.; Glaser, J.; Liu, M.T.; Lane, T.E.; Keirstead, H.S. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp. Neurol. 2003, 184, 456–463. [Google Scholar] [CrossRef]
- Tang, R.; Botchway, B.O.A.; Meng, Y.; Zhang, Y.; Zhou, C.; Jiang, J.; Liu, X. The inhibition of inflammatory signaling pathway by secretory leukocyte protease inhibitor can improve spinal cord injury. Cell. Mol. Neurobiol. 2020, 40, 1067–1073. [Google Scholar] [CrossRef]
- Wallings, R.L.; Herrick, M.K.; Tansey, M.G. Lrrk2 at the interface between peripheral and central immune function in Parkinson’s. Front. Neurosci. 2020, 14, 443. [Google Scholar] [CrossRef]
- Borghi, S.M.; Fattori, V.; Hohmann, M.S.N.; Verri, W.A. Contribution of spinal cord oligodendrocytes to neuroinflammatory diseases and pain. Curr. Med. Chem. 2019, 26, 5781–5810. [Google Scholar] [CrossRef]
- Mietto, B.S.; Mostacada, K.; Martinez, A.M. Neurotrauma and inflammation: Cns and pns responses. Mediat. Inflamm. 2015, 2015, 251204. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Nair, A.; Gupta, M. Mast cells in neurodegenerative disease. Front. Cell. Neurosci. 2019, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 2021, 21, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.A.; Kannarkat, G.T.; Cintron, A.F.; Butkovich, L.M.; Fraser, K.B.; Chang, J.; Grigoryan, N.; Factor, S.A.; West, A.B.; Boss, J.M.; et al. Lrrk2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinson’s Dis. 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Pineau, I.; Sun, L.; Bastien, D.; Lacroix, S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an il-1 receptor/myd88-dependent fashion. Brain Behav. Immun. 2010, 24, 540–553. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K. Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics 2018, 15, 554–577. [Google Scholar] [CrossRef] [Green Version]
- Bloom, O.; Herman, P.E.; Spungen, A.M. Systemic inflammation in traumatic spinal cord injury. Exp. Neurol. 2020, 325, 113143. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Dumont, C.M.; Carlson, M.A.; Munsell, M.K.; Ciciriello, A.J.; Strnadova, K.; Park, J.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019, 86, 312–322. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippone, A.; Mannino, D.; Cucinotta, L.; Paterniti, I.; Esposito, E.; Campolo, M. LRRK2 Inhibition by PF06447475 Antagonist Modulates Early Neuronal Damage after Spinal Cord Trauma. Antioxidants 2022, 11, 1634. https://doi.org/10.3390/antiox11091634
Filippone A, Mannino D, Cucinotta L, Paterniti I, Esposito E, Campolo M. LRRK2 Inhibition by PF06447475 Antagonist Modulates Early Neuronal Damage after Spinal Cord Trauma. Antioxidants. 2022; 11(9):1634. https://doi.org/10.3390/antiox11091634
Chicago/Turabian StyleFilippone, Alessia, Deborah Mannino, Laura Cucinotta, Irene Paterniti, Emanuela Esposito, and Michela Campolo. 2022. "LRRK2 Inhibition by PF06447475 Antagonist Modulates Early Neuronal Damage after Spinal Cord Trauma" Antioxidants 11, no. 9: 1634. https://doi.org/10.3390/antiox11091634
APA StyleFilippone, A., Mannino, D., Cucinotta, L., Paterniti, I., Esposito, E., & Campolo, M. (2022). LRRK2 Inhibition by PF06447475 Antagonist Modulates Early Neuronal Damage after Spinal Cord Trauma. Antioxidants, 11(9), 1634. https://doi.org/10.3390/antiox11091634