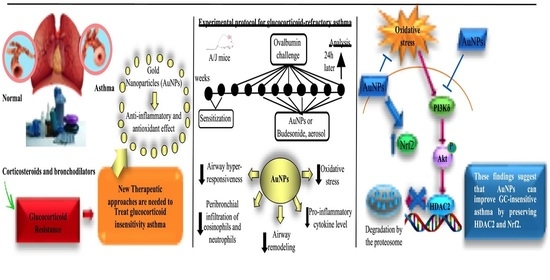
Gold Nanoparticles Inhibit Steroid-Insensitive Asthma in Mice Preserving Histone Deacetylase 2 and NRF2 Pathways
Abstract
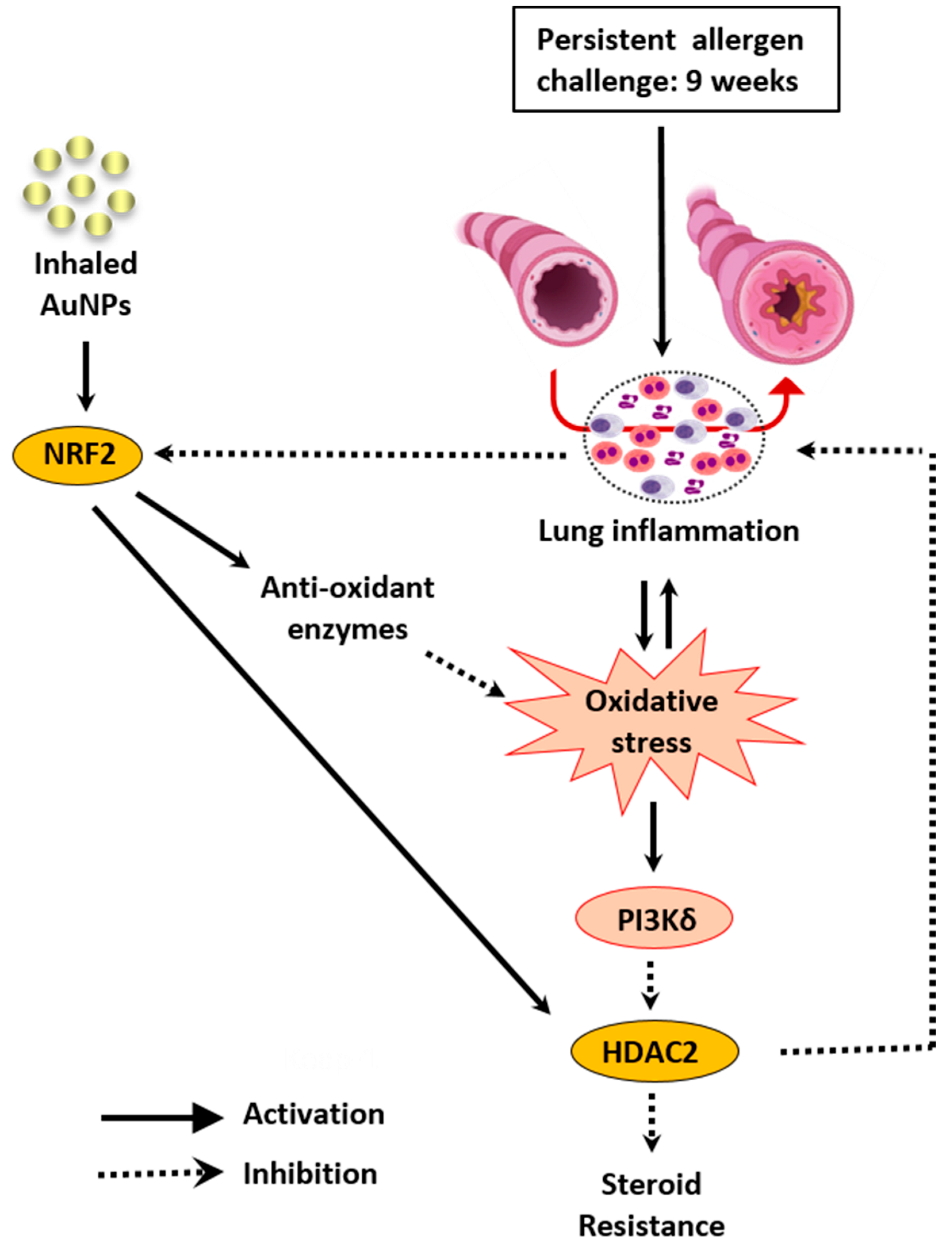
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of AuNPs
2.2. Animals and Experimental Procedures
2.3. Invasive Assessment of Respiratory Mechanics
2.4. Lung Tissue Digestion and Leucocyte Enumeration
2.5. Determination of Myeloperoxidase Activity in the Lung (MPO)
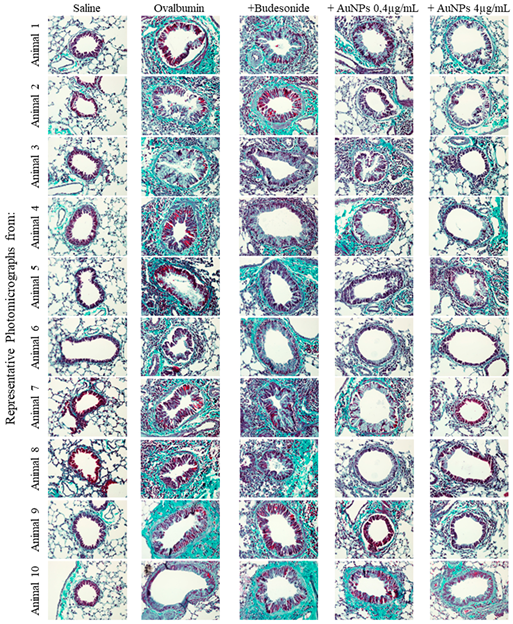
2.6. Histopathological Evaluation of Pulmonary Inflammation and Airway Remodeling
2.7. Cytokine and Chemokine Measurements
2.8. Redox Parameters
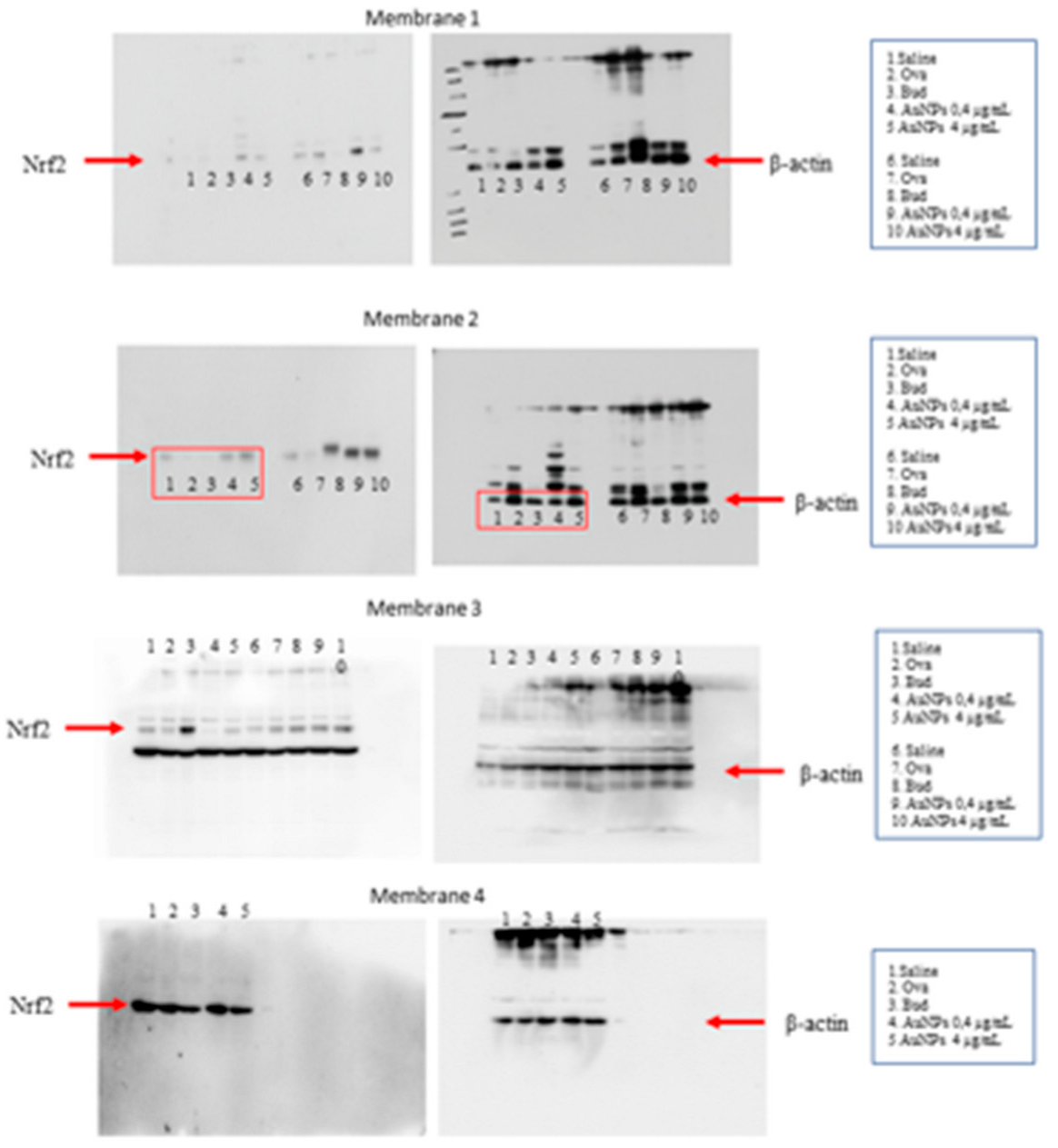
2.9. Western Blotting
2.10. Statistical Analyses
3. Results
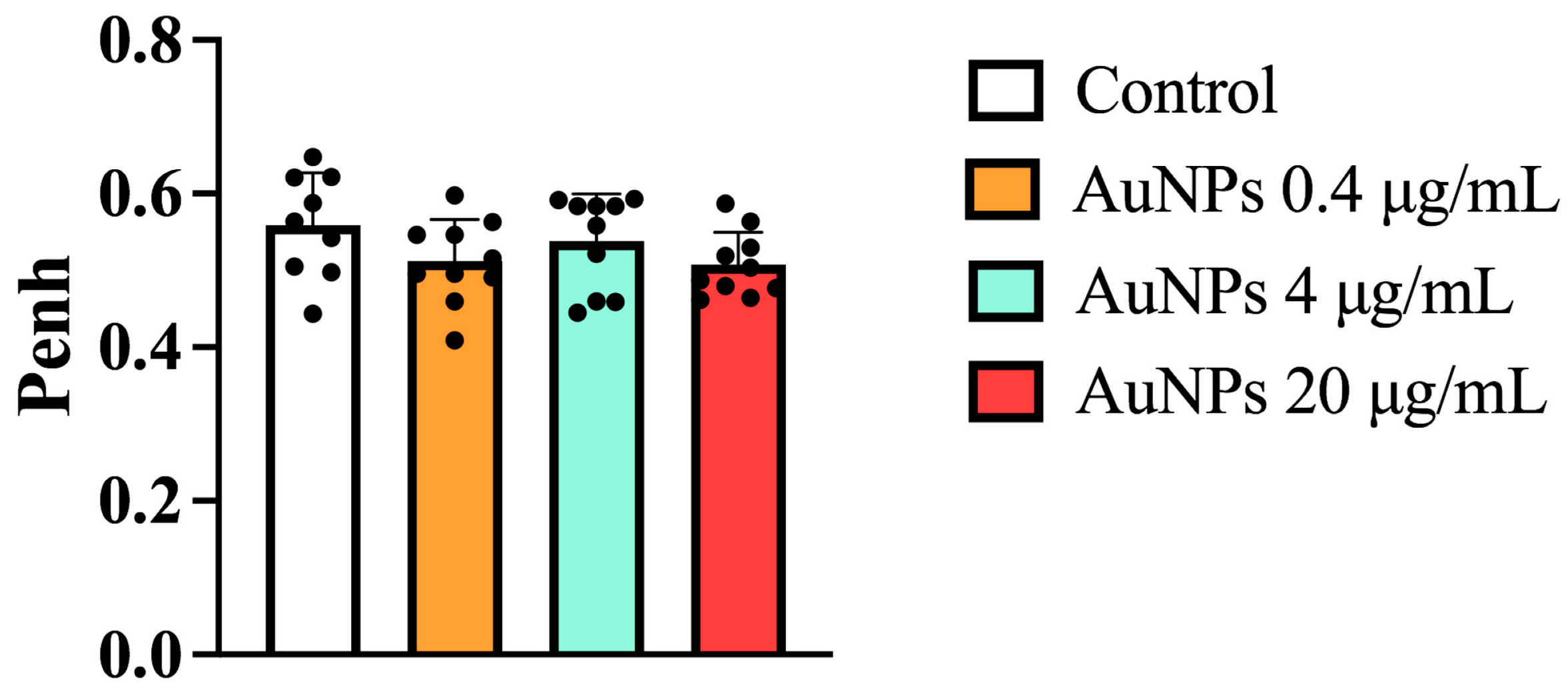
3.1. Effect of AuNPs but Not Budesonide on Airway Hyper-Reactivity (AHR) Caused by Allergen Provocation
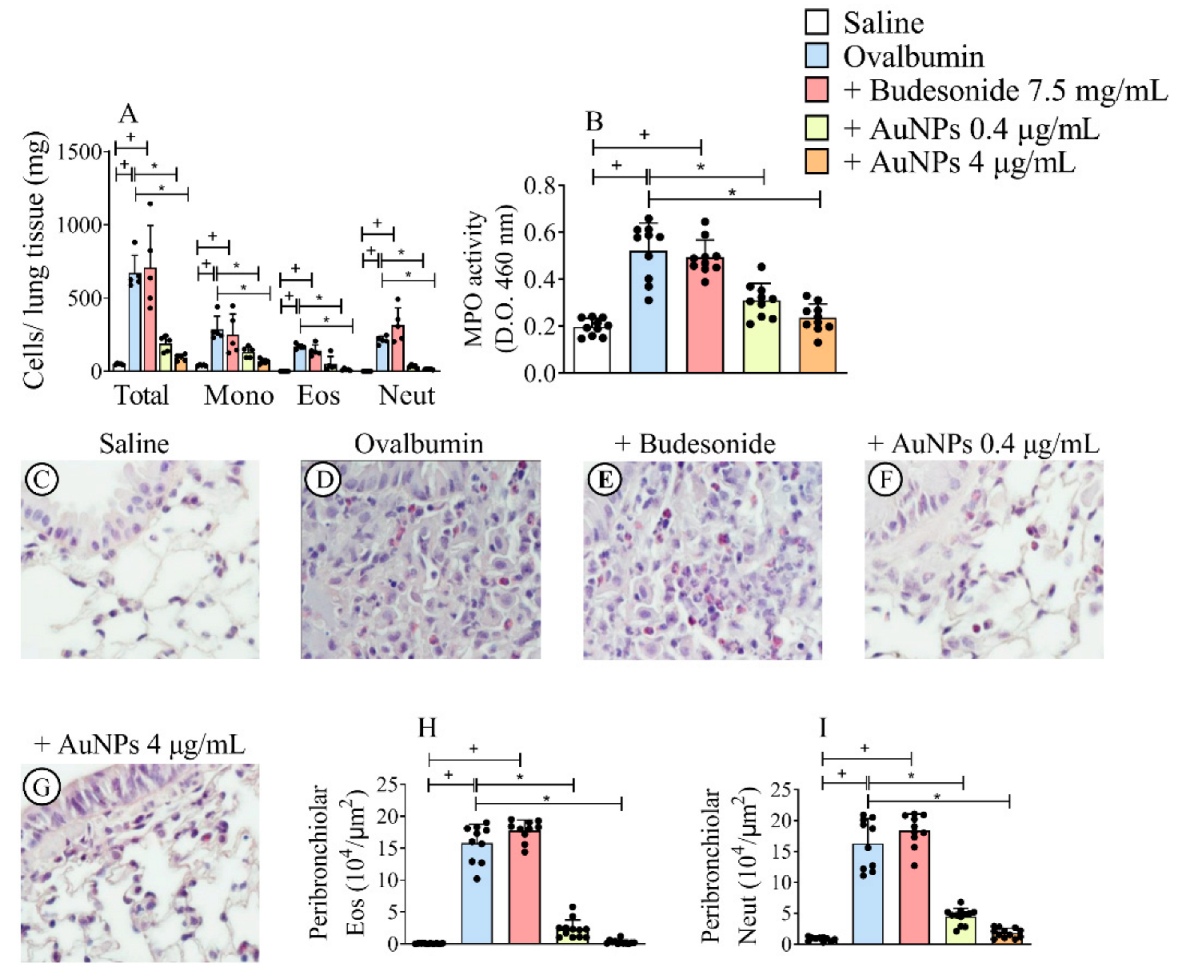
3.2. Effect of AuNPs but Not Budesonide on Inflammatory Changes Caused by Allergen Provocation
3.3. AuNPs Inhibit Allergen-Induced Mucus Production and Peribronchiolar Collagen Deposition Caused by Allergen Provocation
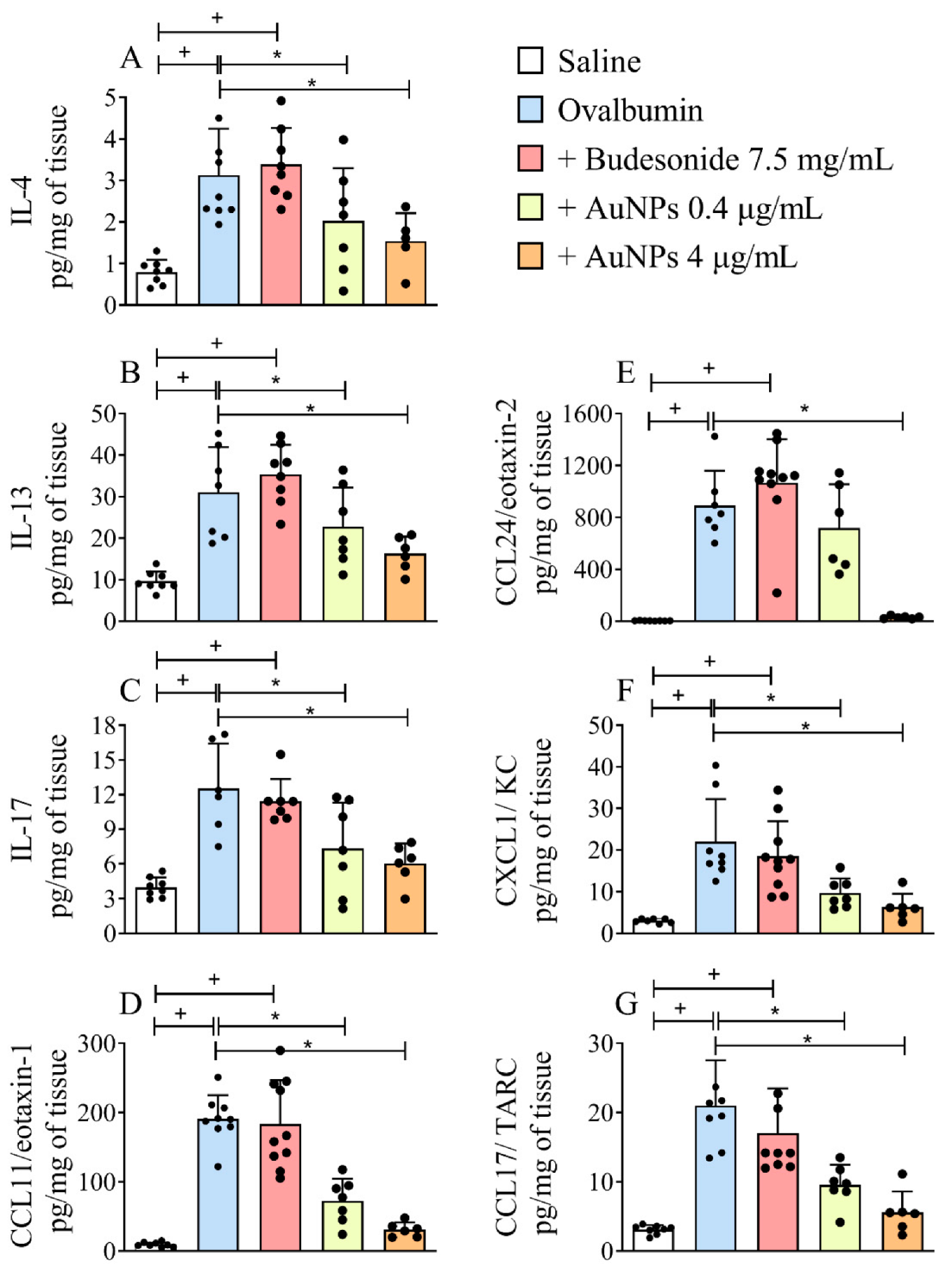
3.4. Effect of AuNPs but Not Budesonide on Cytokine and Chemokine Generation Caused by Allergen Provocation
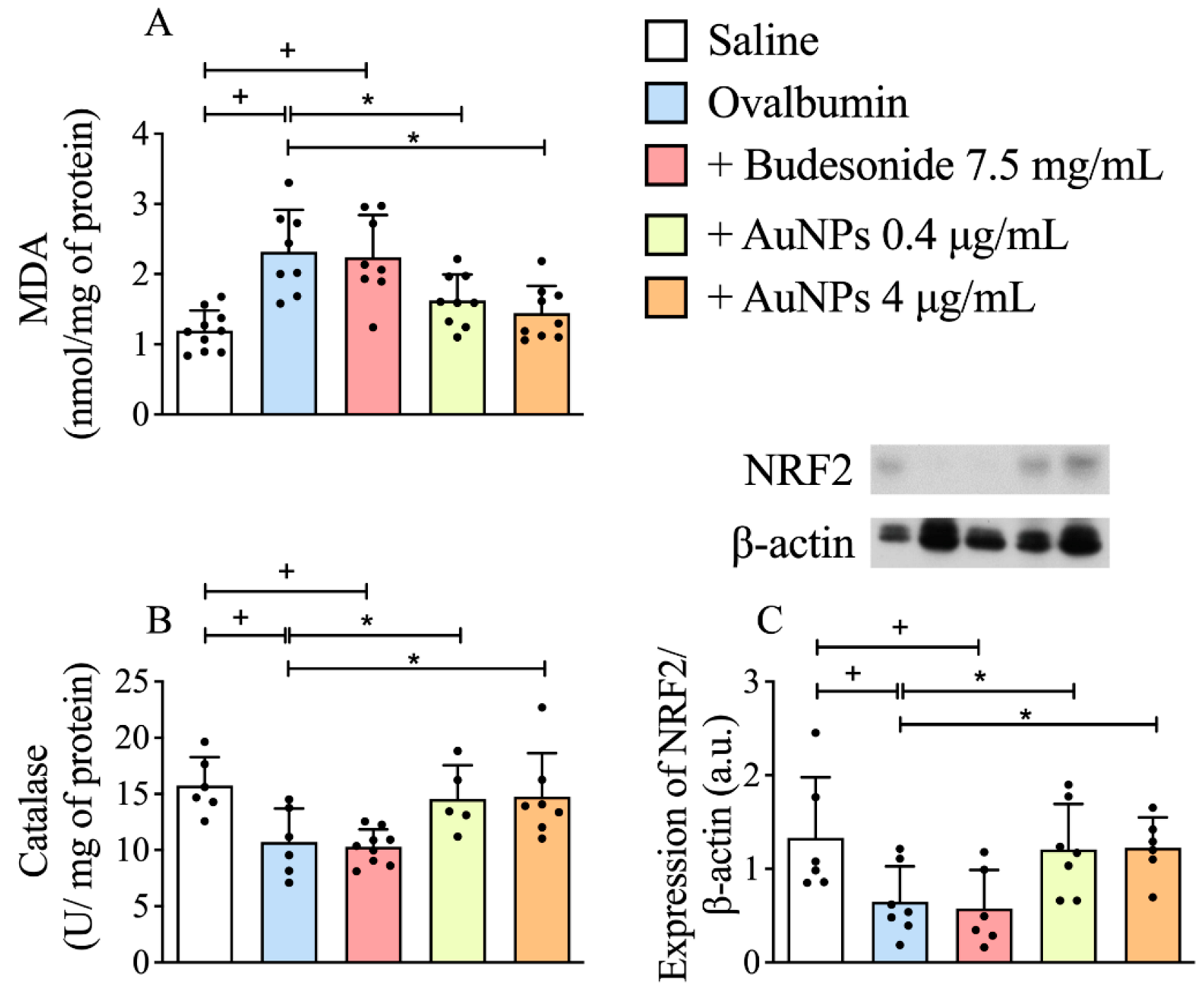
3.5. Effect of AuNPs on Redox Imbalance Related to Corticosteroid Resistance in A/J Mice
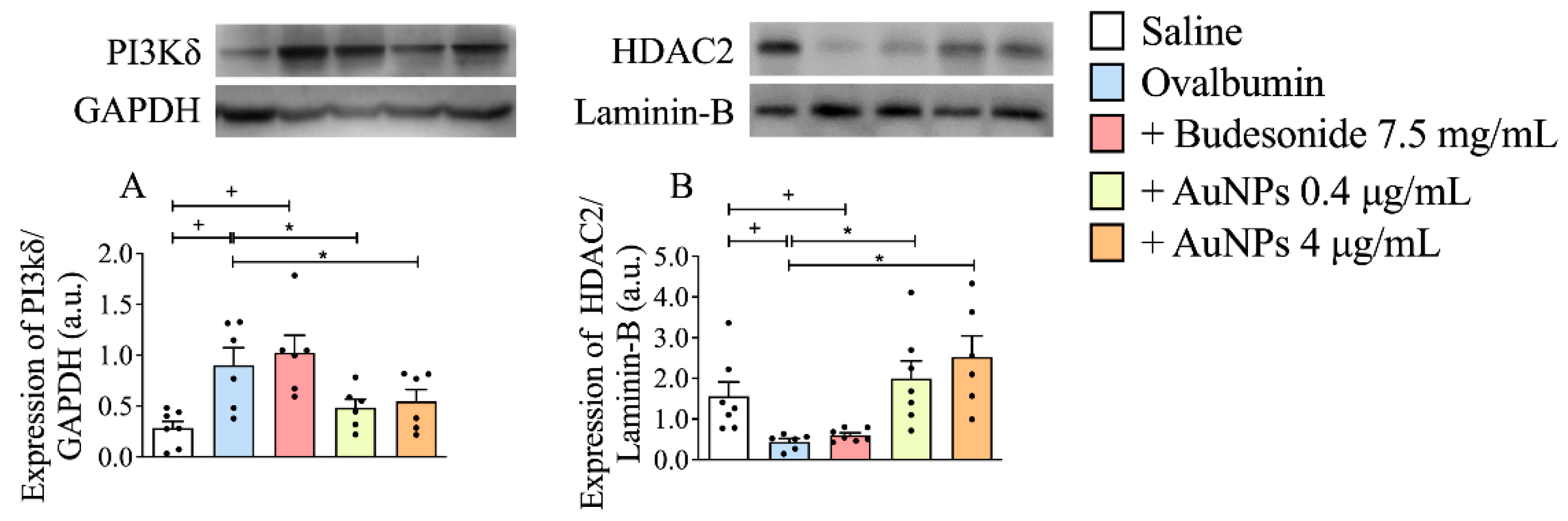
3.6. Effect of AuNPs on the Activated PI3Kδ/HDAC2 Pathway following Long-Term Allergen Exposure in A/J Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma (Gina) Strategy 2021—Executive Summary and Rationale for Key Changes. Am. J. Respir. Crit. Care Med. 2021, 205, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Wendell, S.G.; Fan, H.; Zhang, C. G Protein—Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020, 72, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Kean, W.F.; Kean, I. Clinical pharmacology of gold. Inflammopharmacology 2008, 16, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Hartung, E.F. The Treatment of Rheumatoid Arthritis Including Gold Salts Therapy. Bull. N. Y. Acad. Med. 1943, 19, 693–703. [Google Scholar] [PubMed]
- Trávníček, Z.; Starha, P.; Vančo, J.; Šilha, T.; Hošek, J.; Suchý, P.; Pražanová, G. Anti-inflammatory Active Gold(I) Complexes Involving 6-Substituted-Purine Derivatives. J. Med. Chem. 2012, 55, 4568–4579. [Google Scholar] [CrossRef]
- Pison, U.; Welte, T.; Giersig, M.; Groneberg, D.A. Nanomedicine for respiratory diseases. Eur. J. Pharmacol. 2006, 533, 341–350. [Google Scholar] [CrossRef]
- Bahadori, M.; Mohammadi, F. Nanomedicine for Respiratory Diseases. Tanaffos 2012, 11, 18–22. [Google Scholar]
- Almeida, J.P.M.; Figueroa, E.R.; Drezek, R.A. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine 2014, 10, 503–514. [Google Scholar] [CrossRef]
- Bartczak, D.; Muskens, O.L.; Sanchez-Elsner, T.; Kanaras, A.G.; Millar, T.M. Manipulation of in Vitro Angiogenesis Using Peptide-Coated Gold Nanoparticles. ACS Nano 2013, 7, 5628–5636. [Google Scholar] [CrossRef]
- Di Bella, D.; Ferreira, J.P.S.; Silva, R.D.N.O.; Echem, C.; Milan, A.; Akamine, E.H.; Carvalho, M.H.; Rodrigues, S.F. Gold nanoparticles reduce inflammation in cerebral microvessels of mice with sepsis. J. Nanobiotechnol. 2021, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Kim, H.J.; Ha, Y.-J.; Park, Y.N.; Lee, S.-K.; Park, Y.-B.; Yoo, K.-H. Targeted Chemo-Photothermal Treatments of Rheumatoid Arthritis Using Gold Half-Shell Multifunctional Nanoparticles. ACS Nano 2013, 7, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.; Li, R.; Butler, B.; Burg, A.; Tse, H.; Larson-Casey, J.; Carter, A.; Wright, C.; Rogers, L.; Tipple, T. Auranofin-Mediated NRF2 Induction Attenuates Interleukin 1 Beta Expression in Alveolar Macrophages. Antioxidants 2021, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, J.; Qu, X. Nano-Gold as Artificial Enzymes: Hidden Talents. Adv. Mater. 2014, 26, 4200–4217. [Google Scholar] [CrossRef]
- Barreto, E.; Serra, M.F.; Santos, R.V.D.; Santos, C.E.D.; Hickmann, J.; Cotias, A.C.; Pao, C.R.; Trindade, S.G.; Schimidt, V.; Giacomelli, C.; et al. Local Administration of Gold Nanoparticles Prevents Pivotal Pathological Changes in Murine Models of Atopic Asthma. J. Biomed. Nanotechnol. 2015, 11, 1038–1050. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Monsalves-Alvarez, M.; Henriquez, S.; Llanos, M.N.; Troncoso, R. Glucocorticoid resistance in chronic diseases. Steroids 2016, 115, 182–192. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013, 131, 636–645. [Google Scholar] [CrossRef]
- Olsen, P.; Ferreira, T.P.T.; Serra, M.F.; Farias-Filho, F.A.; Fonseca, B.D.P.; Viola, J.P.B.; Cordeiro, R.S.B.; Silva, P.M.R.; Costa, J.C.S.; Martins, M.A. Lidocaine-derivative JMF2-1 prevents ovalbumin-induced airway inflammation by regulating the function and survival of T cells. Clin. Exp. Allergy 2011, 41, 250–259. [Google Scholar] [CrossRef]
- Serra, M.F.; Anjos-Valotta, E.A.; Olsen, P.; Couto, G.C.; Jurgilas, P.B.; Cotias, A.C.; Pão, C.R.; Ferreira, T.P.T.; Arantes, A.C.S.; Pires, A.L.A.; et al. Nebulized Lidocaine Prevents Airway Inflammation, Peribronchial Fibrosis, and Mucus Production in a Murine Model of Asthma. Anesthesiology 2012, 117, 580–591. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. [Google Scholar]
- Draper, H.H.; Hadley, M. Malondialdehyde Determination as Index of Lipid Peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; pp. 421–431. [Google Scholar]
- Serra, M.F.; Cotias, A.C.; Pão, C.R.R.; Daleprane, J.B.; Jurgilas, P.B.; Couto, G.C.; Anjos-Valotta, E.A.; Cordeiro, R.S.B.; Carvalho, V.F.; Silva, P.M.R.; et al. Repeated Allergen Exposure in A/J Mice Causes Steroid-Insensitive Asthma via a Defect in Glucocorticoid Receptor Bioavailability. J. Immunol. 2018, 201, 851–860. [Google Scholar] [CrossRef]
- Amelink, M.; de Groot, J.C.; de Nijs, S.B.; Lutter, R.; Zwinderman, A.H.; Sterk, P.J.; Brinke, A.T.; Bel, E.H. Severe adult-onset asthma: A distinct phenotype. J. Allergy Clin. Immunol. 2013, 132, 336–341. [Google Scholar] [CrossRef]
- Wenzel, S. Severe asthma: From characteristics to phenotypes to endotypes. Clin. Exp. Allergy 2012, 42, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.M. Do Mouse Models of Allergic Asthma Mimic Clinical Disease? Int. Arch. Allergy Immunol. 2004, 133, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Gonzalo, J.-A.; Coyle, A.J.; Gutierrez-Ramos, J.-C. Mouse models of allergic airway disease. Adv. Immunol. 2001, 77, 263–295. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, K.; Kojima, M. Mouse Model of Airway Remodeling: Strain Differences. Am. J. Respir. Crit. Care Med. 2003, 168, 959–967. [Google Scholar] [CrossRef]
- Ostroukhova, M.; Seguin-Devaux, C.; Oriss, T.B.; Dixon-McCarthy, B.; Yang, L.; Ameredes, B.T.; Corcoran, T.E.; Ray, A. Tolerance Induced by Inhaled Antigen Involves Cd4(+) T Cells Expressing Membrane-Bound Tgf-Beta and Foxp3. J. Clin. Investig. 2004, 114, 28–38. [Google Scholar] [CrossRef]
- Lewkowich, I.P.; Herman, N.S.; Schleifer, K.W.; Dance, M.P.; Chen, B.L.; Dienger, K.M.; Sproles, A.A.; Shah, J.S.; Köhl, J.; Belkaid, Y.; et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 2005, 202, 1549–1561. [Google Scholar] [CrossRef]
- Azevedo, C.T.; Cotias, A.C.; Arantes, A.C.S.; Ferreira, T.P.T.; Martins, M.A.; Olsen, P.C. Assessment of Allergen-Responsive Regulatory T Cells in Experimental Asthma Induced in Different Mouse Strains. Mediat. Inflamm. 2021, 2021, 7584483. [Google Scholar] [CrossRef]
- Reichardt, S.D.; Amouret, A.; Muzzi, C.; Vettorazzi, S.; Tuckermann, J.P.; Lühder, F.; Reichardt, H.M. The Role of Glucocorticoids in Inflammatory Diseases. Cells 2021, 10, 2921. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just TH2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef]
- Lewis, B.W.; Ford, M.L.; Rogers, L.K.; Britt, R.D. Oxidative Stress Promotes Corticosteroid Insensitivity in Asthma and COPD. Antioxidants 2021, 10, 1335. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.R.; Marshall, R.P.; Warnock, L.C.; Bolton, S.; Hastie, A.; Symon, F.; Hargadon, B.; Marshall, H.; Richardson, M.; Brightling, C.E.; et al. Responsiveness to oral prednisolone in severe asthma is related to the degree of eosinophilic airway inflammation. Clin. Exp. Allergy 2017, 47, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Busacker, A.; Balzar, S.; Trudeau, J.; Wenzel, S.E. Distinguishing severe asthma phenotypes: Role of age at onset and eosinophilic inflammation. J. Allergy Clin. Immunol. 2004, 113, 101–108. [Google Scholar] [CrossRef]
- Panettieri, R.A. The Role of Neutrophils in Asthma. Immunol. Allergy Clin. N. Am. 2018, 38, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Crotty, T.B.; Kephart, G.M.; Hyma, B.A.; Colby, T.V.; Reed, C.E.; Hunt, L.W.; Gleich, G.J. Sudden-Onset Fatal Asthma: A Distinct Entity with Few Eosinophils and Relatively More Neutrophils in the Airway Submucosa? Am. Rev. Respir. Dis. 1993, 148, 713–719. [Google Scholar] [CrossRef]
- Ueda, K.; Nishimoto, Y.; Kimura, G.; Masuko, T.; Barnes, P.J.; Ito, K.; Kizawa, Y. Repeated lipopolysaccharide exposure causes corticosteroid insensitive airway inflammation via activation of phosphoinositide-3-kinase δ pathway. Biochem. Biophys. Rep. 2016, 7, 367–373. [Google Scholar] [CrossRef][Green Version]
- Kimura, G.; Ueda, K.; Eto, S.; Watanabe, Y.; Masuko, T.; Kusama, T.; Barnes, P.J.; Ito, K.; Kizawa, Y. Toll-like Receptor 3 Stimulation Causes Corticosteroid-Refractory Airway Neutrophilia and Hyperresponsiveness in Mice. Chest 2013, 144, 99–105. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Shiau, A.-L.; Chen, S.-Y.; Chen, Y.-H.; Cheng, P.-C.; Chang, M.-Y.; Chen, D.-H.; Chou, C.-H.; Wang, C.-R.; Wu, C.-L. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 2007, 56, 544–554. [Google Scholar] [CrossRef]
- Lee, H.; Lee, M.-Y.; Bhang, S.H.; Kim, B.-S.; Kim, Y.S.; Ju, J.H.; Kim, K.S.; Hahn, S.K. Hyaluronate–Gold Nanoparticle/Tocilizumab Complex for the Treatment of Rheumatoid Arthritis. ACS Nano 2014, 8, 4790–4798. [Google Scholar] [CrossRef]
- Rossios, C.; To, Y.; Osoata, G.; Ito, M.; Barnes, P.; Ito, K. Corticosteroid insensitivity is reversed by formoterol via phosphoinositide-3-kinase inhibition. Br. J. Pharmacol. 2012, 167, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.; Erzurum, S.C. Redox Control of Asthma: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2010, 12, 93–124. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.M.; Jones, D.P.; Brown, L.A.S. Glutathione Redox Control of Asthma: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2012, 17, 375–408. [Google Scholar] [CrossRef]
- Boutten, A.; Goven, D.; Artaud-Macari, E.; Boczkowski, J.; Bonay, M. NRF2 targeting: A promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol. Med. 2011, 17, 363–371. [Google Scholar] [CrossRef]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N.; et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005, 202, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanoparticle Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Bajak, E.; Fabbri, M.; Ponti, J.; Gioria, S.; Ojea-Jiménez, I.; Collotta, A.; Mariani, V.; Gilliland, D.; Rossi, F.; Gribaldo, L. Changes in Caco-2 cells transcriptome profiles upon exposure to gold nanoparticles. Toxicol. Lett. 2015, 233, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-B.; Lai, T.-H.; Shieh, J.-M.; Tsou, C.-J. Gold nanoparticles induce heme oxygenase-1 expression through Nrf2 activation and Bach1 export in human vascular endothelial cells. Int. J. Nanomed. 2015, 10, 5925–5939. [Google Scholar] [CrossRef]
- Goldstein, A.; Soroka, Y.; Frušić-Zlotkin, M.; Lewis, A.; Kohen, R. The bright side of plasmonic gold nanoparticles; activation of Nrf2, the cellular protective pathway. Nanoscale 2016, 8, 11748–11759. [Google Scholar] [CrossRef]
- Lai, T.; Wu, M.; Zhang, C.; Che, L.; Xu, F.; Wang, Y.; Wu, Y.; Xuan, N.; Cao, C.; Du, X.; et al. HDAC2 attenuates airway inflammation by suppressing IL-17A production in HDM-challenged mice. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L269–L279. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Han, S.G.; Lee, J.S.; Ahn, K.; Kim, Y.S.; Kim, J.K.; Lee, J.H.; Shin, J.H.; Jeon, K.S.; Cho, W.S.; Song, N.W.; et al. Size-dependent clearance of gold nanoparticles from lungs of Sprague–Dawley rats after short-term inhalation exposure. Arch. Toxicol. 2015, 89, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Hung, Y.-C.; Liau, I.; Huang, G.S. Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, M.F.; Cotias, A.C.; Pimentel, A.S.; Arantes, A.C.S.d.; Pires, A.L.A.; Lanzetti, M.; Hickmann, J.M.; Barreto, E.; Carvalho, V.F.; Silva, P.M.R.e.; et al. Gold Nanoparticles Inhibit Steroid-Insensitive Asthma in Mice Preserving Histone Deacetylase 2 and NRF2 Pathways. Antioxidants 2022, 11, 1659. https://doi.org/10.3390/antiox11091659
Serra MF, Cotias AC, Pimentel AS, Arantes ACSd, Pires ALA, Lanzetti M, Hickmann JM, Barreto E, Carvalho VF, Silva PMRe, et al. Gold Nanoparticles Inhibit Steroid-Insensitive Asthma in Mice Preserving Histone Deacetylase 2 and NRF2 Pathways. Antioxidants. 2022; 11(9):1659. https://doi.org/10.3390/antiox11091659
Chicago/Turabian StyleSerra, Magda F., Amanda C. Cotias, Andreza S. Pimentel, Ana Carolina S. de Arantes, Ana Lucia A. Pires, Manuella Lanzetti, Jandir M. Hickmann, Emiliano Barreto, Vinicius F. Carvalho, Patrícia M. R. e Silva, and et al. 2022. "Gold Nanoparticles Inhibit Steroid-Insensitive Asthma in Mice Preserving Histone Deacetylase 2 and NRF2 Pathways" Antioxidants 11, no. 9: 1659. https://doi.org/10.3390/antiox11091659
APA StyleSerra, M. F., Cotias, A. C., Pimentel, A. S., Arantes, A. C. S. d., Pires, A. L. A., Lanzetti, M., Hickmann, J. M., Barreto, E., Carvalho, V. F., Silva, P. M. R. e., Cordeiro, R. S. B., & Martins, M. A. (2022). Gold Nanoparticles Inhibit Steroid-Insensitive Asthma in Mice Preserving Histone Deacetylase 2 and NRF2 Pathways. Antioxidants, 11(9), 1659. https://doi.org/10.3390/antiox11091659