AMPK-Nrf2 Signaling Pathway in Phrenic Motoneurons following Cervical Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Groups and Surgical Preparation
2.3. Tissue Harvesting and Sample Processing
2.4. Immunohistochemistry and In Situ Enzymatic Reaction
2.5. Protein Extraction and Western Blotting
2.6. Data Analyses and Statistics
3. Results
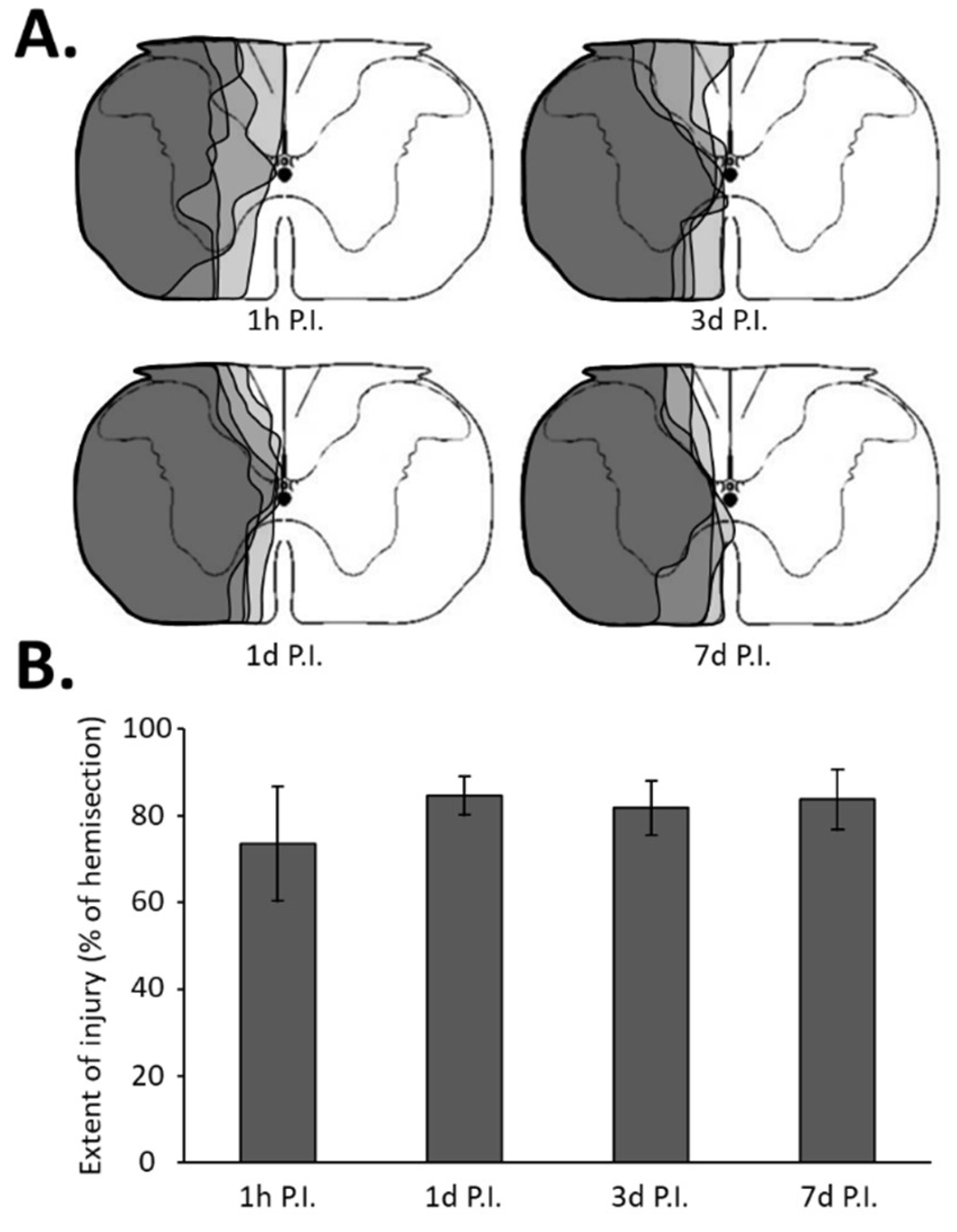
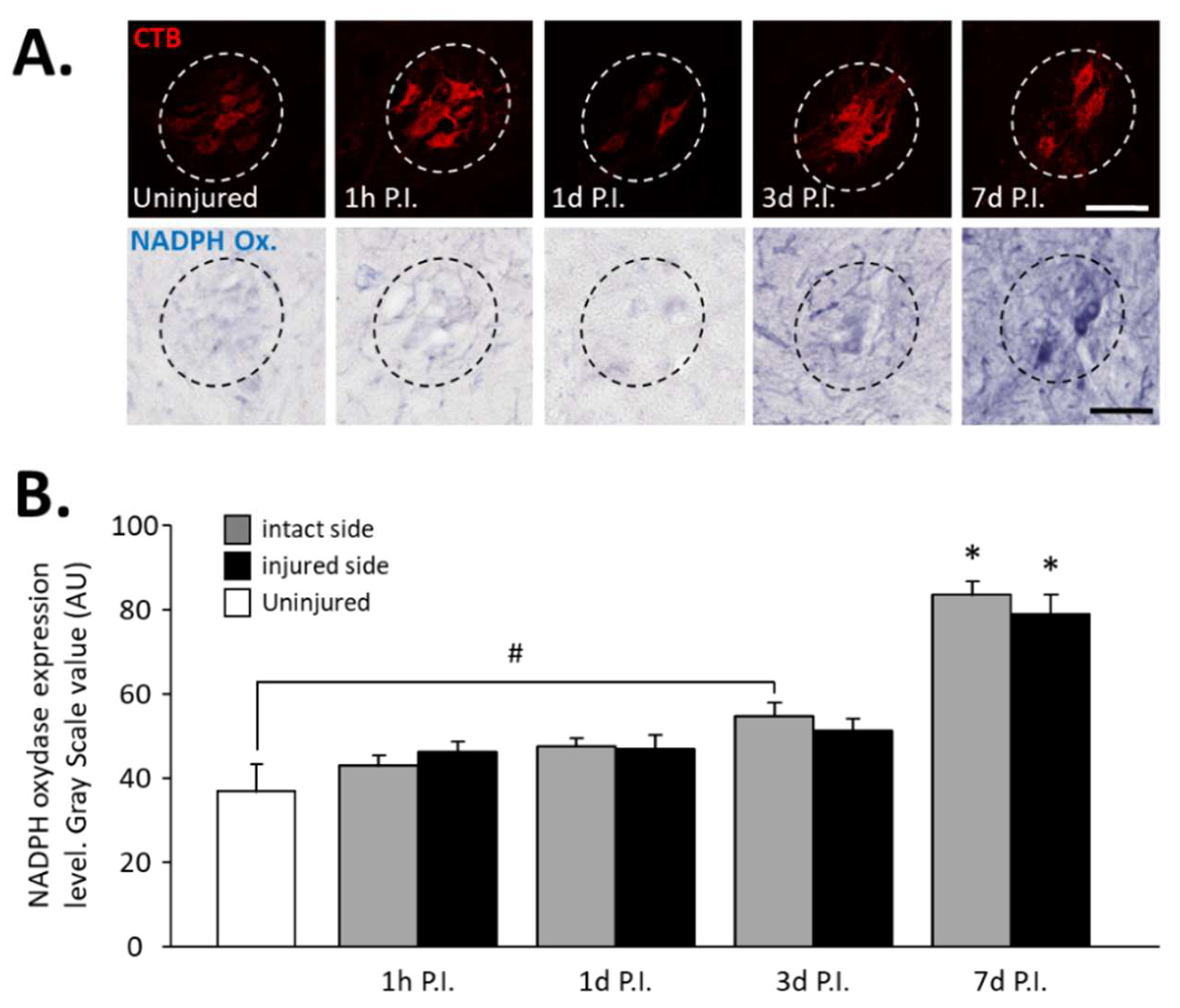
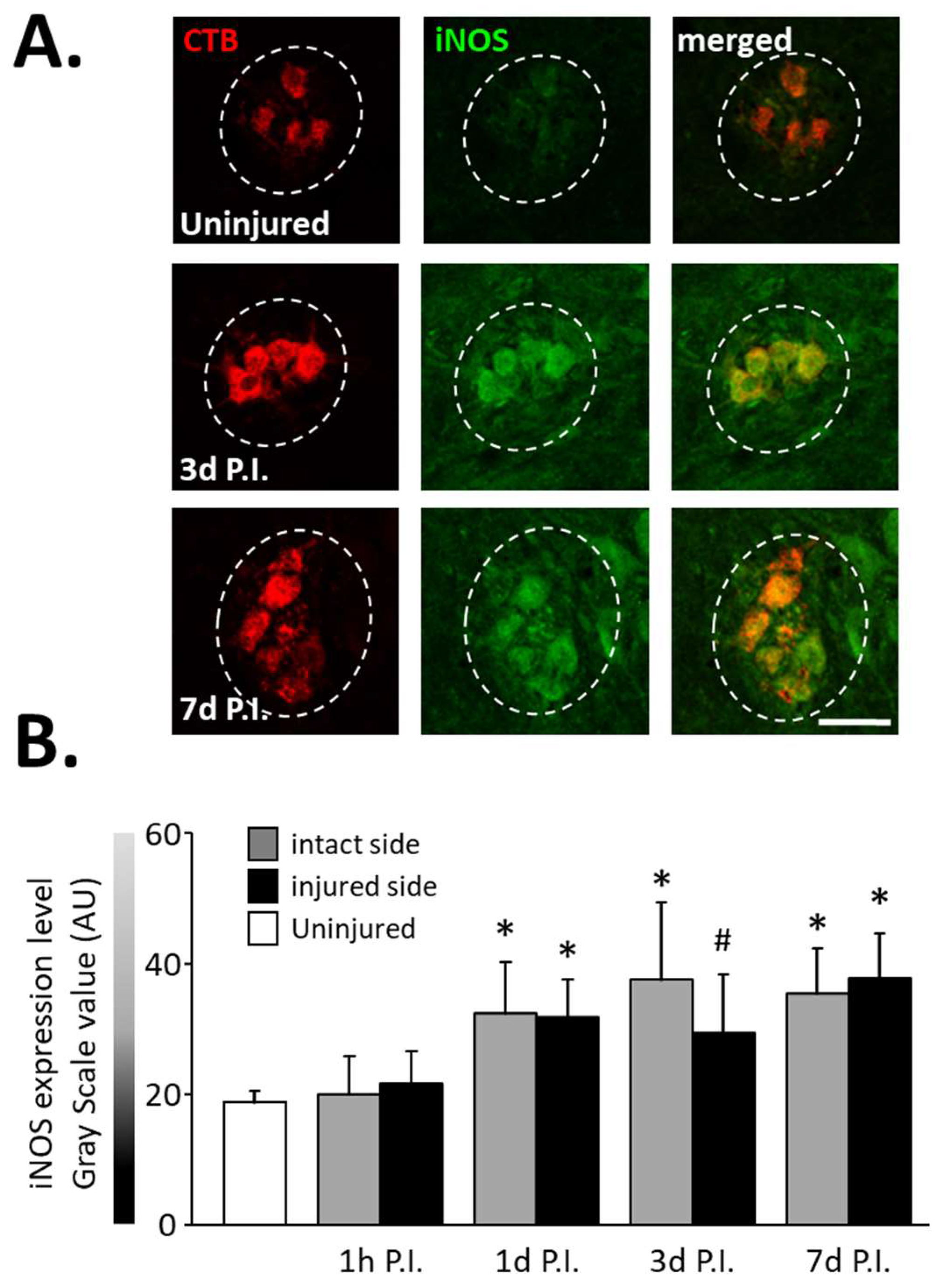
3.1. Inflammatory Processes in Phrenic Motoneurons
3.2. Effect of SCI on AMPK Phosphorylation
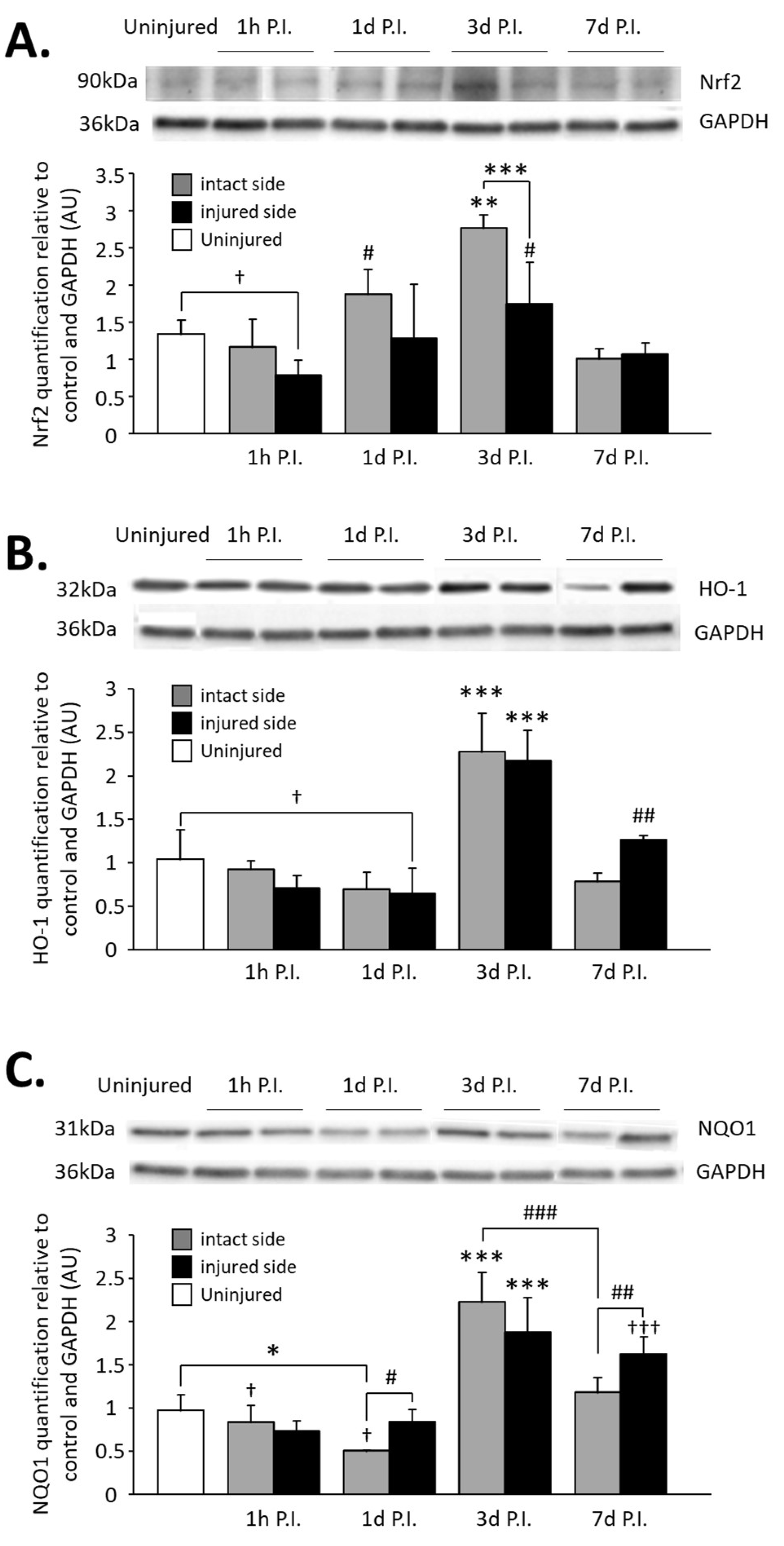
3.3. Impact on Nrf2 Expression in Phrenic Motoneurons
3.4. Impact on Nrf2 Signaling Pathway in the C3–C6 Spinal Cord
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winslow, C.; Rozovsky, J. Effect of spinal cord injury on the respiratory system. Am. J. Phys. Med. Rehabil. 2003, 82, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Nantwi, K.D.; El-Bohy, A.A.; Schrimsher, G.W.; Reier, P.J.; Goshgarian, H.G. Spontaneous Functional Recovery in a Paralyzed Hemidiaphragm Following Upper Cervical Spinal Cord Injury in Adult Rats. Neurorehabilit. Neural Repair 1999, 13, 225–234. [Google Scholar] [CrossRef]
- Golder, F.J.; Reier, P.J.; Bolser, D.C. Altered respiratory motor drive after spinal cord injury: Supraspinal and bilateral effects of a unilateral lesion. J. Neurosci. 2001, 21, 8680–8689. [Google Scholar] [CrossRef] [PubMed]
- Goshgarian, H.G. Invited Review: The crossed phrenic phenomenon: A model for plasticity in the respiratory pathways following spinal cord injury. J. Appl. Physiol. 2003, 94, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.A.; Lee, K.-Z.; Fuller, D.D.; Reier, P.J. Spinal circuitry and respiratory recovery following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Vinit, S.; Gauthier, P.; Stamegna, J.C.; Kastner, A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J. Neurotrauma 2006, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Keomani, E.; Deramaudt, T.B.; Petitjean, M.; Bonay, M.; Lofaso, F.; Vinit, S. A murine model of cervical spinal cord injury to study post-lesional respiratory neuroplasticity. J. Vis. Exp. 2014, 87, e51235. [Google Scholar] [CrossRef]
- Porter, W.T. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J. Physiol. 1895, 17, 455–485. [Google Scholar] [CrossRef]
- Michel-Flutot, P.; Jesus, I.; Vanhee, V.; Bourcier, C.H.; Emam, L.; Ouguerroudj, A.; Lee, K.-Z.; Zholudeva, L.V.; Lane, M.A.; Mansart, A.; et al. Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats. Biology 2022, 11, 473. [Google Scholar] [CrossRef]
- Feldman, J.L.; Del Negro, C.A.; Gray, P.A. Understanding the rhythm of breathing: So near, yet so far. Annu. Rev. Physiol. 2013, 75, 423–452. [Google Scholar] [CrossRef] [Green Version]
- Gandevia, S.C.; Rothwell, J.C. Activation of the human diaphragm from the motor cortex. J. Physiol. 1987, 384, 109–118. [Google Scholar] [CrossRef]
- Feldman, J.L.; Loewy, A.D.; Speck, D.F. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: An autoradiographic study. J. Neurosci. 1985, 5, 1993–2000. [Google Scholar] [CrossRef]
- Lipski, J.; Bektas, A.; Porter, R. Short latency inputs to phrenic motoneurones from the sensorimotor cortex in the cat. Exp. Brain Res. 1986, 61, 280–290. [Google Scholar] [CrossRef]
- Alexandrov, V.G.; Ivanova, T.G.; Alexandrova, N.P. Prefrontal control of respiration. J. Physiol. Pharmacol. 2007, 58 (Suppl. 5), 17–23. [Google Scholar]
- Vandeweerd, J.-M.; Hontoir, F.; De Knoop, A.; De Swert, K.; Nicaise, C. Retrograde Neuroanatomical Tracing of Phrenic Motor Neurons in Mice. J. Vis. Exp. 2018, 132, e56758. [Google Scholar] [CrossRef]
- Lane, M.A.; White, T.E.; Coutts, M.A.; Jones, A.L.; Sandhu, M.S.; Bloom, D.C.; Bolser, D.C.; Yates, B.J.; Fuller, D.D.; Reier, P.J. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J. Comp. Neurol. 2008, 511, 692–709. [Google Scholar] [CrossRef]
- Lane, M.A. Spinal respiratory motoneurons and interneurons. Respir. Physiol. Neurobiol. 2011, 179, 3–13. [Google Scholar] [CrossRef]
- Satkunendrarajah, K.; Karadimas, S.K.; Laliberte, A.M.; Montandon, G.; Fehlings, M.G. Cervical excitatory neurons sustain breathing after spinal cord injury. Nature 2018, 562, 419–422. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflamm. 2021, 18, 284. [Google Scholar] [CrossRef]
- Windelborn, J.A.; Mitchell, G.S. Glial activation in the spinal ventral horn caudal to cervical injury. Respir. Physiol. Neurobiol. 2012, 180, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulsebosch, C.E. Recent advances in pathophysiology and treatment of spinal cord injury. Adv. Physiol. Educ. 2002, 26, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Njapo, S.A.; Rastogi, V.; Hedna, V.S. Taming glutamate excitotoxicity: Strategic pathway modulation for neuroprotection. CNS Drugs 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Deramaudt, T.B.; Dill, C.; Bonay, M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med. Mal. Infect. 2013, 43, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Amin, F.U.; Khan, M.; Abid, M.N.; Rehman, S.U.; Kim, T.H.; Kim, M.W.; Kim, M.O. Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J. Neuroinflamm. 2016, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef]
- Samarghandian, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Khan, H.; Forouzanfar, F.; Aramjoo, H.; Farkhondeh, T. A Pivotal Role of the Nrf2 Signaling Pathway in Spinal Cord Injury: A Prospective Therapeutics Study. CNS Neurol. Disord. Drug Targets 2020, 19, 207–219. [Google Scholar] [CrossRef]
- Guo, X.; Kang, J.; Wang, Z.; Wang, Y.; Liu, M.; Zhu, D.; Yang, F.; Kang, X. Nrf2 signaling in the oxidative stress response after spinal cord injury. Neuroscience 2022, 498, 311–324. [Google Scholar] [CrossRef]
- Jiang, T.; He, Y. Recent Advances in the Role of Nuclear Factor Erythroid-2-Related Factor 2 in Spinal Cord Injury: Regulatory Mechanisms and Therapeutic Options. Front. Aging Neurosci. 2022, 14, 549. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Ali, M.; Bonay, M.; Vanhee, V.; Vinit, S.; Deramaudt, T.B. Comparative effectiveness of 4 natural and chemical activators of Nrf2 on inflammation, oxidative stress, macrophage polarization, and bactericidal activity in an in vitro macrophage infection model. PLoS ONE 2020, 15, e0234484. [Google Scholar] [CrossRef]
- Shao, Z.; Lv, G.; Wen, P.; Cao, Y.; Yu, D.; Lu, Y.; Li, G.; Su, Z.; Teng, P.; Gao, K.; et al. Silencing of PHLPP1 promotes neuronal apoptosis and inhibits functional recovery after spinal cord injury in mice. Life Sci. 2018, 209, 291–299. [Google Scholar] [CrossRef]
- Mao, L.; Wang, H.; Wang, X.; Liao, H.; Zhao, X. Transcription factor Nrf2 protects the spinal cord from inflammation produced by spinal cord injury. J. Surg. Res. 2011, 170, e105–e115. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Xu, L.; Wu, D.; Wu, H.; Li, C.Y. Reduced Nrf2 and Phase II enzymes expression in immune-mediated spinal cord motor neuron injury. Neurol. Res. 2010, 32, 460–465. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success after Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Zivkovic, S.; Ayazi, M.; Hammel, G.; Ren, Y. For Better or for Worse: A Look into Neutrophils in Traumatic Spinal Cord Injury. Front. Cell. Neurosci. 2021, 15, 648076. [Google Scholar] [CrossRef]
- Mantilla, C.B.; Zhan, W.Z.; Sieck, G.C. Retrograde labeling of phrenic motoneurons by intrapleural injection. J. Neurosci. Methods 2009, 182, 244–249. [Google Scholar] [CrossRef] [Green Version]
- MacFarlane, P.M.; Vinit, S.; Mitchell, G.S. Spinal nNOS regulates phrenic motor facilitation by a 5-HT2B receptor- and NADPH oxidase-dependent mechanism. Neuroscience 2014, 269, 67–78. [Google Scholar] [CrossRef]
- Nichols, N.L.; Vinit, S.; Bauernschmidt, L.; Mitchell, G.S. Respiratory function after selective respiratory motor neuron death from intrapleural CTB-saporin injections. Exp. Neurol. 2014, 267, 18–29. [Google Scholar] [CrossRef]
- MacFarlane, P.M.; Vinit, S.; Mitchell, G.S. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: Differential requirement for spinal NADPH oxidase activity. Neuroscience 2011, 178, 45–55. [Google Scholar] [CrossRef]
- Fuller, D.D.; Sandhu, M.S.; Doperalski, N.J.; Lane, M.A.; White, T.E.; Bishop, M.D.; Reier, P.J. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir. Physiol. Neurobiol. 2009, 165, 245–253. [Google Scholar] [CrossRef]
- Bowes, A.L.; Yip, P.K. Modulating inflammatory cell responses to spinal cord injury: All in good time. J. Neurotrauma 2014, 31, 1753–1766. [Google Scholar] [CrossRef]
- Rana, S.; Zhan, W.-Z.; Sieck, G.C.; Mantilla, C.B. Cervical spinal hemisection alters phrenic motor neuron glutamatergic mRNA receptor expression. Exp. Neurol. 2022, 353, 114030. [Google Scholar] [CrossRef]
- Allen, L.L.; Seven, Y.B.; Baker, T.L.; Mitchell, G.S. Cervical spinal contusion alters Na(+)-K(+)-2Cl- and K(+)-Cl- cation-chloride cotransporter expression in phrenic motor neurons. Respir. Physiol. Neurobiol. 2019, 261, 15–23. [Google Scholar] [CrossRef]
- Allen, L.L.; Nichols, N.L.; Asa, Z.A.; Emery, A.T.; Ciesla, M.C.; Santiago, J.V.; Holland, A.E.; Mitchell, G.S.; Gonzalez-Rothi, E.J. Phrenic motor neuron survival below cervical spinal cord hemisection. Exp. Neurol. 2021, 346, 113832. [Google Scholar] [CrossRef]
- Djakovic, S.N.; Schwarz, L.A.; Barylko, B.; DeMartino, G.N.; Patrick, G.N. Regulation of the Proteasome by Neuronal Activity and Calcium/Calmodulin-dependent Protein Kinase II*. J. Biol. Chem. 2009, 284, 26655–26665. [Google Scholar] [CrossRef]
- Ditunno, J.F.; Little, J.W.; Tessler, A.; Burns, A.S. Spinal shock revisited: A four-phase model. Spinal Cord 2004, 42, 383–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Chen, H.; Zhuang, R.; Zhang, H.; Wang, Y.; Hu, X.; Xu, Y.; Li, J.; Li, Y.; Wang, X.; et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int. J. Biol. Sci. 2021, 17, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, S.; Gao, K.; Zhou, Z.; Wang, C.; Shen, Z.; Guo, Y.; Li, Z.; Wan, Z.; Liu, C.; et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience 2017, 348, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Niu, J.; Dang, X. Neuroprotection of melatonin on spinal cord injury by activating autophagy and inhibiting apoptosis via SIRT1/AMPK signaling pathway. Biotechnol. Lett. 2020, 42, 2059–2069. [Google Scholar] [CrossRef]
- Lin, S.; Tian, H.; Lin, J.; Xu, C.; Yuan, Y.; Gao, S.; Song, C.; Lv, P.; Mei, X. Zinc promotes autophagy and inhibits apoptosis through AMPK/mTOR signaling pathway after spinal cord injury. Neurosci. Lett. 2020, 736, 135263. [Google Scholar] [CrossRef]
- Kong, G.; Zhou, L.; Serger, E.; Palmisano, I.; De Virgiliis, F.; Hutson, T.H.; McLachlan, E.; Freiwald, A.; La Montanara, P.; Shkura, K.; et al. AMPK controls the axonal regenerative ability of dorsal root ganglia sensory neurons after spinal cord injury. Nat. Metab. 2020, 2, 918–933. [Google Scholar] [CrossRef]
- Mao, L.; Wang, H.D.; Wang, X.L.; Tian, L.; Xu, J.Y. Disruption of Nrf2 exacerbated the damage after spinal cord injury in mice. J. Trauma Acute Care Surg. 2012, 72, 189–198. [Google Scholar] [CrossRef]
- Wang, X.; de Rivero Vaccari, J.P.; Wang, H.; Diaz, P.; German, R.; Marcillo, A.E.; Keane, R.W. Activation of the nuclear factor E2-related factor 2/antioxidant response element pathway is neuroprotective after spinal cord injury. J. Neurotrauma 2012, 29, 936–945. [Google Scholar] [CrossRef]
- Zhao, W.; Gasterich, N.; Clarner, T.; Voelz, C.; Behrens, V.; Beyer, C.; Fragoulis, A.; Zendedel, A. Astrocytic Nrf2 expression protects spinal cord from oxidative stress following spinal cord injury in a male mouse model. J. Neuroinflamm. 2022, 19, 134. [Google Scholar] [CrossRef]
- Liu, D.; Xu, G.Y.; Pan, E.; McAdoo, D.J. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience 1999, 93, 1383–1389. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Xu, Y.; Ruan, W.; Wang, H.; Zhang, Y.; Saavedra, J.M.; Zhang, L.; Huang, Z.; Pang, T. A Dual AMPK/Nrf2 Activator Reduces Brain Inflammation after Stroke by Enhancing Microglia M2 Polarization. Antioxid. Redox Signal. 2018, 28, 141–163. [Google Scholar] [CrossRef]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel-Flutot, P.; Efthimiadi, L.; Djerbal, L.; Deramaudt, T.B.; Bonay, M.; Vinit, S. AMPK-Nrf2 Signaling Pathway in Phrenic Motoneurons following Cervical Spinal Cord Injury. Antioxidants 2022, 11, 1665. https://doi.org/10.3390/antiox11091665
Michel-Flutot P, Efthimiadi L, Djerbal L, Deramaudt TB, Bonay M, Vinit S. AMPK-Nrf2 Signaling Pathway in Phrenic Motoneurons following Cervical Spinal Cord Injury. Antioxidants. 2022; 11(9):1665. https://doi.org/10.3390/antiox11091665
Chicago/Turabian StyleMichel-Flutot, Pauline, Laurie Efthimiadi, Lynda Djerbal, Therese B. Deramaudt, Marcel Bonay, and Stéphane Vinit. 2022. "AMPK-Nrf2 Signaling Pathway in Phrenic Motoneurons following Cervical Spinal Cord Injury" Antioxidants 11, no. 9: 1665. https://doi.org/10.3390/antiox11091665
APA StyleMichel-Flutot, P., Efthimiadi, L., Djerbal, L., Deramaudt, T. B., Bonay, M., & Vinit, S. (2022). AMPK-Nrf2 Signaling Pathway in Phrenic Motoneurons following Cervical Spinal Cord Injury. Antioxidants, 11(9), 1665. https://doi.org/10.3390/antiox11091665






