Mechanisms Underlying the Protective Effect of Maternal Zinc (ZnSO4 or Zn-Gly) against Heat Stress-Induced Oxidative Stress in Chicken Embryo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Birds, and Diets
2.2. Sample Collection
2.3. Assessment of the Oxidation Status
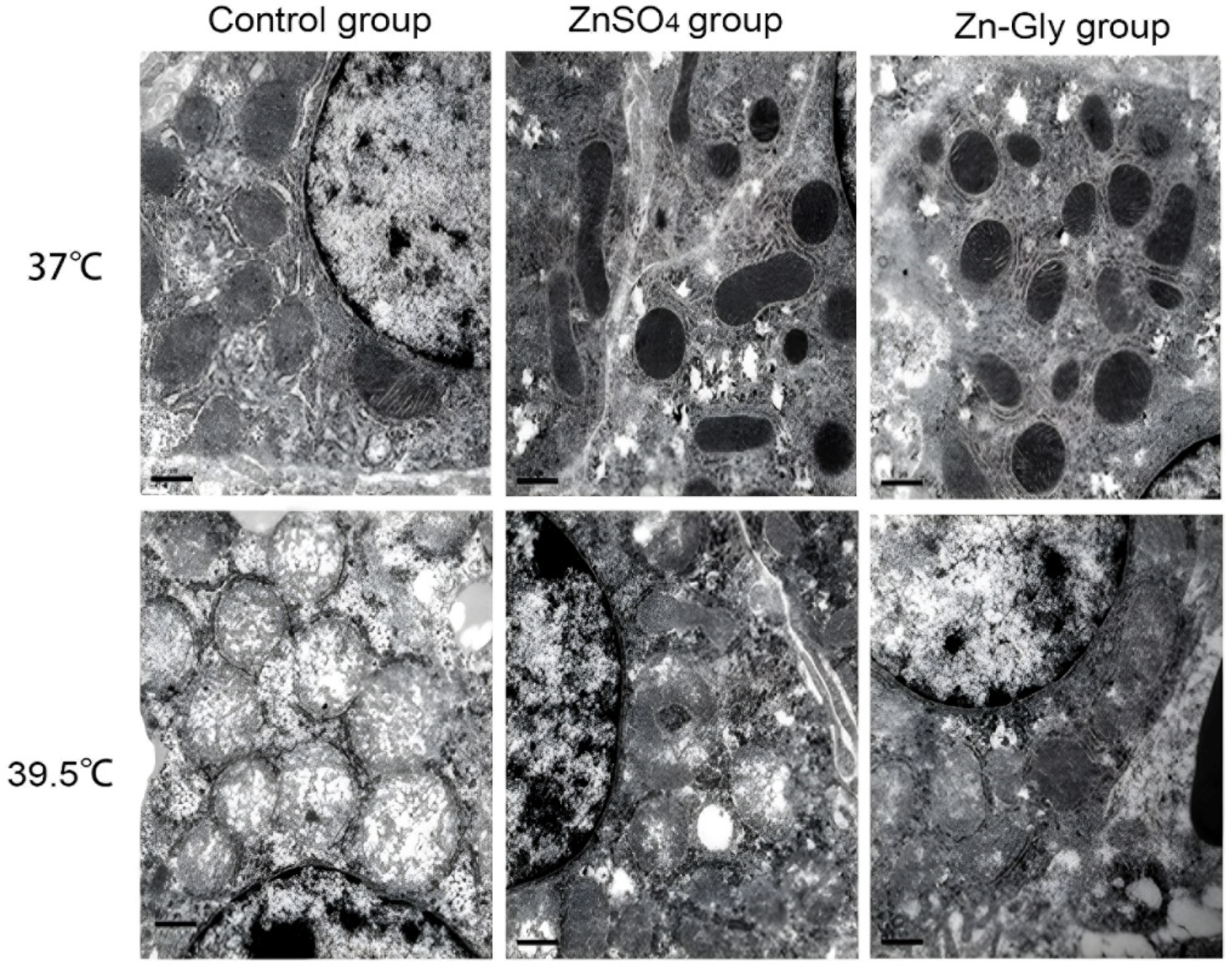
2.4. Mitochondrial Morphology
2.5. Mitochondrial Membrane Potential (MMP)
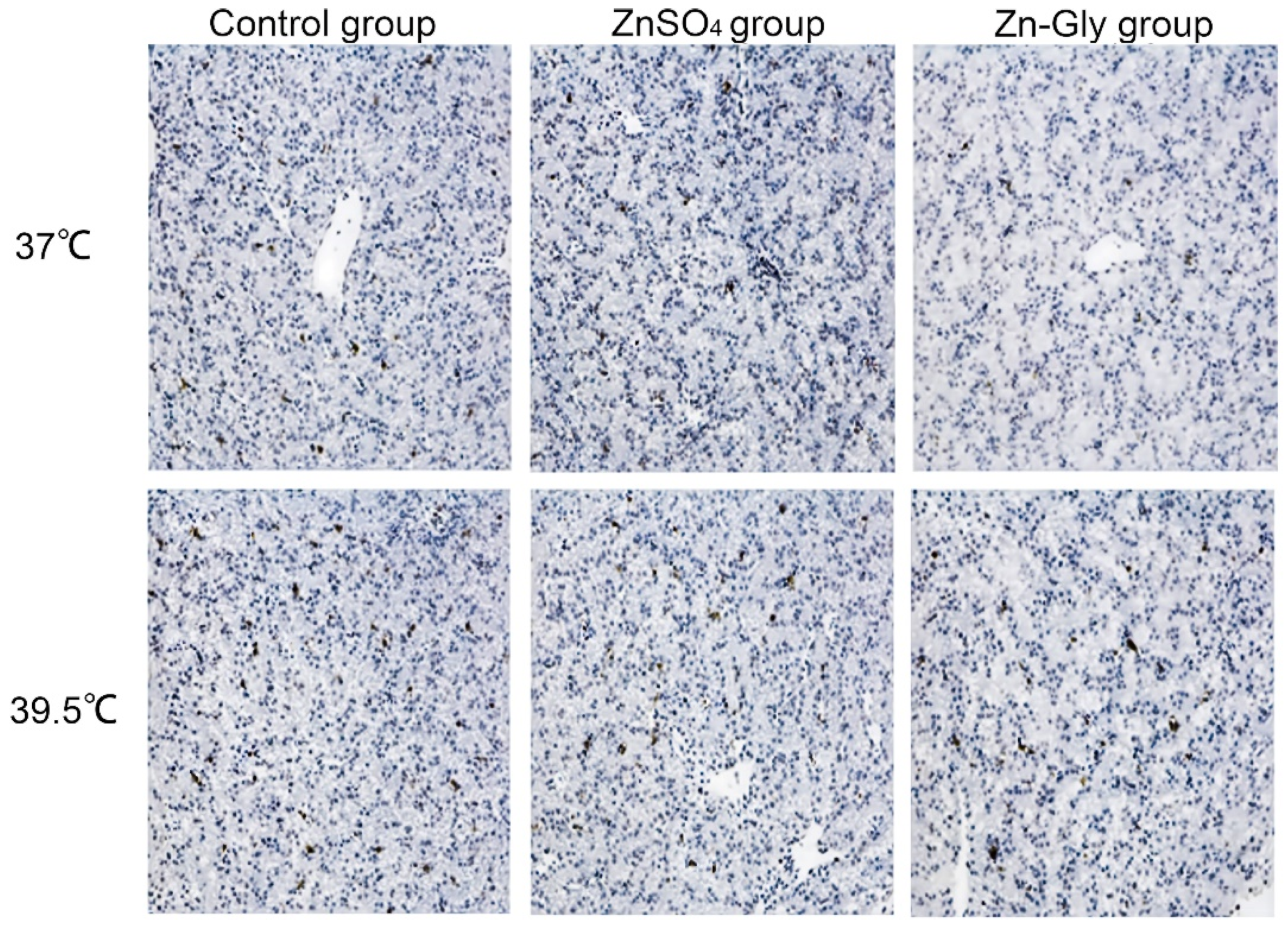
2.6. Assessment of Hepatocyte Apoptosis and Caspase3 Activity
2.7. qRT-PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Embryo Mortality
3.2. Liver Oxidation Indicators
3.3. Liver Antioxidant Indicators
3.4. Hepatocellular Mitochondrial Morphology
3.5. Hepatocellular MMP, Apoptosis index (AI), and Caspase3 Activity
3.6. Expressions of Embryonic Hepatocyte Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molenaar, R.; Hulet, R.; Meijerhof, R.; Maatjens, C.M.; Kemp, B.; van den Brand, H. High eggshell temperatures during incubation decrease growth performance and increase the incidence of ascites in broiler chickens. Poult. Sci. 2011, 90, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Jung, U.; Voy, B.; Dridi, S. Radical Response: Effects of Heat Stress-Induced Oxidative Stress on Lipid Metabolism in the Avian Liver. Antioxidants 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Vashan, S.J.; Golian, A.; Yaghobfar, A. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int. J. Biometeorol. 2016, 60, 1183–1192. [Google Scholar] [CrossRef]
- Uerlings, J.; Song, Z.G.; Hu, X.Y.; Wang, S.K.; Lin, H.; Buyse, J.; Everaert, N. Heat exposure affects jejunal tight junction remodeling independently of adenosine monophosphate-activated protein kinase in 9-day-old broiler chicks. Poult. Sci. 2018, 97, 3681–3690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.S.; Wang, Q.; Li, K.X.; Guo, T.Y.; Xiao, X.; Wang, Y.X.; Zhan, X.A. Effects of Maternal Zinc Glycine on Mortality, Zinc Concentration, and Antioxidant Status in a Developing Embryo and 1-Day-Old Chick. Biol. Trace Elem. Res. 2018, 181, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, A.; Rossi, R.; Rufini, S. Zinc status in athletes: Relation to diet and exercise. Sports Med. 2001, 31, 577–582. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Piacenza, F.; Basso, A.; Malavolta, M. Zinc, metallothioneins and immunosenescence: Effect of zinc supply as nutrigenomic approach. Biogerontology 2011, 12, 455–465. [Google Scholar] [CrossRef]
- Richards, M.P. Trace mineral metabolism in the avian embryo. Poult. Sci. 1997, 76, 152–164. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, X.; Lu, L.; Li, W.; Zhang, L.; Ji, C.; Lin, X.; Liu, H.C.; Odle, J.; Luo, X. Maternal dietary zinc supplementation enhances the epigenetic-activated antioxidant ability of chick embryos from maternal normal and high temperatures. Oncotarget 2017, 8, 19814–19824. [Google Scholar] [CrossRef]
- Star, L.; van der Klis, J.D.; Rapp, C.; Ward, T.L. Bioavailability of organic and inorganic zinc sources in male broilers. Poult. Sci. 2012, 91, 3115–3120. [Google Scholar] [CrossRef]
- Kwiecień, M.; Winiarska-Mieczan, A.; Milczarek, A.; Klebaniuk, R. Biological Response of Broiler Chickens to Decreasing Dietary Inclusion Levels of Zinc Glycine Chelate. Biol. Trace Elem. Res. 2017, 175, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Piao, Y.; Nagaoka, K.; Watanabe, G.; Taya, K.; Li, C. Protective effects of nuclear factor erythroid 2-related factor 2 on whole body heat stress-induced oxidative damage in the mouse testis. Reprod Biol. Endocrinol. 2013, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.N.; Chen, Y.; Cai, J.; Sternberg, P., Jr. Increased glutathione synthesis through an ARE-Nrf2-dependent pathway by zinc in the RPE: Implication for protection against oxidative stress. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Mackei, M.; Molnár, A.; Nagy, S.; Pál, L.; Kővágó, C.; Gálfi, P.; Dublecz, K.; Husvéth, F.; Neogrády, Z.; Mátis, G. Effects of Acute Heat Stress on a Newly Established Chicken Hepatocyte-Nonparenchymal Cell Co-Culture Model. Animals 2020, 10, 409. [Google Scholar] [CrossRef]
- Sozcu, A.; Ipek, A. Acute and chronic eggshell temperature manipulations during hatching term influence hatchability, broiler performance, and ascites incidence. Poult. Sci. 2015, 94, 319–327. [Google Scholar] [CrossRef]
- Blamberg, D.L.; Blackwood, U.B.; Supplee, W.C.; Combs, G.F. Effect of zinc deficiency in hens on hatchability and embryonic development. Proc. Soc. Exp. Biol. Med. 1960, 104, 217–220. [Google Scholar] [CrossRef]
- Willemsen, H.; Kamers, B.; Dahlke, F.; Han, H.; Song, Z.; Ansari Pirsaraei, Z.; Tona, K.; Decuypere, E.; Everaert, N. High- and low-temperature manipulation during late incubation: Effects on embryonic development, the hatching process, and metabolism in broilers. Poult. Sci. 2010, 89, 2678–2690. [Google Scholar] [CrossRef]
- Paravani, E.V.; Odetti, L.M.; Simoniello, M.F.; Poletta, G.L. Molecular analysis and bioinformatic characterization of cooper, zinc-superoxide dismutase (Cu/Zn-sod) gene of Caiman latirostris. Mol. Biol. Rep. 2020, 47, 8849–8857. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox. Signal 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rahman, M.S. Elevated seasonal temperature disrupts prooxidant-antioxidant homeostasis and promotes cellular apoptosis in the American oyster, Crassostrea virginica, in the Gulf of Mexico: A field study. Cell Stress Chaperones 2021, 26, 917–936. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur Lek. 2020, 48, 124–127. [Google Scholar]
- Attia, Y.A.; Al-Harthi, M.A.; Abo El-Maaty, H.M. The Effects of Different Oil Sources on Performance, Digestive Enzymes, Carcass Traits, Biochemical, Immunological, Antioxidant, and Morphometric Responses of Broiler Chicks. Front. Vet. Sci. 2020, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharm. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef] [PubMed]
- Galati, S.; Boni, C.; Gerra, M.C.; Lazzaretti, M.; Buschini, A. Autophagy: A Player in response to Oxidative Stress and DNA Damage. Oxid. Med. Cell Longev. 2019, 2019, 5692958. [Google Scholar] [CrossRef]
- Garbuz, D.G. Regulation of heat shock gene expression in response to stress. Mol. Biol. Mosk 2017, 51, 400–417. [Google Scholar] [CrossRef]
- Miao, Q.; Si, X.; Xie, Y.; Chen, L.; Liu, Z.; Liu, L.; Tang, X.; Zhang, H. Effects of acute heat stress at different ambient temperature on hepatic redox status in broilers. Poult. Sci. 2020, 99, 4113–4122. [Google Scholar] [CrossRef]
- Cong, X.; Zhang, Q.; Li, H.; Jiang, Z.; Cao, R.; Gao, S.; Tian, W. Puerarin ameliorates heat stress-induced oxidative damage and apoptosis in bovine Sertoli cells by suppressing ROS production and upregulating Hsp72 expression. Theriogenology 2017, 88, 215–227. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Brookes, P.S. Mitochondrial H(+) leak and ROS generation: An odd couple. Free Radic. Biol. Med. 2005, 38, 12–23. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Collins, P.; Jones, C.; Choudhury, S.; Damelin, L.; Hodgson, H. Increased expression of uncoupling protein 2 in HepG2 cells attenuates oxidative damage and apoptosis. Liver Int. 2005, 25, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, A.; de Lacoba, M.G.; Rial, E. The mitochondrial uncoupling proteins. Genome Biol. 2002, 3, 3015. [Google Scholar] [CrossRef] [PubMed]
- Dewson, G.; Kluck, R.M. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J. Cell Sci. 2009, 122 Pt 16, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, N.; Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Kang, M.I.; Kobayashi, A.; Yamamoto, M.; Kensler, T.W.; Talalay, P. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA 2004, 101, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Cheng, Y.; Wu, H.; Kong, L.; Wang, S.; Xu, Z.; Zhang, Z.; Tan, Y.; Keller, B.B.; Zhou, H.; et al. Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy. Diabetes 2017, 66, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Feng, L.; Zhou, X.Q. Copper exposure induces toxicity to the antioxidant system via the destruction of Nrf2/ARE signaling and caspase-3-regulated DNA damage in fish muscle: Amelioration by myo-inositol. Aquat. Toxicol. 2015, 159, 245–255. [Google Scholar] [CrossRef]
- Alscher, D.M.; Braun, N.; Biegger, D.; Stuelten, C.; Gawronski, K.; Mürdter, T.E.; Kuhlmann, U.; Fritz, P. Induction of metallothionein in proximal tubular cells by zinc and its potential as an endogenous antioxidant. Kidney Blood Press Res. 2005, 28, 127–133. [Google Scholar] [CrossRef]
- Suvorova, I.N.; Davydov, V.V. Age-dependent changes of superoxide dismutase, glutathione peroxidase, and catalase activity in the brain of rats during immobilized stress. Ukr. Biokhim. Zh. 1999 2004, 76, 74–78. [Google Scholar]
- Li, T.; He, W.; Liao, X.; Lin, X.; Zhang, L.; Lu, L.; Guo, Y.; Liu, Z.; Luo, X. Zinc alleviates the heat stress of primary cultured hepatocytes of broiler embryos via enhancing the antioxidant ability and attenuating the heat shock responses. Anim. Nutr. 2021, 7, 621–630. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Wang, W.; Yang, L.; Zhu, Y. The Role of Zinc in Poultry Breeder and Hen Nutrition: An Update. Biol. Trace Elem. Res. 2019, 192, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lu, L.; Liao, X.; Zhang, L.; Lin, X.; Luo, X.; Ma, Q. Effect of In Ovo Zinc Injection on the Embryonic Development and Epigenetics-Related Indices of Zinc-Deprived Broiler Breeder Eggs. Biol. Trace Elem. Res. 2018, 185, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.N.; Vieira, S.L.; Berwanger, E.; Angel, C.R.; Kindlein, L.; França, I.; Noetzold, T.L. Zinc requirements of broiler breeder hens. Poult. Sci. 2019, 98, 1288–1301. [Google Scholar] [CrossRef]
- Richards, M.P.; Steele, N.C. Trace element metabolism in the developing avian embryo: A review. J. Exp. Zool. Suppl. 1987, 1, 39–51. [Google Scholar] [PubMed]
- Dewar, W.A.; Teague, P.W.; Downie, J.N. The transfer of minerals from the egg to the chick embryo from the 5th to 18th days of incubation. Br. Poult. Sci. 1974, 15, 119–129. [Google Scholar] [CrossRef]
- Jarosz, Ł.; Marek, A.; Grądzki, Z.; Laskowska, E.; Kwiecień, M. Effect of Zinc Sulfate and Zinc Glycine Chelate on Concentrations of Acute Phase Proteins in Chicken Serum and Liver Tissue. Biol. Trace Elem. Res. 2019, 187, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Olin, K.L.; Shigenaga, M.K.; Ames, B.N.; Golub, M.S.; Gershwin, M.E.; Hendrickx, A.G.; Keen, C.L. Maternal dietary zinc influences DNA strand break and 8-hydroxy-2’-deoxyguanosine levels in infant rhesus monkey liver. Proc. Soc. Exp. Biol. Med. 1993, 203, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Olin, K.L.; Fraga, C.G.; Keen, C.L. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J. Nutr. 1995, 125, 823–829. [Google Scholar] [CrossRef]
- Liu, Z.H.; Lu, L.; Wang, R.L.; Lei, H.L.; Li, S.F.; Zhang, L.Y.; Luo, X.G. Effects of supplemental zinc source and level on antioxidant ability and fat metabolism-related enzymes of broilers. Poult. Sci. 2015, 94, 2686–2694. [Google Scholar] [CrossRef]
- Spiers, J.G.; Tan, L.S.; Anderson, S.T.; Hill, A.F.; Lavidis, N.A.; Chen, H.C. Hepatic Homeostasis of Metal Ions Following Acute Repeated Stress Exposure in Rats. Antioxidants 2021, 11, 85. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, Z.; Tuzcu, M.; Sahin, N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 2012, 50, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, L.; Li, S.F.; Zhang, L.Y.; Luo, X.G. Organic zinc absorption by the intestine of broilers in vivo. Br. J. Nutr. 2017, 117, 1086–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, R. Antioxidant activities of bile pigments. Antioxid. Redox. Signal 2004, 6, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.G.; Wen, C.; Wang, L.C.; Wang, T.; Zhou, Y.M. Effects of zinc-bearing clinoptilolite on growth performance, cecal microflora and intestinal mucosal function of broiler chickens. Anim. Feed. Sci. Technol. 2014, 189, 98–106. [Google Scholar] [CrossRef]
- Sridhar, K.; Nagalakshmi, D.; Rao, D.S.; Rao, S.V.R. Effect of graded concentration of organic Zinc (Zinc glycinate) on antioxidants status and immune response in commercial broilers. Indian J. Anim. Res. 2016, 50, 471–475. [Google Scholar] [CrossRef]
- Malyar, R.M.; Li, H.; Liu, D.; Abdulrahim, Y.; Farid, R.A.; Gan, F.; Ali, W.; Enayatullah, H.; Banuree, S.A.H.; Huang, K.; et al. Selenium/Zinc-Enriched probiotics improve serum enzyme activity, antioxidant ability, inflammatory factors and related gene expression of Wistar rats inflated under heat stress. Life Sci. 2020, 248, 117464. [Google Scholar] [CrossRef]
- Bun, S.D.; Guo, Y.M.; Guo, F.C.; Ji, F.J.; Cao, H. Influence of organic zinc supplementation on the antioxidant status and immune responses of broilers challenged with Eimeria tenella. Poult. Sci. 2011, 90, 1220–1226. [Google Scholar] [CrossRef]
- Huang, Y.L.; Lu, L.; Li, S.F.; Luo, X.G.; Liu, B. Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed a conventional corn-soybean meal diet. J. Anim. Sci. 2009, 87, 2038–2046. [Google Scholar] [CrossRef]
- Kou, H.; Hu, J.; Vijayaraman, S.B.; Wang, A.L.; Zheng, Y.; Chen, J.; He, G.; Miao, Y.; Lin, L. Evaluation of dietary zinc on antioxidant-related gene expression, antioxidant capability and immunity of soft-shelled turtles Pelodiscussinensis. Fish Shellfish. Immunol. 2021, 118, 303–312. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, Y.; Li, J.; Zhang, T.; Wen, J. Effects of Methionine Hydroxy Analog Chelated Cu/Mn/Zn on Laying Performance, Egg Quality, Enzyme Activity and Mineral Retention of Laying Hens. J. Poult. Sci. 2012, 49, 20–25. [Google Scholar] [CrossRef]
- Nitrayova, S.; Windisch, W.; von Heimendahl, E.; Müller, A.; Bartelt, J. Bioavailability of zinc from different sources in pigs. J. Anim. Sci. 2012, 90 (Suppl. 4), 185–187. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, D.; Curtis, T.; Chen, M.; Xu, H. Zinc Protects Oxidative Stress-Induced RPE Death by Reducing Mitochondrial Damage and Preventing Lysosome Rupture. Oxid. Med. Cell Longev. 2017, 2017, 6926485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamalov, G.; Deshmukh, P.A.; Baburyan, N.Y.; Gandhi, M.S.; Johnson, P.L.; Ahokas, R.A.; Bhattacharya, S.K.; Sun, Y.; Gerling, I.C.; Weber, K.T. Coupled calcium and zinc dyshomeostasis and oxidative stress in cardiac myocytes and mitochondria of rats with chronic aldosteronism. J. Cardiovasc. Pharm. 2009, 53, 414–423. [Google Scholar] [CrossRef]
- Miao, X.; Wang, Y.; Sun, J.; Sun, W.; Tan, Y.; Cai, L.; Zheng, Y.; Su, G.; Liu, Q.; Wang, Y. Zinc protects against diabetes-induced pathogenic changes in the aorta: Roles of metallothionein and nuclear factor (erythroid-derived 2)-like 2. Cardiovasc. Diabetol. 2013, 12, 54. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Cao, Y.; Li, C. Zinc might prevent heat-induced hepatic injury by activating the Nrf2-antioxidant in mice. Biol. Trace Elem. Res. 2015, 165, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Jaiswal, A.K. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007, 282, 16502–16510. [Google Scholar] [CrossRef] [Green Version]
| Ingredients (%) | g/kg of Total Diet |
|---|---|
| Maize | 646 |
| Soybean meal | 250 |
| CaHPO4 | 18 |
| Limestone | 70 |
| Salt | 3 |
| DL-methionine | 3 |
| Premix 1 | 10 |
| Nutrient Composition | |
| ME 2 (MJ/kg) | 11.20 |
| Crude protein | 161.1 |
| Calcium | 30.4 |
| Total phosphorus | 6.3 |
| Lysine | 8.0 |
| Met + Cys | 8.2 |
| Zine (mg/kg) | 24 |
| Genes | Genbank Accession | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| SOD | U28407.1 | 5’-CTGGAAATGCTGGACCTCGTTTAG-3’ | 3’-CAGCTCATTTCCCACTGCCATCTT-5’ |
| MT | X06749.1 | 5’-GAGCCGAACCGACCCGAACT-3’ | 3’-CTTGCACGACCCAGCACAGGA-5’ |
| BAX | FJ977571.1 | 5’-GGATTCTCACAGTAGGAGGATGGAT-3’ | 3’-GGCCACCAGTGAAGGCAAAC-5’ |
| UCP3 | NM_204107.1 | 5’-GCAGAGAAACAGAGCGGGATTTGA-3’ | 3’-GGCTCCTGGCTCACGGATAGA-5’ |
| Nrf2 | NM_205117.1 | 5’-GTGCCGCAGGGCAATGCTAGT-3’ | 3’-GCCAGCAGGAGGGTCTTTCTTTG-5’ |
| 18S | AF173612.1 | 5’-CCGGACACGGACAGGATTGACA-3’ | 3’-CAGACAAATCGCTCCACCAACTAAG-5’ |
| Temperature | Maternal Zinc | Late Mortality | Whole-Term Mortality |
|---|---|---|---|
| 37 °C 1 | Control | 6.00 ± 0.58 a | 8.57 ± 0.51 a |
| ZnSO4 | 5.34 ± 0.21 a | 7.55 ± 0.54 ab | |
| Zn-Gly | 3.86 ± 0.10 b | 6.02 ± 0.89 b | |
| 39.5 °C 1 | Control | 6.03 ± 0.89 a | 8.60 ± 0.97 a |
| ZnSO4 | 4.89 ± 0.80 ab | 7.53 ± 1.26 ab | |
| Zn-Gly | 3.85 ± 1.20 b | 6.01 ± 0.72 b | |
| 37 °C 2 | 5.07 ± 0.99 | 7.38 ± 1.25 | |
| 39.5 °C 2 | 4.92 ± 1.27 | 7.38 ± 1.43 | |
| Control 3 | 6.01 ± 0.68 a | 8.59 ± 0.69 a | |
| ZnSO4 3 | 5.12 ± 0.58 a | 7.54 ± 0.87 a | |
| Zn-Gly 3 | 3.86 ± 0.76 b | 6.02 ± 0.72 b | |
| p-value | |||
| Temperature | 0.687 | 0.996 | |
| Maternal zinc | 0.001 | 0.001 | |
| Temperature × maternal zinc | 0.833 | 0.999 | |
| Temperature | Maternal Zinc | ROS, nmol/mg port | MDA, nmol/mg port | PC, nmol/mg port | 8-OHdG, nmol/mg port | HSP70, ng/g |
|---|---|---|---|---|---|---|
| 37 °C 1 | Contro | 1396 ± 56.61 | 0.60 ± 0.05 bc | 0.99 ± 0.19 bc | 14.14 ± 0.91 cd | 14.14 ± 0.91 cd |
| ZnSO4 | 1325 ± 61.28 | 0.58 ± 0.02 bc | 0.88 ± 0.13 cd | 14.09 ± 0.52 cd | 14.09 ± 0.52 cd | |
| Zn-Gly | 1196 ± 52.02 | 0.53 ± 0.07 c | 0.71 ± 0.05 d | 13.08 ± 0.45 d | 13.08 ± 0.45 d | |
| 39.5 °C 1 | Control | 1553 ± 48.64 | 0.97 ± 0.13 a | 1.49 ± 0.14 a | 33.08 ± 1.12 a | 33.08 ± 1.12 a |
| ZnSO4 | 1544 ± 32.14 | 0.69 ± 0.01 b | 1.17 ± 0.00 b | 22.66 ± 0.84 b | 22.66 ± 0.84 b | |
| Zn-Gly | 1495 ± 74.95 | 0.56 ± 0.04 c | 1.0 ± 0.04 bc | 15.16 ± 1.45 c | 15.16 ± 1.45 c | |
| 37 °C 2 | 1295 ± 102.68 b | 0.57 ± 0.05 b | 0.86 ± 0.17 b | 13.77 ± 0.78 b | 13.77 ± 0.78 b | |
| 39.5 °C 2 | 1531 ± 54.74 a | 0.74 ± 0.19 a | 1.22 ± 0.23 a | 23.64 ± 7.74 a | 23.64 ± 7.74 a | |
| Control 3 | 1475 ± 97.61 a | 0.78 ± 0.22 a | 1.24 ± 0.31 a | 23.61 ± 10.17 a | 23.61 ± 10.17 a | |
| ZnSO4 3 | 1436 ± 127.85 a | 0.64 ± 0.06 b | 1.02 ± 0.18 b | 18.39 ± 4.64 b | 18.39 ± 4.64 b | |
| Zn-Gly 3 | 1324 ± 169.28 b | 0.54 ± 0.05 c | 0.85 ± 0.16 c | 14.12 ± 1.49 c | 14.12 ± 1.49 c | |
| p-value | ||||||
| Temperature | <0.001 | <0.001 | <0.001 | 0.012 | <0.001 | |
| Maternal zinc | 0.003 | <0.001 | <0.001 | 0.001 | <0.001 | |
| Temperature × maternal zinc | 0.108 | 0.002 | 0.216 | 0.975 | <0.001 | |
| Temperature | Maternal Zinc | T-SOD, U/mg port | CuZn-SOD, U/mg port | GSH-Px, U/mg port | CAT, U/mg port | T-AOC, U/mg port | MT, ng/g |
|---|---|---|---|---|---|---|---|
| 37 °C 1 | Control | 93.26 ± 3.05 d | 63.67 ± 0.79 c | 73.52 ± 3.30 b | 8.02 ± 0.46 bc | 0.86 ± 0.07 c | 93.98 ± 0.37 c |
| ZnSO4 | 114.7 ± 4.18 ab | 67.29 ± 1.51 b | 76.32 ± 2.71 b | 8.71 ± 0.5 ab | 1.14 ± 0.05 ab | 96.84 ± 0.41 b | |
| Zn-Gly | 119.6 ± 4.91 a | 71.01 ± 0.80 a | 80.48 ± 0.16 a | 9.10 ± 0.57 a | 1.19 ± 0.07 a | 102.49 ± 0.87 a | |
| 39.5 °C 1 | Control | 86.13 ± 1.74 e | 60.86 ± 0.17 d | 64.57 ± 0.44 d | 6.84 ± 0.45 d | 0.71 ± 0.05 d | 79.54 ± 2.19 e |
| ZnSO4 | 102.7 ± 1.08 c | 65.87 ± 1.84 b | 69.38 ± 1.45 c | 7.52 ± 0.37 cd | 0.84 ± 0.04 c | 90.75 ± 0.54 d | |
| Zn-Gly | 112.7 ± 3.73 b | 70.57 ± 0.46 a | 73.40 ± 1.29 b | 8.46 ± 0.33 ab | 1.05 ± 0.11 b | 93.60 ± 2.35 c | |
| 37 °C 2 | 109.2 ± 12.66 a | 67.32 ± 3.32 a | 76.77 ± 3.71 a | 8.61 ± 0.65 a | 1.06 ± 0.16 a | 98.63 ± 3.91 a | |
| 39.5 °C 2 | 100.7 ± 11.16 b | 65.77 ± 4.31 b | 69.12 ± 3.96 b | 7.60 ± 0.78 b | 0.87 ± 0.16 b | 87.96 ± 6.64 b | |
| Control 3 | 89.69 ± 4.49 c | 62.27 ± 1.62 c | 69.04 ± 5.33 c | 7.43 ± 0.76 c | 0.78 ± 0.10 c | 86.76 ± 8.03 c | |
| ZnSO4 3 | 107.8 ± 6.90 b | 66.58 ± 1.69 b | 72.85 ± 4.27 b | 8.11 ± 0.76 b | 0.99 ± 0.17 b | 93.80 ± 3.36 b | |
| Zn-Gly 3 | 116.2 ± 5.45 a | 70.79 ± 0.63 a | 76.94 ± 3.96 a | 8.78 ± 0.54 a | 1.12 ± 0.11 a | 99.16 ± 4.81 a | |
| p-value | |||||||
| Temperature | <0.001 | 0.011 | <0.001 | 0.001 | <0.001 | <0.001 | |
| Maternal zinc | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | |
| Temperature × maternal zinc | 0.315 | 0.212 | 0.614 | 0.507 | 0.138 | <0.001 | |
| Temperature | Maternal Zinc | MMP, mV | AI, % | Caspase3, U/mg Prot |
|---|---|---|---|---|
| 37 °C 1 | Control | 12.06 ± 0.18 | 2.64 ± 0.21 d | 56.35 ± 1.80 |
| ZnSO4 | 13.13 ± 0.33 | 1.43 ± 0.15 e | 52.82 ± 1.28 | |
| Zn-Gly | 14.93 ± 0.84 | 0.24 ± 0.03 f | 49.25 ± 1.46 | |
| 39.5 °C 1 | Control | 10.93 ± 0.60 | 7.38 ± 0.34 a | 68.17 ± 1.69 |
| ZnSO4 | 12.51 ± 0.31 | 5.60 ± 0.30 b | 63.59 ± 3.68 | |
| Zn-Gly | 13.68 ± 1.42 | 3.61 ± 0.24 c | 59.25 ± 2.81 | |
| 37 °C 2 | 13.53 ± 1.38 a | 1.44 ± 1.01 b | 52.81 ± 3.18 b | |
| 39.5 °C 2 | 12.37 ± 1.43 b | 5.53 ± 1.59 a | 63.67 ± 4.58 a | |
| Control 3 | 11.49 ± 0.74 c | 5.01 ± 2.46 a | 62.26 ± 6.66 a | |
| ZnSO4 3 | 12.82 ± 0.45 b | 3.52 ± 2.15 b | 57.44 ± 6.20 b | |
| Zn-Gly 3 | 14.39 ± 1.21 a | 1.92 ± 1.74 c | 54.25 ± 5.83 c | |
| p-value | ||||
| Temperature | 0.013 | <0.001 | <0.001 | |
| Maternal zinc | <0.001 | <0.001 | <0.001 | |
| Temperature × maternal zinc | 0.745 | <0.001 | 0.781 | |
| Temperature | Maternal Zinc | CuZn-SOD | MT | Nrf2 | UCP | Bax |
|---|---|---|---|---|---|---|
| 37 °C 1 | Control | 0.64 ± 0.03 d | 0.87 ± 0.06 c | 0.93 ± 0.05 | 0.85 ± 0.13 d | 1.37 ± 0.05 c |
| ZnSO4 | 0.85 ± 0.05 b | 0.98 ± 0.03 c | 1.12 ± 0.10 | 0.99 ± 0.11 d | 0.99 ± 0.11 d | |
| Zn-Gly | 1.19 ± 0.03 a | 1.72 ± 0.18 a | 1.23 ± 0.05 | 1.03 ± 0.02 d | 0.95 ± 0.09 d | |
| 39.5 °C 1 | Control | 0.60 ± 0.01 d | 0.82 ± 0.05 c | 1.12 ± 0.11 | 1.52 ± 0.15 c | 2.43 ± 0.27 a |
| ZnSO4 | 0.71 ± 0.00 c | 0.88 ± 0.10 c | 1.33 ± 0.02 | 2.08 ± 0.26 b | 2.08 ± 0.26 b | |
| Zn-Gly | 1.16 ± 0.02 a | 1.54 ± 0.09 b | 1.51 ± 0.15 | 2.43 ± 0.27 a | 1.52 ± 0.15 c | |
| 37 °C 2 | 0.88 ± 0.25 a | 1.19 ± 0.41 a | 1.09 ± 0.14 b | 0.96 ± 0.12 b | 1.09 ± 0.21 b | |
| 39.5 °C 2 | 0.86 ± 0.26 b | 1.08 ± 0.35 b | 1.32 ± 0.18 a | 2.01 ± 0.45 a | 2.01 ± 0.45 a | |
| Control 3 | 0.63 ± 0.03 b | 0.85 ± 0.05 b | 1.03 ± 0.13 c | 1.18 ± 0.39 c | 1.90 ± 0.61 a | |
| ZnSO4 3 | 0.78 ± 0.08 b | 0.93 ± 0.09 b | 1.24 ± 0.12 b | 1.46 ± 0.61 b | 1.46 ± 0.61 b | |
| Zn-Gly 3 | 1.18 ± 0.03 a | 1.63 ± 0.16 a | 1.37 ± 0.18 a | 1.73 ± 0.79 a | 1.23 ± 0.33 c | |
| p-value | ||||||
| Temperature | <0.001 | 0.038 | <0.001 | <0.001 | <0.001 | |
| Maternal zinc | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Temperature × maternal zinc | 0.004 | 0.536 | 0.661 | 0.010 | 0.035 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xie, L.; Ding, X.; Wang, Y.; Xu, Y.; Li, D.; Liang, S.; Wang, Y.; Zhang, L.; Fu, A.; et al. Mechanisms Underlying the Protective Effect of Maternal Zinc (ZnSO4 or Zn-Gly) against Heat Stress-Induced Oxidative Stress in Chicken Embryo. Antioxidants 2022, 11, 1699. https://doi.org/10.3390/antiox11091699
Zhang Y, Xie L, Ding X, Wang Y, Xu Y, Li D, Liang S, Wang Y, Zhang L, Fu A, et al. Mechanisms Underlying the Protective Effect of Maternal Zinc (ZnSO4 or Zn-Gly) against Heat Stress-Induced Oxidative Stress in Chicken Embryo. Antioxidants. 2022; 11(9):1699. https://doi.org/10.3390/antiox11091699
Chicago/Turabian StyleZhang, Yunfeng, Lingyu Xie, Xiaoqing Ding, Yuanyuan Wang, Yibin Xu, Danlei Li, Shuang Liang, Yongxia Wang, Ling Zhang, Aikun Fu, and et al. 2022. "Mechanisms Underlying the Protective Effect of Maternal Zinc (ZnSO4 or Zn-Gly) against Heat Stress-Induced Oxidative Stress in Chicken Embryo" Antioxidants 11, no. 9: 1699. https://doi.org/10.3390/antiox11091699
APA StyleZhang, Y., Xie, L., Ding, X., Wang, Y., Xu, Y., Li, D., Liang, S., Wang, Y., Zhang, L., Fu, A., & Zhan, X. (2022). Mechanisms Underlying the Protective Effect of Maternal Zinc (ZnSO4 or Zn-Gly) against Heat Stress-Induced Oxidative Stress in Chicken Embryo. Antioxidants, 11(9), 1699. https://doi.org/10.3390/antiox11091699







