Physiological and Comparative Transcriptome Analysis Reveals the Mechanism by Which Exogenous 24-Epibrassinolide Application Enhances Drought Resistance in Potato (Solanum tuberosum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Water Content and ABA Content
2.3. Photosynthesis and Chlorophyll Fluorescence Measurements
2.4. Content of Photosynthetic Pigments Content
2.5. NBT and DAB Staining
2.6. Quantification of MDA and Activities of SOD, POD, and CAT
2.7. Relative Conductivity
2.8. Proline, Soluble Protein, and Soluble Sugar Content
2.9. RNA Sequencing and Analysis of Differentially Expressed Genes
2.10. GO and KEGG Enrichment
2.11. Quantitative Real-Time PCR (qRT-PCR) Validation
2.12. Statistical Analysis
3. Results
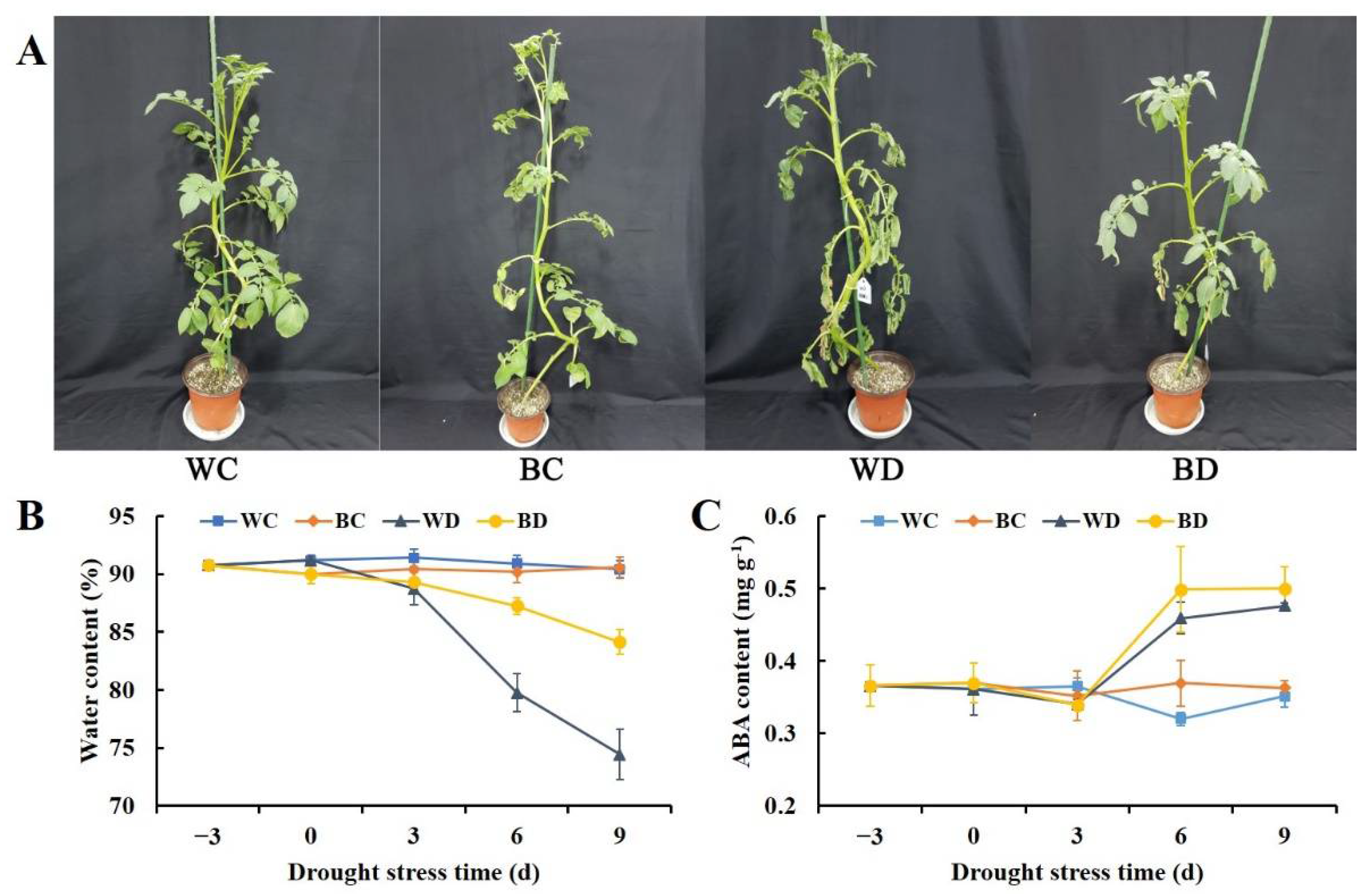
3.1. Exogenous Application of EBR Delayed Plant Water Loss and Increased the ABA Content under Drought
3.2. Exogenous Application of EBR Enhanced the Photosynthetic Capacity under Drought
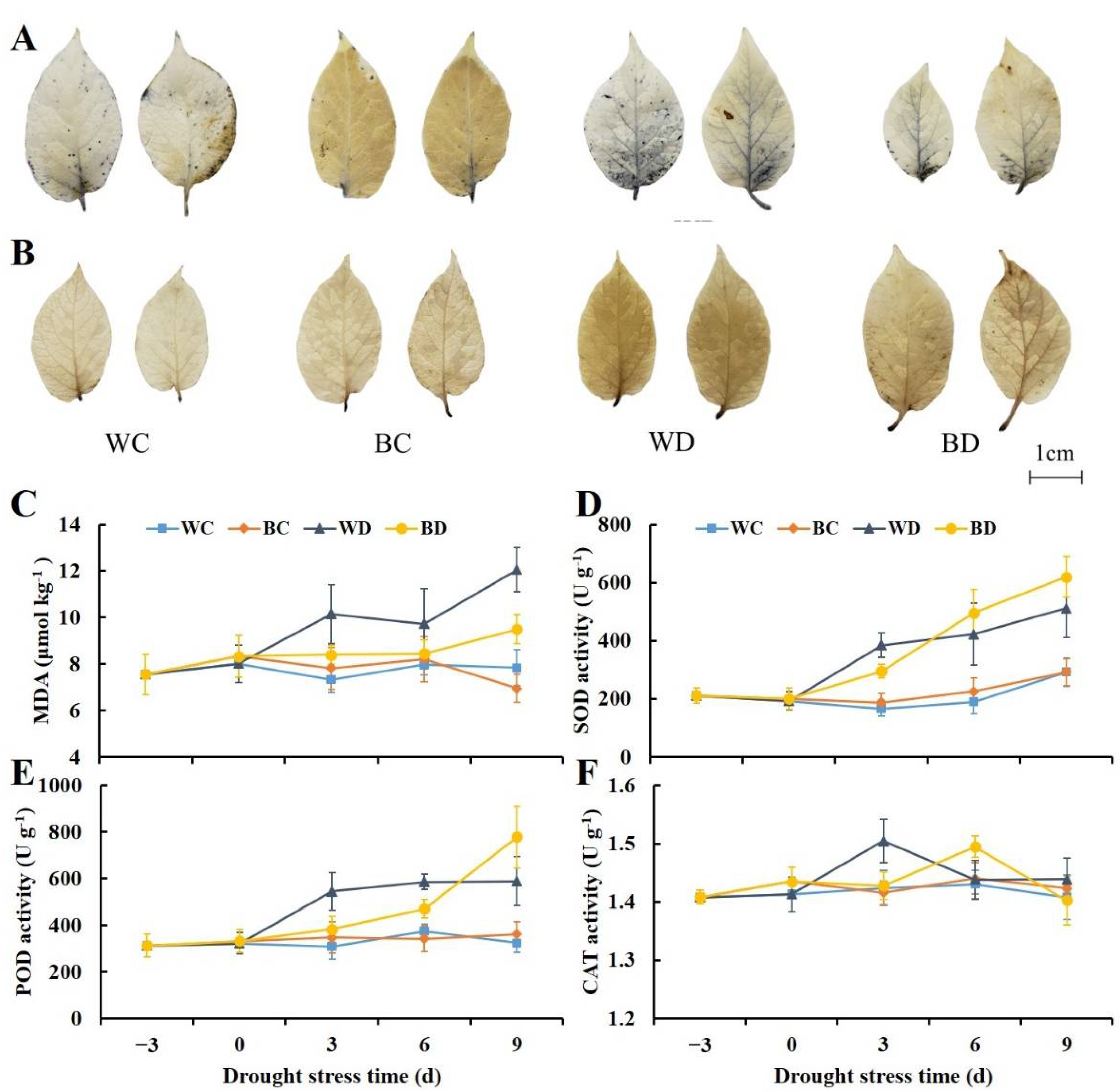
3.3. Exogenous Application of EBR Enhanced Antioxidant Enzyme Activity and the Scavenging of ROS under Drought
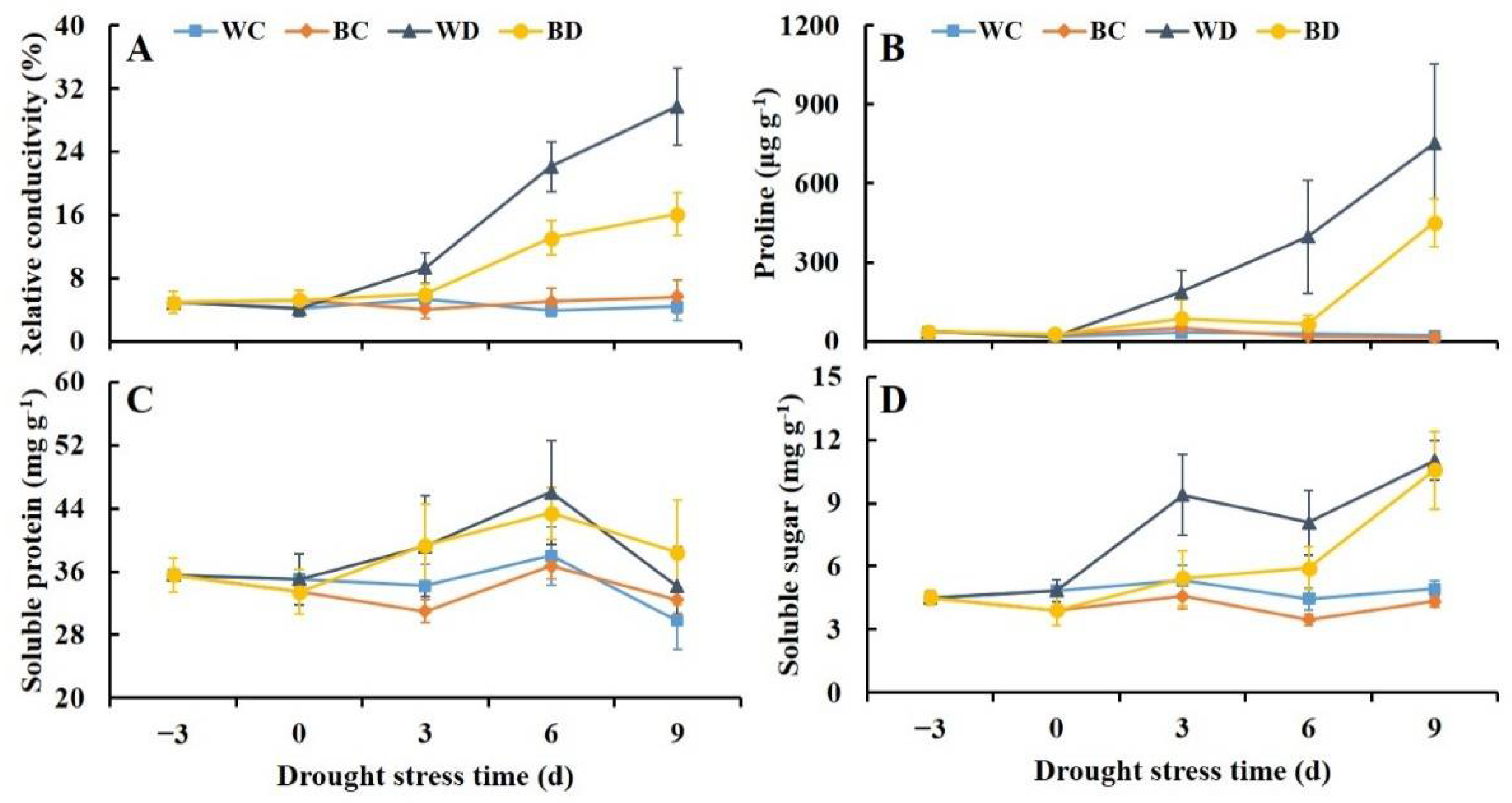
3.4. Exogenous Application of EBR Alleviated Increases in Relative Conductivity and Osmotic Solutes under Drought
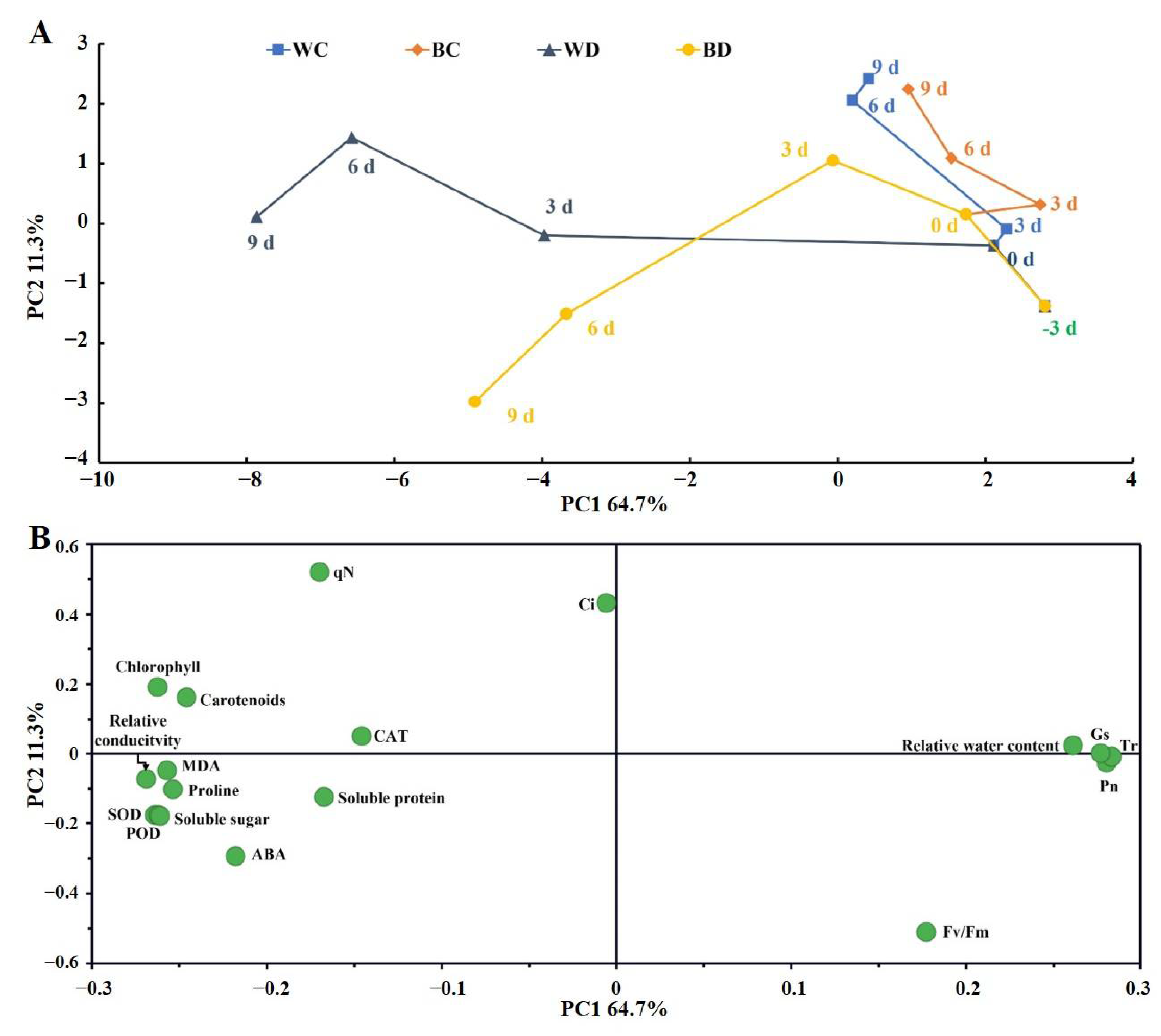
3.5. Time-Related Trajectory Analysis
3.6. RNA-Seq and Differential Gene Expression
3.7. Verification of RNA-Seq Data
3.8. GO and KEGG Enrichment of DEGs
3.9. Drought-Responsive Genes under EBR Applications
3.10. Expression Patterns of Genes Involved in BR and ABA Biosynthesis
4. Discussion
4.1. Response of Hormone Signals under Drought Stress after EBR Pretreatment
4.2. Effect of EBR on Growth and the Photosynthesis of Potato under Drought Stress
4.3. Effect of EBR on the ROS Scavenging System of Potato under Drought Stress
4.4. Effect of EBR on the Osmotic Solutes of Potato under Drought Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.Z.; Wang, P.; Tang, D.; Yang, Z.M.; Lu, F.; Qi, J.J.; Tawari, N.R.; Shang, Y.; Li, C.H.; Huang, S.W. The genetic basis of inbreeding depression in potato. Nat. Genet. 2019, 51, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Yang, Z.M.; Tang, D.; Zhu, Y.H.; Wang, P.; Li, D.W.; Zhu, G.T.; Xiong, X.Y.; Shang, Y.; Li, C.H.; et al. Genome design of hybrid potato. Cell 2021, 184, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
- Gervais, T.; Creelman, A.; Li, X.Q.; Bizimungu, B.; Koeyer, D.D.; Dahal, K. Potato response to drought stress: Physiological and growth basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Rodrigues, J.; Inzé, D.; Nelissen, H.; Saibo, N.J.M. Source-sink regulation in crops under water deficit. Trends Plant Sci. 2019, 24, 652–663. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Xia, H.; Ni, Z.Y.; Hu, R.P.; Lin, L.J.; Deng, H.H.; Wang, J.; Tang, Y.; Sun, G.C.; Wang, X.; Li, H.X.; et al. Melatonin alleviates drought stress by a non-enzymatic and enzymatic antioxidative system in kiwifruit seedlings. Int. J. Mol. Sci. 2020, 21, 852. [Google Scholar] [CrossRef]
- Talbi, S.; Romero-Puertas, M.C.; Hernández, A.; Terrón, L.; Ferchichi, A.; Sandalio, L.M. Drought tolerance in a Saharian plant Oudneya africana: Role of antioxidant defences. Environ. Exp. Bot. 2015, 111, 114–126. [Google Scholar] [CrossRef]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the basal role of ABA-roles outside of stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, D.; Janeczko, A.; Dziurka, M.; Pociecha, E.; Oklestkova, J.; Szarejko, I. Barley brassinosteroid mutants provide an insight into phytohormonal homeostasis in plant reaction to drought stress. Front. Plant Sci. 2016, 7, 1824. [Google Scholar] [CrossRef]
- Nolan, T.M.; Nemanja, V.; Liu, D.; Eugenia, R.; Yin, Y.H. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and physiological responses in plant growth and abiotic stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Wang, Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, H.X.; Lin, L.; Wang, F.; Sui, N. Roles of brassinosteroids in plant growth and abiotic stress response. Plant Growth Regul. 2021, 93, 29–38. [Google Scholar] [CrossRef]
- Zhang, M.C.; Zhai, Z.X.; Tian, X.L.; Duan, L.S.; Li, Z.H. Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul. 2008, 56, 257–264. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M.; Athar, H.R. Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.). Plant Growth Regul. 2008, 55, 51–64. [Google Scholar] [CrossRef]
- Cui, X.Y.; Gao, Y.; Guo, J.; Yu, T.F.; Zheng, W.J.; Liu, Y.W.; Chen, J.; Xu, Z.S.; Ma, Y.Z. BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol. 2019, 180, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Fariduddin, Q.; Khanam, S.; Hasan, S.A.; Ali, B.; Hayat, S.; Ahmad, A. Effect of 28-homobrassinolide on the drought stress-induced changes in photosynthesis and antioxidant system of Brassica juncea L. Acta Physiol. Plant. 2009, 31, 889–897. [Google Scholar] [CrossRef]
- Yuan, G.F.; Jia, C.G.; Li, Z.; Sun, B.; Zhang, L.P.; Liu, N.; Wang, Q.M. Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci. Hortic. 2010, 126, 103–108. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Sun, S.A.; Yao, X.F.; Liu, X.; Qiao, Z.H.; Liu, Y.; Li, X.D.; Jiang, X.Y. Brassinolide can improve drought tolerance of maize seedlings under drought stress: By inducing the photosynthetic performance, antioxidant capacity and ZmMYB gene expression of maize seedlings. J. Soil Sci. Plant Nut. 2022, 22, 2092–2104. [Google Scholar] [CrossRef]
- Gill, M.B.; Cai, K.F.; Zhang, G.P.; Zeng, F.R. Brassinolide alleviates the drought-induced adverse effects in barley by modulation of enzymatic antioxidants and ultrastructure. Plant Growth Regul. 2017, 82, 447–455. [Google Scholar] [CrossRef]
- Gruszka, D.; Pociecha, E.; Jurczyk, B.; Dziurka, M.; Oliwa, J.; Sadura, I.; Janeczko, A. Insights into metabolic reactions of semi-dwarf, barley brassinosteroid mutants to drought. Int. J. Mol. Sci. 2020, 21, 5096. [Google Scholar] [CrossRef]
- Wang, X.X.; Gao, Y.A.; Wang, Q.J.; Chen, M.; Ye, X.L.; Li, D.M.; Chen, X.D.; Li, L.; Gao, D.S. 24-Epibrassinolide-alleviated drought stress damage influences antioxidant enzymes and autophagy changes in peach (Prunus persicae L.) leaves. Plant Physiol. Bioch. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Castañeda-Murillo, C.C.; Rojas-Ortiz, J.G.; Sánchez-Reinoso, A.D.; Chávez-Arias, C.C.; Restrepo-Díaz, H. Foliar brassinosteroid analogue (DI-31) sprays increase drought tolerance by improving plant growth and photosynthetic efficiency in lulo plants. Heliyon 2022, 8, e08977. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.; Jiang, H.; Tang, B.; Zhang, M.; Li, Z.; and Yin, Y. The AP2/ERF transcription factor TINY modulates brassinosteroid-regulated plant growth and drought responses in Arabidopsis. Plant Cell 2019, 31, 1788–1806. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, F.F.; Xie, Q. Balancing growth and adaptation to stress: Crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Yu, J.Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Benefits of brassinosteroid crosstalk. Trends Plant Sci. 2012, 17, 594–605. [Google Scholar] [CrossRef]
- Wang, R.J.; Wang, R.X.; Liu, M.M.; Yuan, W.W.; Zhao, Z.Y.; Liu, X.Q.; Peng, Y.M.; Yang, X.R.; Sun, Y.; Tang, W.Q. Nucleocytoplasmic trafficking and turnover mechanisms of BRASSINAZOLE RESISTANT1 in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2101838118. [Google Scholar] [CrossRef]
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T.; et al. Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat. Commun. 2018, 9, 4680. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, B.; Xie, Z.; Nolan, T.; Ye, H.; Song, G.Y.; Walley, J.; Yin, Y. GSK3-like kinase BIN2 phosphorylates RD26 to potentiate drought signaling in Arabidopsis. Plant J. 2019, 100, 923–937. [Google Scholar] [CrossRef]
- Chen, J.N.; Nolan, T.M.; Ye, H.X.; Zhang, M.C.; Tong, H.N.; Xin, P.Y.; Chu, J.F.; Chu, C.C.; Li, Z.H.; Yin, Y.H. Arabidopsis WRKY46, WRKY54 and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought response. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhang, N.; Yang, J.W.; Si, H.J. Functional analysis of potato CPD gene: A rate-limiting enzyme in brassinosteroid biosynthesis under polyethylene glycol-induced osmotic stress. Crop Sci. 2016, 56, 2675–2687. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, Y.; Yan, J.W.; Di, P.C.; Li, J.; Sun, X.J.; Han, G.Q.; Ni, L.; Jiang, M.Y.; Yuan, J.H.; et al. Brassinosteroid-Signaling Kinase 1 Phosphorylating Calcium/Calmodulin-Dependent Protein Kinase Functions in Drought Tolerance in Maize. New Phytol. 2021, 231, 695–712. [Google Scholar] [CrossRef]
- Ramraj, V.M.; Vyas, B.N.; Godrej, N.B.; Mistry, K.B.; Swam, B.N.; Ingh, N.S. Effects of 28-homobrassinolide on yields of wheat, rice, groundnut, mustard, potato and cotton. J. Agr. Sci. 1997, 128, 405–413. [Google Scholar] [CrossRef]
- Huang, S.H.; Zheng, C.Y.; Zhao, Y.; Li, Q.; Liu, J.W.; Deng, R.; Lei, T.T.; Wang, S.F.; Wang, X.F. RNA interference knockdown of the brassinosteroid receptor BRI1 in potato (Solanum tuberosum L.) reveals novel functions for brassinosteroid signaling in controlling tuberization. Sci. Hortic. 2021, 290, 110516. [Google Scholar] [CrossRef]
- Korableva, N.P.; Platonova, T.A.; Dogonadze, M.Z.; Evsunina, A.S. Brassinolide effect on growth of apical meristems, ethylene production, and abscisic acid content in potato tubers. Biol. Plant. 2002, 45, 39–43. [Google Scholar] [CrossRef]
- Li, L.Q.; Deng, M.M.; Lyu, C.C.; Zhang, J.; Peng, J.; Cai, C.C.; Yang, S.M.; Lu, L.M.; Ni, S.; Liu, F.; et al. Quantitative phosphoproteomics analysis reveals that protein modification and sugar metabolism contribute to sprouting in potato after BR treatment. Food Chem. 2020, 325, 126875. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Xia, S.T.; Yi, S.; Wang, H.Q.; Luo, W.G.; Su, S.Y.; Xiao, L.T. Brassinolide increases potato root growth in vitro in a dose-dependent way and alleviates salinity stress. Biomed Res. Int. 2016, 2016, 8231873. [Google Scholar] [CrossRef] [PubMed]
- Kolomeichuk, L.V.; Efmova, M.V.; Zlobin, I.E.; Kreslavski, V.D.; Murgan, O.K.; Kovtun, I.S.; Khripach, V.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. 24-Epibrassinolide alleviates the toxic efects of NaCl on photosynthetic processes in potato plants. Photosynth. Res. 2020, 146, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Tian, Y.X.; Jiang, M.; Yuan, Q.; Chen, Q.; Zhang, Y.; Luo, Y.; Zhang, F.; Tang, H.R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera). RSC Adv. 2018, 8, 33845–33854. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhao, Y.; Shan, W.; Kuang, J.; Lu, W.; Su, X.; Tao, N.; Lakshmanan, P.; Chen, J. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Iram, A. Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora 2005, 200, 535–546. [Google Scholar] [CrossRef]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Ma, R.; Liu, W.G.; Li, S.G.; Zhu, X.; Yang, J.W.; Zhang, N.; Si, H.J. Genome-wide identification, characterization and expression analysis of the CIPK gene family in potato (Solanum tuberosum L.) and the role of StCIPK10 in response to drought and osmotic stress. Int. J. Mol. Sci. 2021, 22, 13535. [Google Scholar] [CrossRef]
- Chen, Y.K.; Canhui Li, C.H.; Yi, J.; Yang, Y.; Lei, C.X.; Gong, M. Transcriptome response to drought, rehydration and re-dehydration in potato. Int. J. Mol. Sci. 2020, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, T.; Wu, Z.; Wang, J.; Zhang, C.; Wang, H.; Wang, Z.X.; Wang, X. The intrinsically disordered protein BKI1 is essential for inhibiting BRI1 signaling in plants. Mol. Plant 2015, 8, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Liu, J.; Xu, J.F.; Duan, S.G.; Wang, Q.R.; Li, G.C.; Jin, L.P. Transcriptome profiling reveals effects of drought stress on gene expression in diploid potato genotype P3-198. Int. J. Mol. Sci. 2019, 20, 852. [Google Scholar] [CrossRef]
- Aliche, E.B.; Theeuwen, T.P.J.M.; Oortwijn, M.; Visser, R.G.F.; Linden, C.G. Carbon partitioning mechanisms in potato under drought stress. Plant Physiol. Bioch. 2020, 146, 211–219. [Google Scholar] [CrossRef]
- Li, K.R.; Wang, H.H.; Han, G.; Wang, Q.J.; Fan, J. Effects of brassinolide on the survival, growth and drought resistance of Robinia Pseudoacacia seedlings under water-stress. New Forest. 2008, 35, 255–266. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef]
- Pilon, C.; Snider, J.L.; Sobolev, V.; Chastain, D.R.; Sorensen, R.B.; Meeks, C.D.; Massa, A.N.; Walk, T.; Singh, B.; Earl, H.J. Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). J. Plant Physiol. 2018, 231, 124–134. [Google Scholar] [CrossRef]
- Luo, F.; Cai, J.H.; Kong, X.M.; Zhou, Q.; Zhou, X.; Zhao, Y.B.; Ji, S.J. Transcriptome profiling reveals the roles of pigment mechanisms in postharvest broccoli yellowing. Hortic. Res. 2019, 6, 74. [Google Scholar] [CrossRef]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51, 329–337. [Google Scholar] [CrossRef] [Green Version]


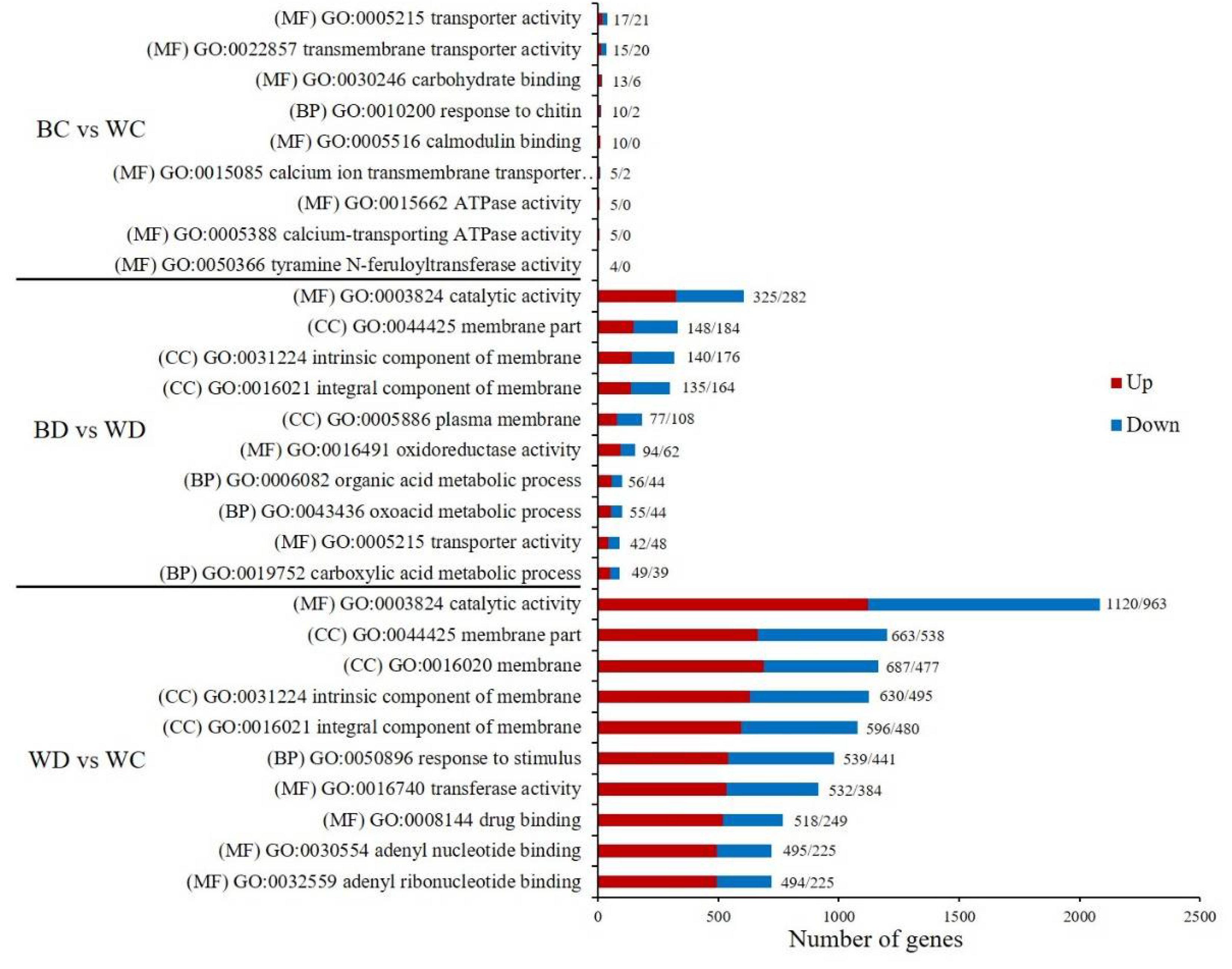

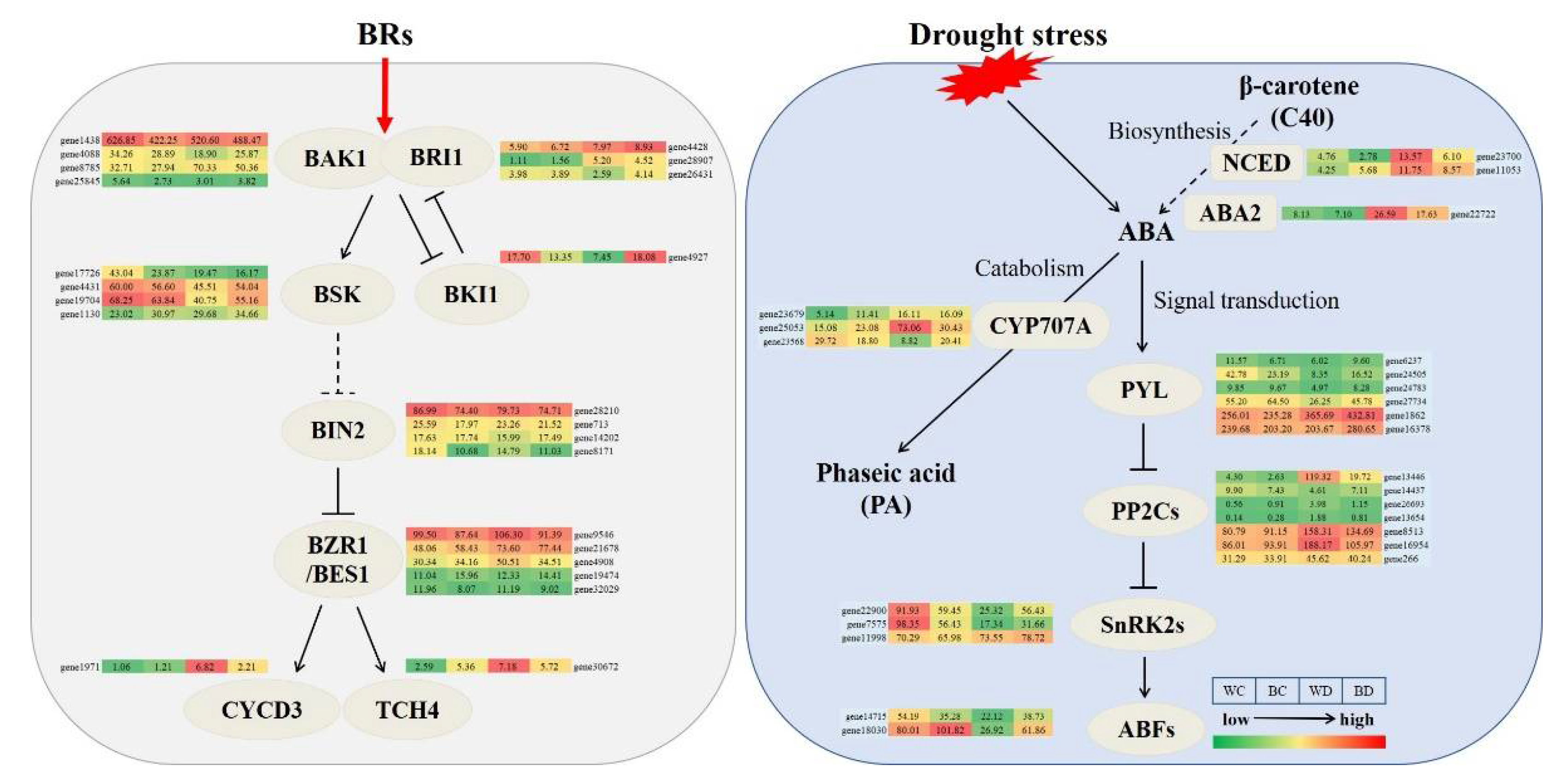
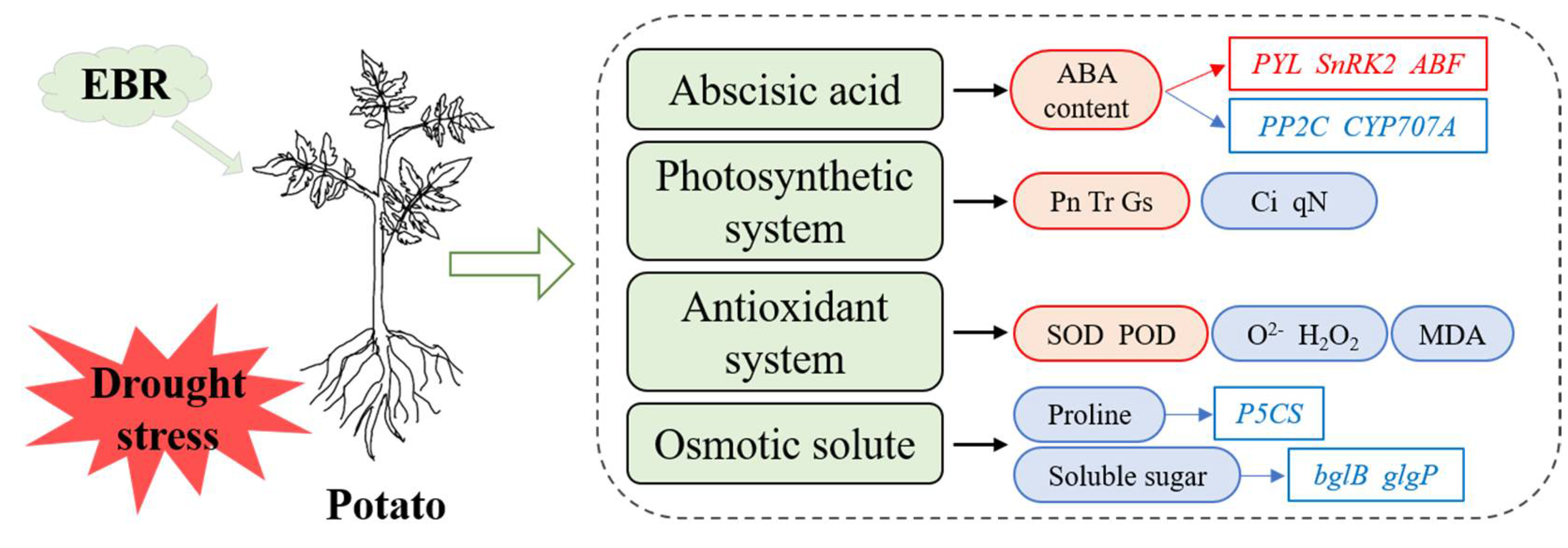
| Comparison | KEGG Pathway | Upregulated DEGs Number | Downregulated DEGs Number | p-Value |
|---|---|---|---|---|
| BC vs. WC | MAPK signaling pathway-plant | 3 | 2 | 0.0349 |
| Plant-pathogen interaction | 5 | 1 | 0.0444 | |
| Plant hormone signal transduction | 2 | 5 | 0.0494 | |
| BD vs. WD | Circadian rhythm-plant | 3 | 4 | 0.0010 |
| Starch and sucrose metabolism | 4 | 6 | 0.0013 | |
| Alpha-linolenic acid metabolism | 7 | 0 | 0.0045 | |
| Plant hormone signal transduction | 15 | 5 | 0.0057 | |
| Flavonoid biosynthesis | 4 | 2 | 0.0111 | |
| Carotenoid biosynthesis | 2 | 2 | 0.0387 | |
| WD vs. WC | Photosynthesis-antenna proteins | 12 | 0 | 0.0000 |
| Starch and sucrose metabolism | 16 | 12 | 0.0000 | |
| Carbon fixation in photosynthetic organisms | 10 | 6 | 0.0005 | |
| Photosynthesis | 14 | 2 | 0.0021 | |
| Plant hormone signal transduction | 24 | 39 | 0.0016 | |
| Alpha-linolenic acid metabolism | 3 | 13 | 0.0074 | |
| Flavonoid biosynthesis | 6 | 9 | 0.0069 | |
| Galactose metabolism | 7 | 7 | 0.0063 | |
| Carotenoid biosynthesis | 7 | 3 | 0.0290 | |
| MAPK signaling pathway-plant | 11 | 21 | 0.0497 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Ma, J.; Huang, W.; Di, H.; Xia, X.; Ma, W.; Ma, J.; Yang, J.; Li, X.; Lian, H.; et al. Physiological and Comparative Transcriptome Analysis Reveals the Mechanism by Which Exogenous 24-Epibrassinolide Application Enhances Drought Resistance in Potato (Solanum tuberosum L.). Antioxidants 2022, 11, 1701. https://doi.org/10.3390/antiox11091701
Zheng H, Ma J, Huang W, Di H, Xia X, Ma W, Ma J, Yang J, Li X, Lian H, et al. Physiological and Comparative Transcriptome Analysis Reveals the Mechanism by Which Exogenous 24-Epibrassinolide Application Enhances Drought Resistance in Potato (Solanum tuberosum L.). Antioxidants. 2022; 11(9):1701. https://doi.org/10.3390/antiox11091701
Chicago/Turabian StyleZheng, Hao, Jie Ma, Wenli Huang, Hongmei Di, Xue Xia, Wei Ma, Jun Ma, Jiao Yang, Xiaomei Li, Huashan Lian, and et al. 2022. "Physiological and Comparative Transcriptome Analysis Reveals the Mechanism by Which Exogenous 24-Epibrassinolide Application Enhances Drought Resistance in Potato (Solanum tuberosum L.)" Antioxidants 11, no. 9: 1701. https://doi.org/10.3390/antiox11091701
APA StyleZheng, H., Ma, J., Huang, W., Di, H., Xia, X., Ma, W., Ma, J., Yang, J., Li, X., Lian, H., Huang, Z., Tang, Y., Zheng, Y., Li, H., Zhang, F., & Sun, B. (2022). Physiological and Comparative Transcriptome Analysis Reveals the Mechanism by Which Exogenous 24-Epibrassinolide Application Enhances Drought Resistance in Potato (Solanum tuberosum L.). Antioxidants, 11(9), 1701. https://doi.org/10.3390/antiox11091701







