Recombinant Expression of Archaeal Superoxide Dismutases in Plant Cell Cultures: A Sustainable Solution with Potential Application in the Food Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cloning of Aeropyrum Pernix K1 and Saccharolobus Solfataricus P2 Mn/Fe-SOD Coding DNA Sequences (CDS)
2.2. Cloning of SodAp and SodSs in Plant Expression Vectors
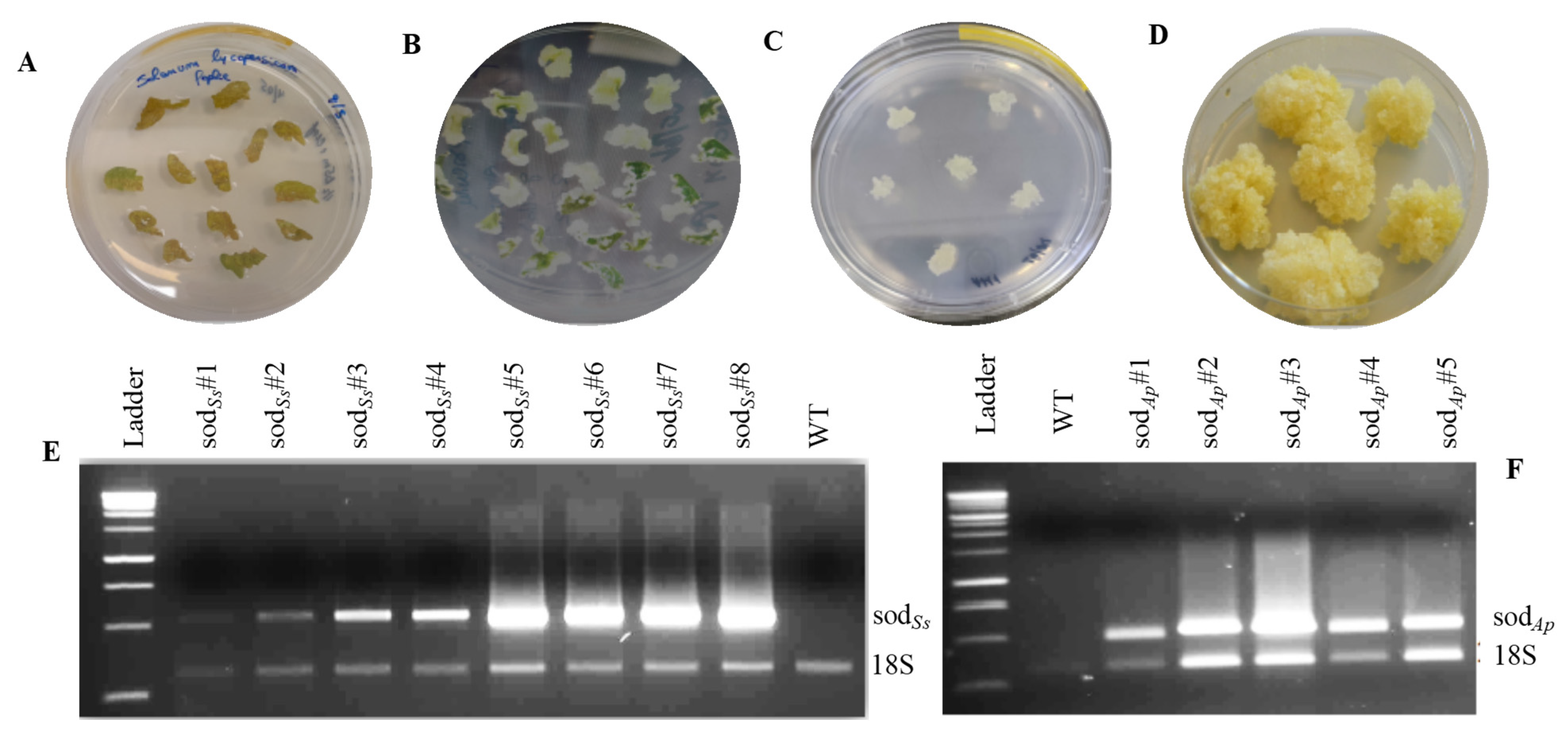
2.3. Genetic Transformation of Tomato Plants by Agrobacterium Tumefaciens
2.4. Molecular Analysis of the Tomato Transformed Lines
2.5. Production of Tomato Cell Extracts
2.6. SOD and NBT Assays
2.7. Molecular Mass Determination
2.8. In Vitro Gastric Digestion Assay on SODSs-Enriched Tomato Cell Extracts
2.9. Resistance of SODSs-Enriched Tomato Cell Extracts to Proteolytic Degradation
2.10. SDS-PAGE and Western Blot Analyses
2.11. ORAC Assay
2.12. Sampling Preparation
2.13. Colour Measurements
2.14. Histamine and Nitrate Determination in Tuna Fillets
2.15. Statistical Analysis
3. Results and Discussion
3.1. Expression of SODAp and SODSs in Tomato Cell Cultures
3.2. Biochemical Characterization of SODAp and SODSs
3.3. Resistance of SODSs-Tomato Cell Extracts to Simulated Gastric Fluid (SGF) and Pancreatic Proteases
3.4. Activity of SODAp-Tomato Cell Extracts on Tuna Slices
3.5. Activity of SODAp-Tomato Cell Extracts on Myoglobin Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. Biomed. Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Di Mambro, V.M.; Borin, M.F.; Fonseca, M.J. Topical formulations with superoxide dismutase: Influence of formulation composition on physical stability and enzymatic activity. J. Pharm. Biomed. Anal. 2003, 32, 97–105. [Google Scholar] [CrossRef]
- Wang, S. Methyl jasmonate reduces water stress in strawberry. J. Plant Growth Regul. 1999, 18, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Padmapriya, V.; Anand, N. Purification and characterization of iron-containing superoxide dismutase from anabaena variabilis Kutz ex Born. et Flah. Biomed. Pharmacol. J. 2010, 3, 349–356. [Google Scholar]
- Liu, J.; Yin, M.; Zhu, H.; Lu, J.; Cui, Z. Purification and characterization of a hyperthermostable Mn-superoxide dismutase from Thermus thermophilus HB27. Extremophiles 2011, 15, 221–226. [Google Scholar] [CrossRef]
- Pinmanee, P.; Sompinit, K.; Arnthong, J.; Suwannarangsee, S.; Jantimaporn, A.; Khongkow, M.; Nimchua, T.; Sukyai, P. Enhancing the productivity and stability of superoxide dismutase from Saccharomyces cerevisiae TBRC657 and its application as a free radical scavenger. Fermentation 2022, 8, 169. [Google Scholar] [CrossRef]
- Catara, G.; Fiume, I.; Iuliano, F.; Maria, G.; Ruggiero, G.; Palmieri, G.; Capasso, A.; Rossi, M. A new kumamolisin-like protease from Alicyclobacillus acidocaldarius: An enzyme active under extreme acidic conditions. Biocatal. Biotransform. 2006, 24, 358–370. [Google Scholar] [CrossRef]
- Gogliettino, M.; Riccio, A.; Cocca, E.; Rossi, M.; Palmieri, G.; Balestrieri, M. A new pepstatin-insensitive thermopsin-like protease overproduced in peptide-rich cultures of Sulfolobus solfataricus. Int. J. Mol. Sci. 2014, 15, 3204–3219. [Google Scholar] [CrossRef]
- Guleria, S.; Jain, R.; Singh, D.; Kumar, S. A thermostable Fe/Mn SOD of Geobacillus sp. PCH100 isolated from glacial soil of Indian trans-Himalaya exhibits activity in the presence of common inhibitors. Int. J. Biol. Macromol. 2021, 179, 576–585. [Google Scholar] [CrossRef]
- Palmieri, G.; Arciello, S.; Bimonte, M.; Carola, A.; Tito, A.; Gogliettino, M.; Cocca, E.; Fusco, C.; Balestrieri, M.; Colucci, M.G.; et al. The extraordinary resistance to UV radiations of a manganese superoxide dismutase of Deinococcus radiodurans offers promising potentialities in skin care applications. J. Biotechnol. 2019, 302, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Stetter, K.O.; Fiala, G.; Huber, G.; Huber, R.; Segerer, A. Hyperthermophilic microorganisms. FEMS Microbiol. Lett. 1990, 75, 117–124. [Google Scholar] [CrossRef]
- Cannio, R.; Catara, G.; Fiume, I.; Balestrieri, M.; Rossi, M.; Palmieri, G. Identification of a cell-bound extracellular protease overproduced by Sulfolobus solfataricus in peptide-rich media. Protein Pept. Lett. 2010, 17, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Gogliettino, M.; Balestrieri, M.; Cocca, E.; Mucerino, S.; Rossi, M.; Petrillo, M.; Mazzella, E.; Palmieri, G. Identification and characterisation of a novel acylpeptide hydrolase from Sulfolobus solfataricus: Structural and functional insights. PLoS ONE 2012, 7, e37921. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Langella, E.; Gogliettino, M.; Saviano, M.; Pocsfalvi, G.; Rossi, M. A novel class of protease targets of phosphatidylethanolamine-binding proteins (PEBP): A study of the acylpeptide hydrolase and the PEBP inhibitor from the archaeon Sulfolobus solfataricus. Mol. Biosyst. 2010, 6, 2498–2507. [Google Scholar] [CrossRef]
- Sako, Y.; Nomura, N.; Uchida, A.; Ishida, Y.; Morii, H.; Koga, Y.; Hoaki, T.; Maruyama, T. Aeropyrum pernix gen. nov., sp. nov., a novel aerobic hyperthermophilic archaeon growing at temperatures up to 100 degrees C. Int. J. Syst. Bacteriol. 1996, 46, 1070–1077. [Google Scholar] [CrossRef]
- Ciaramella, M.; Pisani, F.M.; Rossi, M. Molecular biology of extremophiles: Recent progress on the hyperthermophilic archaeon Sulfolobus. Antonie Van Leeuwenhoek 2002, 81, 85–97. [Google Scholar] [CrossRef]
- Zaparty, M.; Esser, D.; Gertig, S.; Haferkamp, P.; Kouril, T.; Manica, A.; Pham, T.K.; Reimann, J.; Schreiber, K.; Sierocinski, P.; et al. “Hot standards” for the thermoacidophilic archaeon Sulfolobus colfataricus. Extremophiles 2010, 14, 119–142. [Google Scholar] [CrossRef]
- Reichel, C.; Mathur, J.; Ecker, P.; Langenkemper, K.; Koncz, C.; Schell, J.; Reiss, B.; Maas, C. Enhanced green fluorescence by the expression of an aequorea victoria green fluorescent protein mutant in mono- and dicotyledonous plant cells. Proc. Natl. Acad. Sci. USA 1996, 93, 5888–5893. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, M.L.; Vollano, L.; Peruzy, M.F.; Marrone, R.; Mercogliano, R. Determination of nitrate and nitrite levels in infant foods marketed in Southern Italy. CyTA-J. Food 2015, 13, 629–634. [Google Scholar] [CrossRef]
- Restrepo, M.A.; Freed, D.D.; Carrington, J.C. Nuclear transport of plant potyviral proteins. Plant Cell 1990, 2, 987–998. [Google Scholar]
- Conley, A.J.; Joensuu, J.J.; Richman, A.; Menassa, R. Protein body-inducing fusions for high-level productionand purification of recombinant proteins in plants. Plant Biotechnol. J. 2011, 9, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.B. Rare codon clusters at 5’-end influence heterologous expression of archaeal gene in Escherichia coli. Protein Expr. Purif. 2006, 50, 49–57. [Google Scholar] [CrossRef]
- Romao, S. Therapeutic value of oral supplementation with melon superoxide dismutase and wheat gliadin combination. Nutrients 2015, 31, 430–436. [Google Scholar] [CrossRef]
- Sáez-Hernández, R.; Antela, K.U.; Mauri-Aucejo, A.R.; Morales-Rubio, A.; Cervera, M.L. Smartphone-based colorimetric study of adulterated tuna samples. Food Chem. 2022, 389, 133063. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Roncales, P. Carbon monoxide in meat and fish packaging: Advantages and limits. Foods 2018, 7, 12. [Google Scholar] [CrossRef]
- Howes, B.D.; Milazzo, L.; Droghetti, E.; Nocentini, M.; Smulevich, G. Addition of sodium ascorbate to extend the shelf-life of tuna meat fish: A risk or a benefit for consumers? J. Inorg. Biochem. 2019, 200, 110813. [Google Scholar] [CrossRef] [PubMed]
- Chaijan, M. Physicochemical changes of tilapia (Oreochromis niloticus) muscle during salting. Food Chem. 2011, 129, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Hambridge, T. Nitrate and nitrite: Intake assessment. WHO Food Addit. Ser. 2003, 50, 1053–1071. [Google Scholar]
- Blank, J.M.; Farwell, C.J.; Morrissette, J.M.; Schallert, R.J.; Block, B.A. Influence of swimming speed on metabolic rates of juvenile Pacific bluefin tuna and yellowfin tuna. Physiol. Biochem. Zool. 2007, 80, 167–177. [Google Scholar] [CrossRef]
- Marques, S.S.; Magalhaes, L.M.; Mota, A.I.P.; Soares, T.R.P.; Korsak, B.; Reis, S.; Segundo, M.A. Myoglobin microplate assay to evaluate prevention of protein peroxidation. J. Pharm Biomed. Anal. 2015, 114, 305–311. [Google Scholar] [CrossRef]
- Lindley, M.G. The impact of food processing on antioxidants in vegetable oils, fruits and vegetables. Trends Food Sci. Technol. 1998, 9, 336–340. [Google Scholar] [CrossRef]
- Poljsak, B.; Kovač, V.; Milisav, I. Antioxidants, food processing and health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and hot extremozymes: Industrial relevance and current trends. Front. Bioeng. Biotechnol. 2015, 20, 148. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogliettino, M.; Arciello, S.; Cillo, F.; Carluccio, A.V.; Palmieri, G.; Apone, F.; Ambrosio, R.L.; Anastasio, A.; Gratino, L.; Carola, A.; et al. Recombinant Expression of Archaeal Superoxide Dismutases in Plant Cell Cultures: A Sustainable Solution with Potential Application in the Food Industry. Antioxidants 2022, 11, 1731. https://doi.org/10.3390/antiox11091731
Gogliettino M, Arciello S, Cillo F, Carluccio AV, Palmieri G, Apone F, Ambrosio RL, Anastasio A, Gratino L, Carola A, et al. Recombinant Expression of Archaeal Superoxide Dismutases in Plant Cell Cultures: A Sustainable Solution with Potential Application in the Food Industry. Antioxidants. 2022; 11(9):1731. https://doi.org/10.3390/antiox11091731
Chicago/Turabian StyleGogliettino, Marta, Stefania Arciello, Fabrizio Cillo, Anna Vittoria Carluccio, Gianna Palmieri, Fabio Apone, Rosa Luisa Ambrosio, Aniello Anastasio, Lorena Gratino, Antonietta Carola, and et al. 2022. "Recombinant Expression of Archaeal Superoxide Dismutases in Plant Cell Cultures: A Sustainable Solution with Potential Application in the Food Industry" Antioxidants 11, no. 9: 1731. https://doi.org/10.3390/antiox11091731
APA StyleGogliettino, M., Arciello, S., Cillo, F., Carluccio, A. V., Palmieri, G., Apone, F., Ambrosio, R. L., Anastasio, A., Gratino, L., Carola, A., & Cocca, E. (2022). Recombinant Expression of Archaeal Superoxide Dismutases in Plant Cell Cultures: A Sustainable Solution with Potential Application in the Food Industry. Antioxidants, 11(9), 1731. https://doi.org/10.3390/antiox11091731







