Low Concentrations of Oxidized Phospholipids Increase Stress Tolerance of Endothelial Cells
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Analyses of Cells Accumulated in BAL
2.3. Culturing of Cells
2.4. Nuclei Counting Using Hoechst 33,342
2.5. Metabolic Activity Assessment
2.6. ELISA
2.7. Proteomic Analyses of Secreted Proteins
2.7.1. Sample Preparation
2.7.2. In-Solution Protein Reduction, Alkylation, and Digestion
2.7.3. Analysis by NanoLC-MSMS
2.8. Transendothelial Electrical Resistance
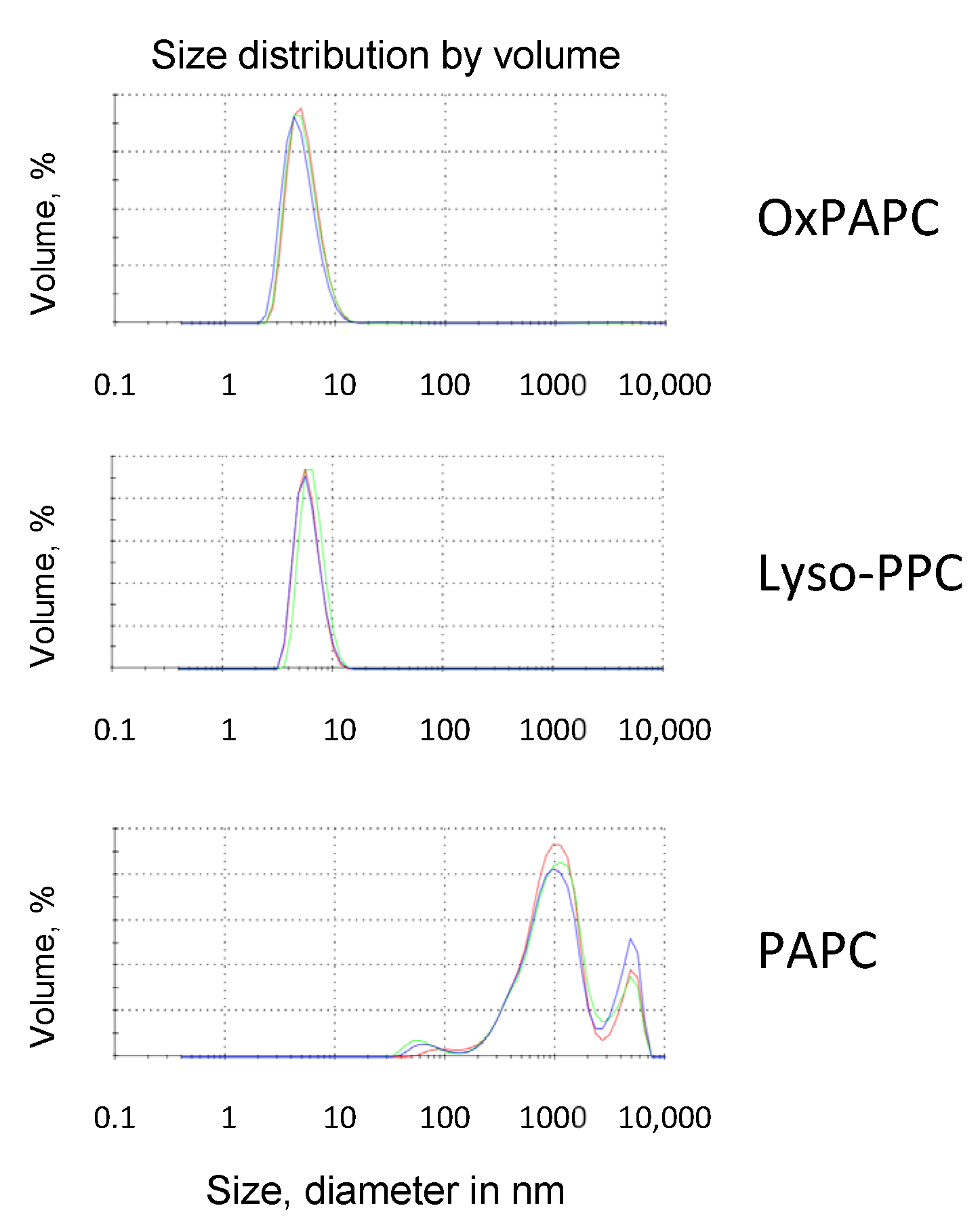
2.9. Dynamic Light Scattering
2.10. Western Blotting
2.11. Statistical Analyses
3. Results
3.1. Phenomenon of Endothelial Protection by OxPLs
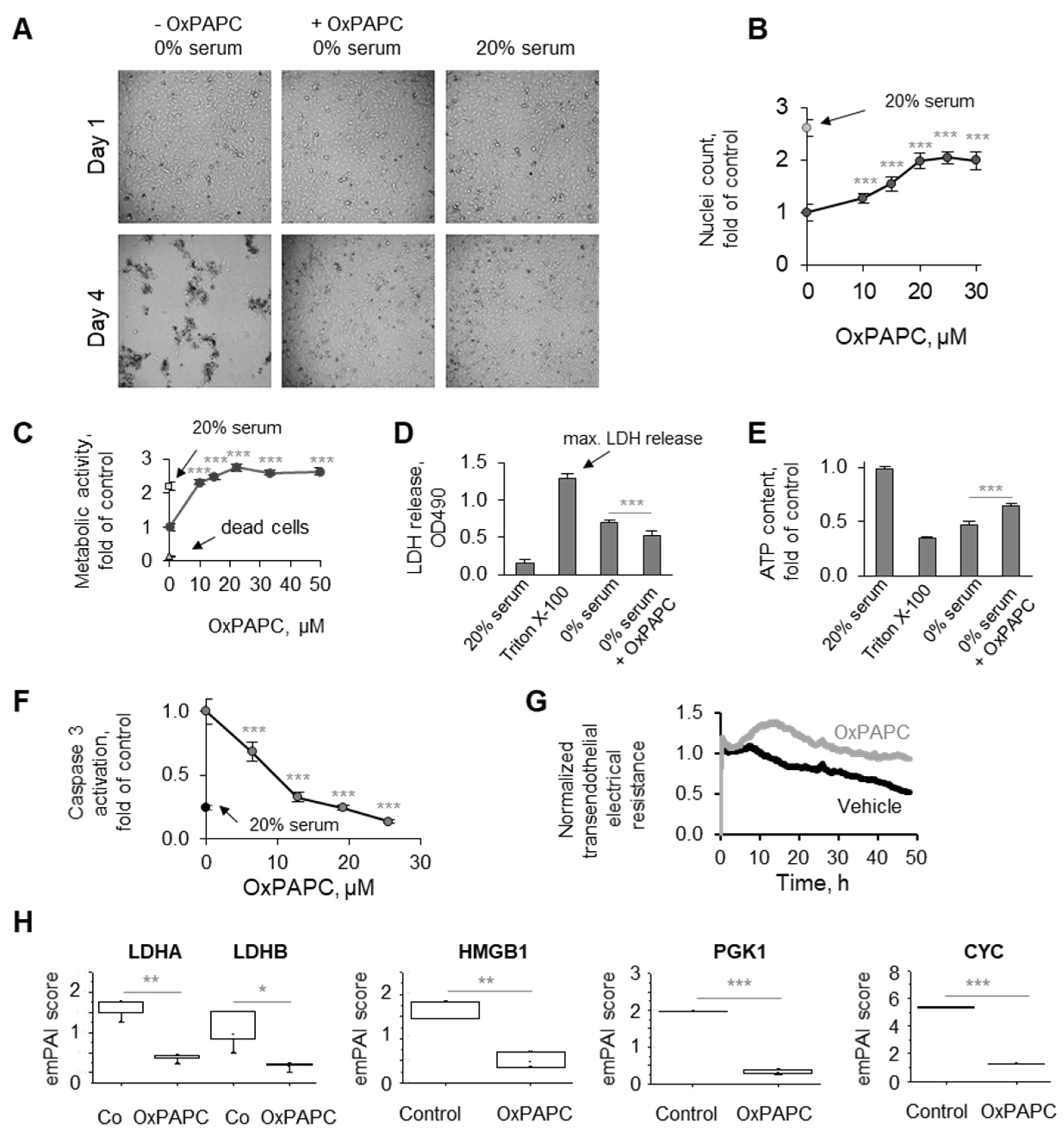
3.1.1. Protective Action of OxPLs Can Be Demonstrated Using Different Experimental Readouts
3.1.2. OxPLs Protect Primary Endothelial Cells Isolated from Different Vessels
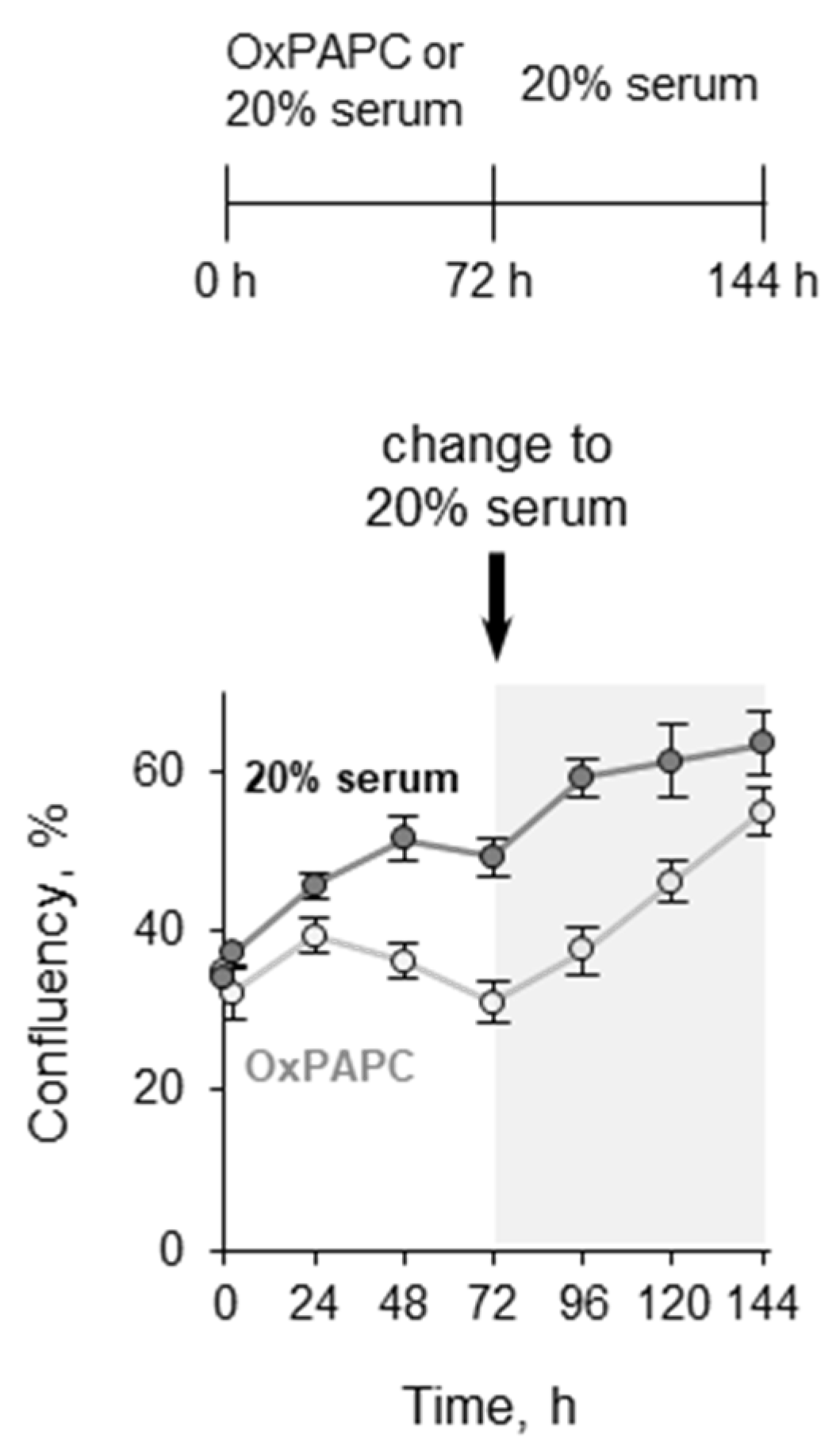
3.1.3. Pretreatment with OxPLs Does Not Inhibit Proliferation of ECs after Serum Replenishment
3.1.4. OxPAPC Protects Cells in the Presence of Toxins
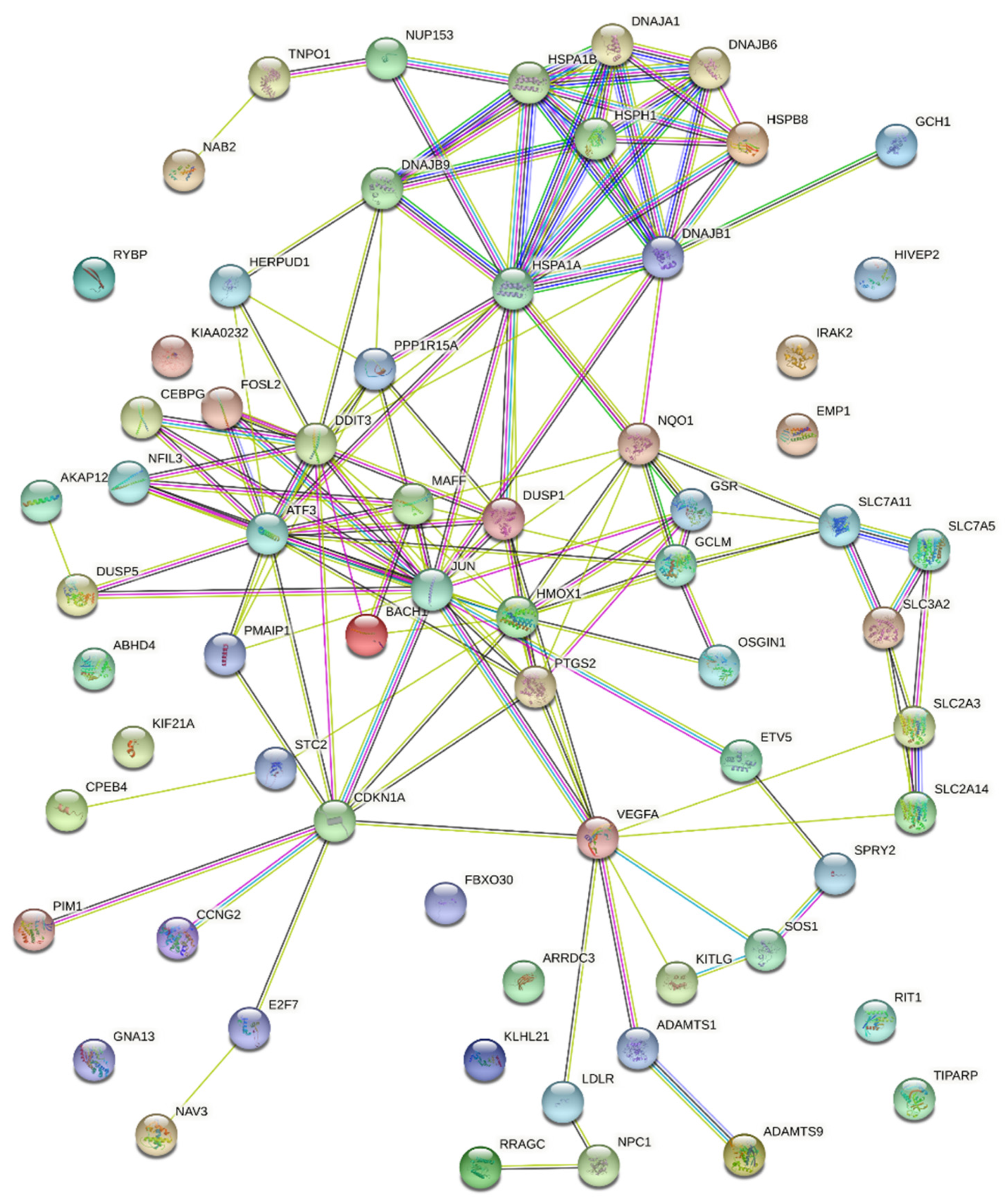
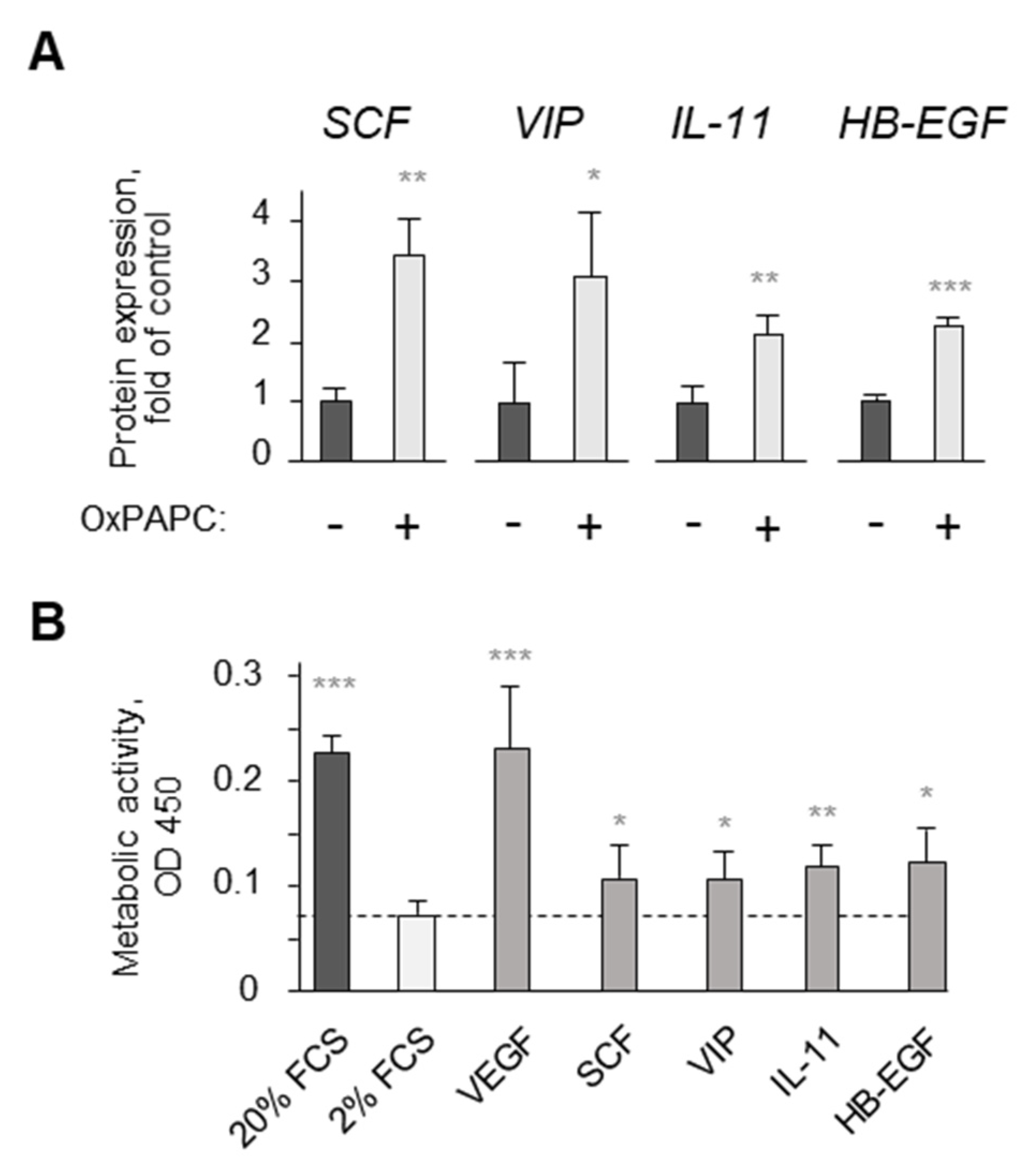
3.2. Potential Mechanisms Mediating Protective Action of OxPLs
| GO-Term/Pathway/Cluster | False Discovery Rate | Genes | |
|---|---|---|---|
| Stress response | GO:0033554; Cellular response to stress | 3.19 × 10−7 | PPP1R15A; HMOX1; GSR; DNAJB9; DNAJB1; IRAK2; CPEB4; STC2; SLC7A11; HSPB8; VEGFA; ETV5; NQO1; E2F7; PMAIP1; ATF3; PTGS2; JUN; RRAGC; HSPA1B; HSPA1A; BACH1; CDKN1A; HERPUD1; DDIT3 |
| HSA-2262752; Cellular responses to stress | 9.87 × 10−8 | PPP1R15A; GSR; DNAJB1; DNAJB6; HSPB8; VEGFA; CEBPG; HSPH1; ATF3; JUN; RRAGC; HSPA1B; DNAJA1; CDKN1A; NUP153; DDIT3 | |
| NRF2 | WP2884; NRF2 pathway | 3.53 × 10−8 | SLC2A3; HMOX1; GSR; DNAJB1; SLC7A11; NQO1; MAFF; GCLM; HSPA1B; SLC2A14 |
| UPR | GO:0006986; Response to unfolded protein | 4.61 × 10−8 | DNAJB9; DNAJB1; STC2; HSPB8; HSPH1; ATF3; HSPA1B; HSPA1A; DNAJA1; HERPUD1; DDIT3 |
| GO:0006457; Protein folding | 0.0081 | DNAJB1; DNAJB6; HSPH1; HSPA1B; HSPA1A; DNAJA1 | |
| HSA-381119; Unfolded protein response (UPR) | 0.0055 | DNAJB9; CEBPG; ATF3; HERPUD1; DDIT3 | |
| HSF1 | HSA-3371453; Regulation of HSF1-mediated heat shock response | 0.0019 | DNAJB1; DNAJB6; HSPB8; HSPH1; HSPA1B; NUP153 |
| Cell death * | GO:0060548; Negative regulation of cell death | 8.50 × 10−7 | HMOX1; KITLG; DUSP1; VEGFA; DNAJB6; CPEB4; NPC1; SLC7A11; NQO1; PTGS2; GCLM; JUN; PIM1; HSPA1B; HSPA1A; SPRY2; DNAJA1; CDKN1A; HERPUD1 |
| GO:0043066; Negative regulation of apoptotic process | 5.19 × 10−6 | HMOX1; KITLG; DUSP1; VEGFA; DNAJB6; CPEB4; NQO1; PTGS2; GCLM; JUN; PIM1; HSPA1B; HSPA1A; SPRY2; DNAJA1; CDKN1A; HERPUD1 | |
| GO:0043068; Positive regulation of programmed cell death | 5.19 × 10−6 | HMOX1; DUSP1; NQO1; PMAIP1; ATF3; OSGIN1; PTGS2; JUN; DNAJA1; AKAP12; CDKN1A; SOS1; GNA13; RYBP; DDIT3 | |
| Amino acid transport | CL:14971; Amino acid transport across the plasma membrane | 0.00090 | SLC2A3; SLC7A5; SLC7A11; SLC3A2; SLC2A14 |
| GO:0089718; Amino acid import across plasma membrane | 0.0039 | SLC7A5; SLC7A11; SLC3A2 | |
| VEGFA ** | WP3888; VEGFA-VEGFR2 signaling pathway | 5.81 × 10−5 | VEGFA; DNAJB9; ADAMTS1; PTGS2; DUSP5; JUN; HSPA1B; DNAJA1; HERPUD1; ADAMTS9; SLC2A14 |
3.3. Structure–Activity Relationship Analysis
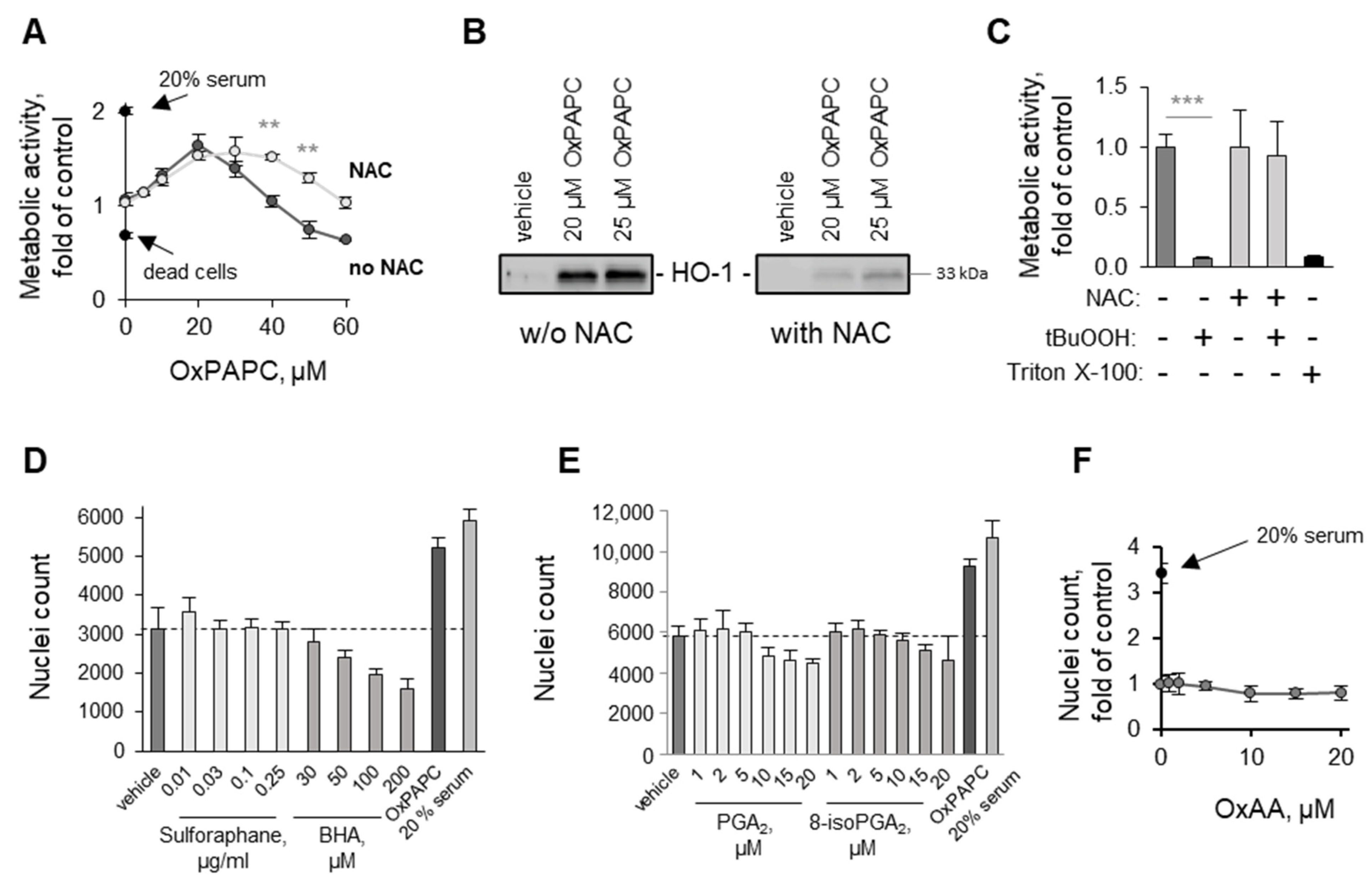
3.3.1. Oxidation of Fatty Acid Residues Is Crucial for Protective Activity
3.3.2. Lyso-PLs Are Protective
3.4. Detergent Properties as a Potential Prerequisite for the Protective Activity of OxPLs and Lyso-PLs
Upon Oxidation, PLs Acquire Detergent-Like Properties
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8(OH)C8-NH-PC | 1-O-hexadecyl-2-deoxy-2-(8-hydroxy-octanoyl)amino-sn-glycero-3-phosphocholine |
| 12(OH)C12-NH-PC | 1-O-hexadecyl-2-deoxy-2-(12-hydroxy-dodecanoyl)amino-sn-glycero-3-phosphocholine |
| 8-iso-PGA2 | 8-iso-prostaglandin A2 |
| α-CD | α-cyclodextrin |
| ara-C | cytosine β-D-arabinofuranoside hydrochloride, arabinoside-C |
| BCH | 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid, 2-amino-2-norbornanecarboxylic acid |
| BHA | butylated hydroxyanisole |
| tBuOOH | tert-butyl hydroperoxide |
| β-CD | β-cyclodextrin |
| CM | conditioned medium |
| CYC | cytochrome c |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| EC | endothelial cell |
| FBS | fetal bovine serum |
| FCS | fetal calf serum |
| GGA | geranylgeranylacetone |
| HBMVEC | human bovine microvascular endothelial cell |
| HB-EGF | heparin-binding EGF-like growth factor |
| HCAEC | human coronary artery endothelial cell |
| HLMVEC | human lung microvascular endothelial cell |
| HMGB1 | high-mobility group B1 protein |
| HPAEC | human pulmonary artery endothelial cell |
| HSF1 | heat-shock factor 1 |
| HSP | heat-shock protein |
| HUVEC | human umbilical vein endothelial cell |
| IL-11 | interleukin-11 |
| KDdiAPC | 1-palmitoyl-2-(4-keto-dodec-3-ene-dioyl)-sn-glycero-3-phosphocholine |
| KOdiAPC | 1-palmitoyl-2-(5-keto-6-octene-dioyl)-sn-glycero-3-phosphocholine |
| LDH | lactate dehydrogenase |
| LDHA | lactate dehydrogenase A chain |
| LDHB | lactate dehydrogenase B chain |
| Lyso-PPC | 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine |
| Lyso-PPE | 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine |
| Lyso-PPG | 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoglycerol |
| Lyso-OPA | 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate |
| Lyso-PPI | 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoinositol |
| Lyso-PPS | 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoserine |
| MβCD | methyl-β-cyclodextrin |
| NAC | N-acetyl cysteine |
| OxAA | oxidized arachidonic acid |
| OxPAPA | oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidic acid |
| OxPAPC | oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine |
| OxPAPE | oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine |
| OxPAPG | oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoglycerol |
| OxPAPS | oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoserine |
| OxPDHPC | oxidized 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine |
| OxPLPC | oxidized 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine |
| OxPL | oxidized phospholipid |
| PAF | platelet-activating factor |
| PAzPC | 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine |
| PAPA | 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidic acid |
| PAPC | 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine |
| PAPE | 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine |
| PAPG | 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoglycerol |
| PAPS | 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoserine |
| PGA2 | prostaglandin A2 |
| PGE2-NH-PC | 1-O-hexadecyl-2-deoxy-2-(prostaglandin E2)amino-sn-glycero-3-phosphocholine |
| PGF2α-NH-PC | 1-O-hexadecyl-2-deoxy-2-(prostaglandin F2α)amino-sn-glycero-3-phosphocholine |
| PGK1 | phosphoglycerate kinase 1 |
| PGPC | 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphocholine |
| PONPC | 1-palmitoyl-2-(9’-oxononanoyl)-sn-glycero-3-phosphocholine |
| POVPC | 1-palmitoyl-2-(5’-oxovaleroyl)-sn-glycero-3-phosphocholine |
| PUFA | polyunsaturated fatty acid |
| RIPA | radioimmunoprecipitation assay |
| SCF | stem-cell factor |
| TER | transendothelial electrical resistance |
| TUDCA | tauroursodeoxycholic acid |
| VEGF | vascular endothelial growth factor |
| VIP | vasoactive intestinal peptide |
References
- Spickett, C.M. Formation of Oxidatively Modified Lipids as the Basis for a Cellular Epilipidome. Front. Endocrinol. 2020, 11, 602771. [Google Scholar] [CrossRef]
- O’donnell, V.B.; Rossjohn, J.; Wakelam, M.J. Phospholipid Signaling in Innate Immune Cells. J. Clin. Investig. 2018, 128, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Oskolkova, O.V.; Bochkov, V.N. Gain of function mechanisms triggering biological effects of oxidized phospholipids. Curr. Opin. Toxicol. 2020, 20–21, 85–94. [Google Scholar] [CrossRef]
- Zhivaki, D.; Kagan, J.C. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat. Rev. Immunol. 2022, 22, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Kadl, A.; Huber, J.; Gruber, F.; Binder, B.R.; Leitinger, N. Protective Role of Phospholipid Oxidation Products in Endotoxin-Induced Tissue Damage. Nature 2002, 419, 77–81. [Google Scholar] [CrossRef]
- Chu, L.H.; Indramohan, M.; Ratsimandresy, R.A.; Gangopadhyay, A.; Morris, E.P.; Monack, D.M.; Dorfleutner, A.; Stehlik, C. The oxidized phospholipid oxpapc protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat. Commun. 2018, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Feng, Q.; Wolleb, H.; Shamshiev, A.; Ebner, C.; Tortola, L.; Broz, P.; Carreira, E.M.; Kopf, M. Cyclopentenone prostaglandins and structurally related oxidized lipid species instigate and share distinct pro- and anti-inflammatory pathways. Cell Rep. 2020, 30, 4399–4417.e7. [Google Scholar] [CrossRef]
- Que, X.; Hung, M.-Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef]
- Sun, X.; Seidman, J.S.; Zhao, P.; Troutman, T.D.; Spann, N.J.; Que, X.; Zhou, F.; Liao, Z.; Pasillas, M.; Yang, X.; et al. Neutralization of oxidized phospholipids ameliorates non-alcoholic steatohepatitis. Cell Metab. 2020, 31, 189–206.e8. [Google Scholar] [CrossRef]
- Yeang, C.; Hasanally, D.; Que, X.; Hung, M.-Y.; Stamenkovic, A.; Chan, D.; Chaudhary, R.; Margulets, V.; Edel, A.L.; Hoshijima, M.; et al. Reduction of myocardial ischaemia-reperfusion injury by inactivating oxidized phospholipids. Cardiovasc. Res. 2019, 115, 179–189. [Google Scholar] [CrossRef]
- Ambrogini, E.; Que, X.; Wang, S.; Yamaguchi, F.; Weinstein, R.S.; Tsimikas, S.; Manolagas, S.C.; Witztum, J.L.; Jilka, R.L. Oxidation-Specific epitopes restrain bone formation. Nat. Commun. 2018, 9, 2193. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S. Potential causality and emerging medical therapies for lipoprotein(a) and its associated oxidized phospholipids in calcific aortic valve stenosis. Circ. Res. 2019, 124, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, G.O.; Hermetter, A. Mediation of apoptosis by oxidized phospholipids. Subcell. Biochem. 2008, 49, 351–367. [Google Scholar] [CrossRef]
- Oskolkova, O.V.; Afonyushkin, T.; Preinerstorfer, B.; Bicker, W.; Von Schlieffen, E.; Hainzl, E.; Demyanets, S.; Schabbauer, G.; Lindner, W.; Tselepis, A.D.; et al. Oxidized Phospholipids Are More Potent Antagonists Of Lipopolysaccharide Than Inducers Of Inflammation. J. Immunol. 2010, 185, 7706–7712. [Google Scholar] [CrossRef] [PubMed]
- Von Schlieffen, E.; Oskolkova, O.V.; Schabbauer, G.; Gruber, F.; Blüml, S.; Genest, M.; Kadl, A.; Marsik, C.; Knapp, S.; Chow, J.; et al. Multi-Hit inhibition of circulating and cell-associated components of the toll-like receptor 4 pathway by oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 356–362. [Google Scholar] [CrossRef]
- Jyrkkänen, H.-K.; Kansanen, E.; Inkala, M.; Kivelä, A.M.; Hurttila, H.; Heinonen, S.E.; Goldsteins, G.; Jauhiainen, S.; Tiainen, S.; Makkonen, H.; et al. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ. Res. 2008, 103, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Oskolkova, O.V.; Hodzic, A.; Karki, P.; Gesslbauer, B.; Ke, Y.; Hofer, D.C.; Bogner-Strauss, J.G.; Galano, J.-M.; Oger, C.; Birukova, A.; et al. Oxidized phospholipids on alkyl-amide scaffold demonstrate anti-endotoxin and endothelial barrier-protective properties. Free Radic. Biol. Med. 2021, 174, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Gesslbauer, B.; Kuerzl, D.; Valpatic, N.; Bochkov, V.N. Unbiased identification of proteins covalently modified by complex mixtures of peroxidized lipids using a combination of electrophoretic mobility band shift with mass spectrometry. Antioxidants 2018, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (empai) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Birukova, A.A.; Smurova, K.; Birukov, K.G.; Kaibuchi, K.; Garcia, J.G.N.; Verin, A.D. Role of rho gtpases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc. Res. 2004, 67, 64–77. [Google Scholar] [CrossRef]
- Birukova, A.A.; Adyshev, D.; Gorshkov, B.; Bokoch, G.M.; Birukov, K.G.; Verin, A.D. Gef-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L540–L548. [Google Scholar] [CrossRef] [PubMed]
- Gargalovic, P.S.; Imura, M.; Zhang, B.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.-P.; He, A.; Truong, A.; Patel, S.; et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl. Acad. Sci. USA 2006, 103, 12741–12746. [Google Scholar] [CrossRef]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme oxygenases in cardiovascular health and disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef] [PubMed]
- Edinger, A.L. Growth factors regulate cell survival by controlling nutrient transporter expression. Biochem. Soc. Trans. 2005, 33, 225–227. [Google Scholar] [CrossRef]
- Gargalovic, P.S.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.-P.; He, A.; Truong, A.; Baruch-Oren, T.; Berliner, J.A.; Kirchgessner, T.G.; et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2490–2496. [Google Scholar] [CrossRef]
- Milkereit, R.; Persaud, A.; Vanoaica, L.; Guetg, A.; Verrey, F.; Rotin, D. Laptm4b recruits the lat1-4f2hc leu transporter to lysosomes and promotes mtorc1 activation. Nat. Commun. 2015, 6, 7250. [Google Scholar] [CrossRef] [PubMed]
- Hoang, K.N.L.; Anstee, J.E.; Arnold, J.N. The diverse roles of heme oxygenase-1 in tumor progression. Front. Immunol. 2021, 12, 658315. [Google Scholar] [CrossRef] [PubMed]
- Shukla, H.; Gaje, G.; Koucheki, A.; Lee, H.Y.; Sun, X.; Trush, M.A.; Zhu, H.; Li, Y.R.; Jia, Z. Nadph-Quinone oxidoreductase-1 mediates benzo-a-pyrene-1,6-quinone-induced cytotoxicity and reactive oxygen species production in human ea.hy926 endothelial cells. Toxicol. Appl. Pharmacol. 2020, 404, 115180. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Philippova, M.; Oskolkova, O.; Kadl, A.; Furnkranz, A.; Karabeg, E.; Afonyushkin, T.; Gruber, F.; Breuss, J.; Minchenko, A.; et al. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ. Res. 2006, 99, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Van Marion, D.M.; Hu, X.; Zhang, D.; Hoogstra-Berends, F.; Seerden, J.-P.G.; Loen, L.; Heeres, A.; Steen, H.; Henning, R.H.; Brundel, B.J. Screening of novel hsp-inducing compounds to conserve cardiomyocyte function in experimental atrial fibrillation. Drug Des. Devel. Ther. 2019, 13, 345–364. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, H.; Chen, Z.; Cao, Q.; Hu, L.; Wu, Y. Effects of geranylgeranylacetone upon cardiovascular diseases. Cardiovasc. Ther. 2018, 36, e12331. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.; Lehraiki, A.; Millet, A.; Rouaud, F.; Plaisant, M.; Jaune, E.; Botton, T.; Ronco, C.; Abbe, P.; Amdouni, H.; et al. Compounds triggering er stress exert anti-melanoma effects and overcome braf inhibitor resistance. Cancer Cell 2016, 29, 805–819. [Google Scholar] [CrossRef]
- Cortez, L.; Sim, V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion 2014, 8, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Yellon, D.M. The risk pathway and beyond. Basic Res. Cardiol. 2018, 113, 2. [Google Scholar] [CrossRef] [PubMed]
- Satitpitakul, V.; Sun, Z.; Suri, K.; Amouzegar, A.; Katikireddy, K.R.; Jurkunas, U.V.; Kheirkhah, A.; Dana, R. Vasoactive intestinal peptide promotes corneal allograft survival. Am. J. Pathol. 2018, 188, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.M.; De Keulenaer, G.W. Autocrine signaling in cardiac remodeling: A rich source of therapeutic targets. J. Am. Heart Assoc. 2021, 10, e019169. [Google Scholar] [CrossRef]
- Afonyushkin, T.; Oskolkova, O.V.; Bochkov, V.N. Oxidized phospholipids stimulate production of stem cell factor via nrf2-dependent mechanisms. Angiogenesis 2018, 21, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Tew, D.G.; Southan, C.; Rice, S.Q.; Lawrence, M.P.; Li, H.; Boyd, H.F.; Moores, K.; Gloger, I.S.; Macphee, C.H. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 591–599. [Google Scholar] [CrossRef]
- Choi, J.; Zhang, W.; Gu, X.; Chen, X.; Hong, L.; Laird, J.M.; Salomon, R.G. Lysophosphatidylcholine is generated by spontaneous deacylation of oxidized phospholipids. Chem. Res. Toxicol. 2011, 24, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Heerklotz, H. Interactions of surfactants with lipid membranes. Q. Rev. Biophys. 2008, 41, 205–264. [Google Scholar] [CrossRef] [PubMed]
- Tsubone, T.M.; Junqueira, H.C.; Baptista, M.S.; Itri, R. Contrasting roles of oxidized lipids in modulating membrane microdomains. Biochim. Biophys. Acta Biomembr. 2019, 1861, 660–669. [Google Scholar] [CrossRef]
- Yeh, M.; Cole, A.L.; Choi, J.; Liu, Y.; Tulchinsky, D.; Qiao, J.-H.; Fishbein, M.C.; Dooley, A.N.; Hovnanian, T.; Mouilleseaux, K.; et al. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ. Res. 2004, 95, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; London, E. Effect of cyclodextrin and membrane lipid structure upon cyclodextrin-lipid interaction. Langmuir Acs J. Surf. Colloids 2013, 29, 14631–14638. [Google Scholar] [CrossRef]
- Morita, M.; Sekine, A.; Urano, Y.; Nishimura, T.; Takabe, W.; Arai, H.; Hamakubo, T.; Kodama, T.; Noguchi, N. Lysophosphatidylcholine promotes srebp-2 activation via rapid cholesterol efflux and srebp-2-independent cytokine release in human endothelial cells. J. Biochem. 2015, 158, 331–338. [Google Scholar] [CrossRef]
- Shi, Y.; Ruan, H. Toward a membrane-centric biology. Front. Immunol. 2020, 11, 1909. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the keap1-nrf2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Frigerio, G.; Favero, C.; Savino, D.; Mercadante, R.; Albetti, B.; Dioni, L.; Vigna, L.; Bollati, V.; Pesatori, A.C.; Fustinoni, S. Plasma metabolomic profiling in 1391 subjects with overweight and obesity from the sphere study. Metabolites 2021, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Kluza, E.; Beldman, T.J.; Shami, A.; Scholl, E.R.; Malinova, T.S.; Grootemaat, A.E.; Van Der Wel, N.N.; Gonçalves, I.; Huveneers, S.; Mulder, W.J.M.; et al. Diverse ultrastructural landscape of atherosclerotic endothelium. Atherosclerosis 2021, 339, 35–45. [Google Scholar] [CrossRef]
- Watson, A.D.; Leitinger, N.; Navab, M.; Faull, K.F.; Hörkkö, S.; Witztum, J.L.; Palinski, W.; Schwenke, D.; Salomon, R.G.; Sha, W.; et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997, 272, 13597–13607. [Google Scholar] [CrossRef]
- Di Lisi, D.; Madonna, R.; Zito, C.; Bronte, E.; Badalamenti, G.; Parrella, P.; Monte, I.; Tocchetti, C.G.; Russo, A.; Novo, G. Anticancer therapy-induced vascular toxicity: Vegf inhibition and beyond. Int. J. Cardiol. 2017, 227, 11–17. [Google Scholar] [CrossRef]
- Slezak, J.; Kura, B.; Ravingerová, T.; Tribulova, N.; Okruhlicova, L.; Barancik, M. Mechanisms of cardiac radiation injury and potential preventive approaches. Can. J. Physiol. Pharmacol. 2015, 93, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, T.; Szczesny, E.; Chlopicki, S. Detrimental effects of chemotherapeutics and other drugs on the endothelium: A call for endothelial toxicity profiling. Pharmacol. Rep. 2015, 67, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Maulucci, G.; Daniel, B.; Cohen, O.; Avrahami, Y.; Sasson, S. Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells. Mol. Aspects Med. 2016, 49, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Nègre-Salvayre, A.; Augé, N.; Camaré, C.; Bacchetti, T.; Ferretti, G.; Salvayre, R. Dual signaling evoked by oxidized ldls in vascular cells. Free Radic. Biol. Med. 2017, 106, 118–133. [Google Scholar] [CrossRef] [PubMed]








| Antibody | Dilution | Protein Size, kDa | Host |
|---|---|---|---|
| α-actin | 1:3000 | 42 | mouse |
| α-AKT | 1:2000 | 60 | mouse |
| α-phospho-AKT | 1:1000 | 60 | rabbit |
| α-ERK1/2 | 1:1000 | 42 | rabbit |
| α-phospho-ERK1/2 | 1:2000 | 42 | rabbit |
| α-STAT3 | 1:1000 | 80 | mouse |
| α-phospho- STAT3 | 1:2000 | 90 | rabbit |
| α-HO-1 | 1:100 | 33 | mouse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauerhofer, C.; Afonyushkin, T.; Oskolkova, O.V.; Hellauer, K.; Gesslbauer, B.; Schmerda, J.; Ke, Y.; Zimmer, A.; Birukova, A.A.; Birukov, K.G.; et al. Low Concentrations of Oxidized Phospholipids Increase Stress Tolerance of Endothelial Cells. Antioxidants 2022, 11, 1741. https://doi.org/10.3390/antiox11091741
Mauerhofer C, Afonyushkin T, Oskolkova OV, Hellauer K, Gesslbauer B, Schmerda J, Ke Y, Zimmer A, Birukova AA, Birukov KG, et al. Low Concentrations of Oxidized Phospholipids Increase Stress Tolerance of Endothelial Cells. Antioxidants. 2022; 11(9):1741. https://doi.org/10.3390/antiox11091741
Chicago/Turabian StyleMauerhofer, Christina, Taras Afonyushkin, Olga V. Oskolkova, Klara Hellauer, Bernd Gesslbauer, Jasmin Schmerda, Yunbo Ke, Andreas Zimmer, Anna A. Birukova, Konstantin G. Birukov, and et al. 2022. "Low Concentrations of Oxidized Phospholipids Increase Stress Tolerance of Endothelial Cells" Antioxidants 11, no. 9: 1741. https://doi.org/10.3390/antiox11091741
APA StyleMauerhofer, C., Afonyushkin, T., Oskolkova, O. V., Hellauer, K., Gesslbauer, B., Schmerda, J., Ke, Y., Zimmer, A., Birukova, A. A., Birukov, K. G., & Bochkov, V. (2022). Low Concentrations of Oxidized Phospholipids Increase Stress Tolerance of Endothelial Cells. Antioxidants, 11(9), 1741. https://doi.org/10.3390/antiox11091741








