Mechanistic Wound Healing and Antioxidant Potential of Moringa oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Extraction of Moringa oleifera Seeds
2.3. Metabolomic Analysis
2.4. Molecular Docking Study
2.5. Animal Model
2.6. In Vitro IL-6, MMP-1, and MMP-2 Determinations
3. Results and Discussion
3.1. QTOF-MS Assisted Dereplication of the Chemical Constituents in M. oleifera Seed Extract
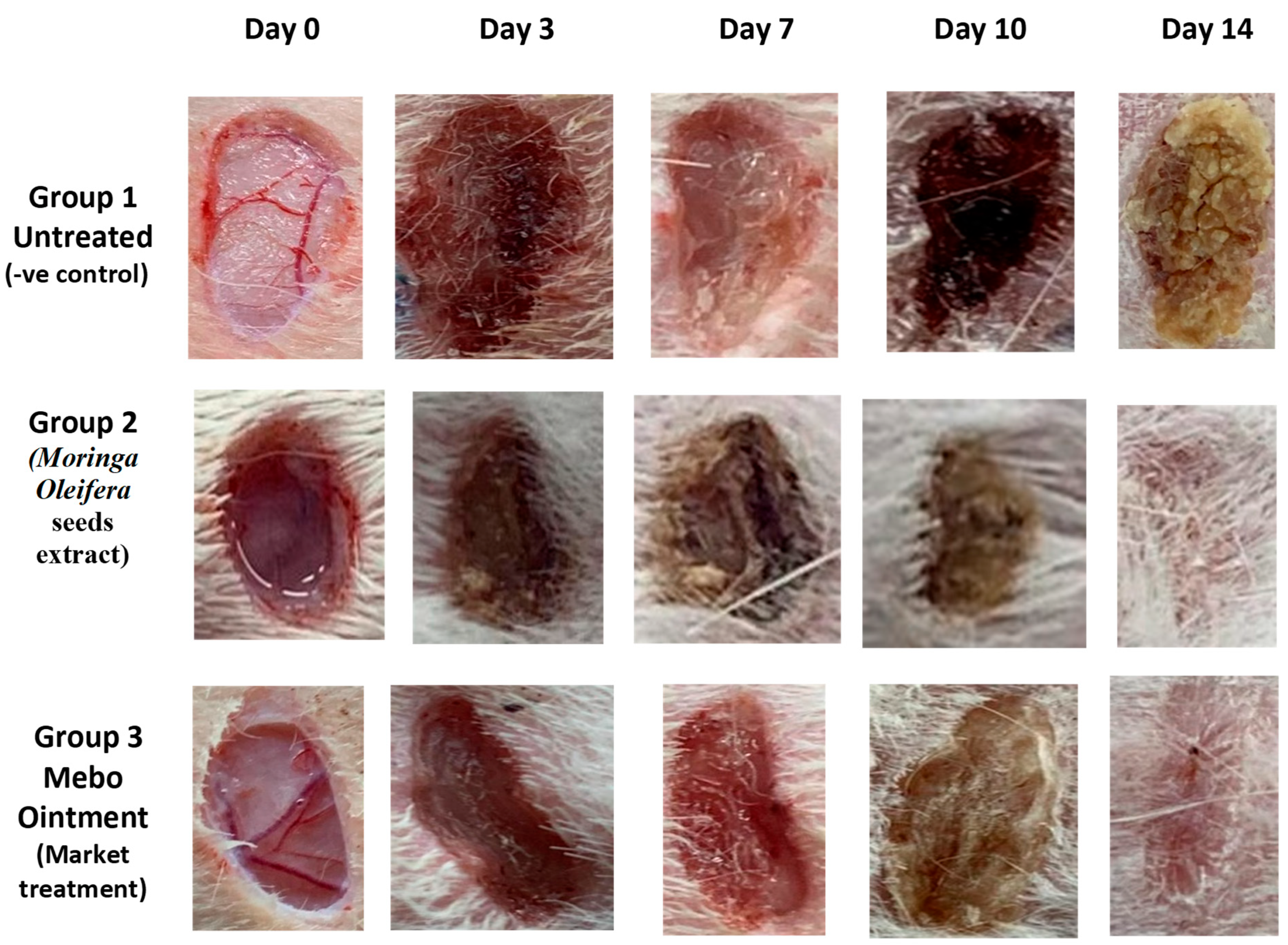
3.2. Wound Closure Process
Estimation of the Wound Closure Rate
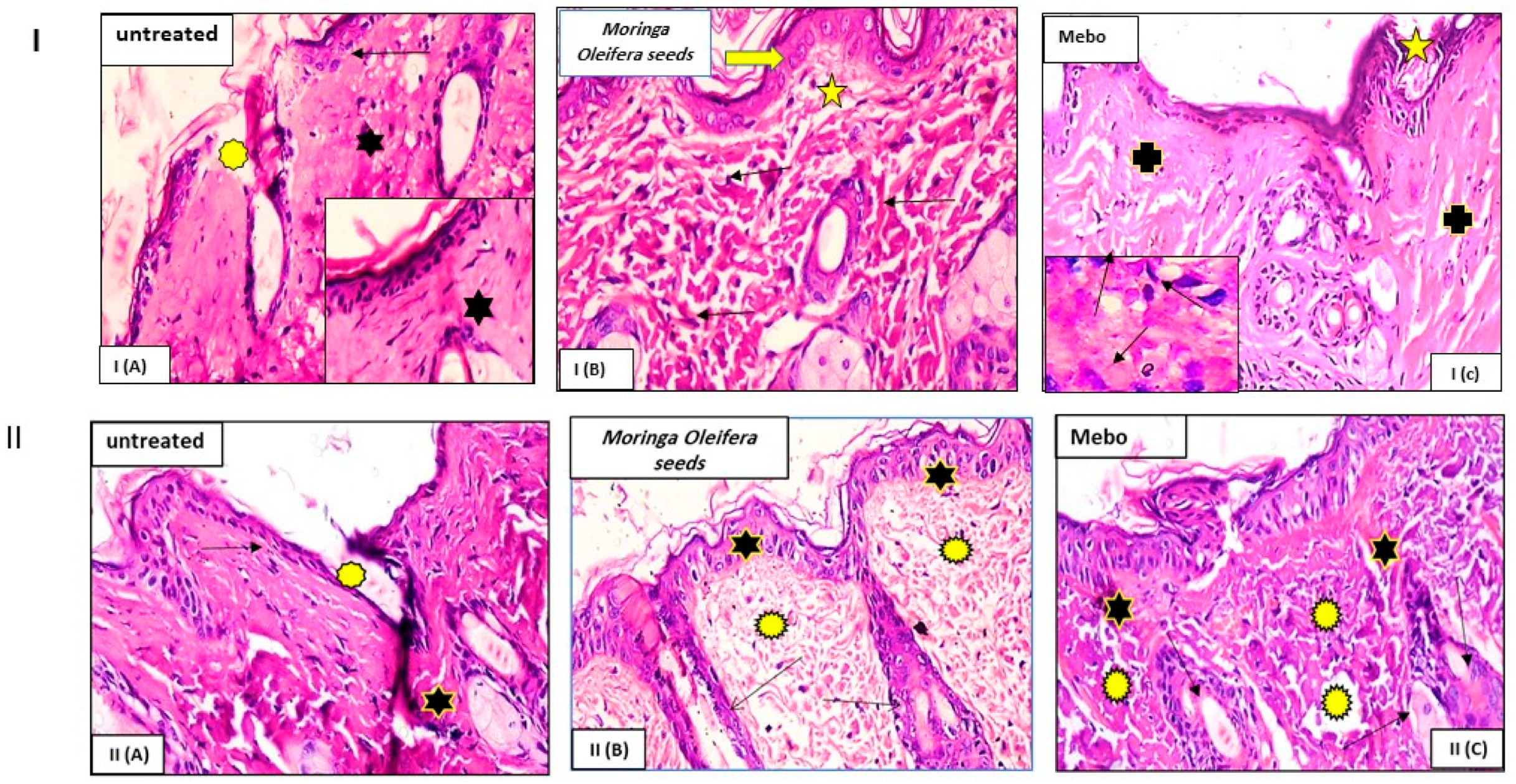
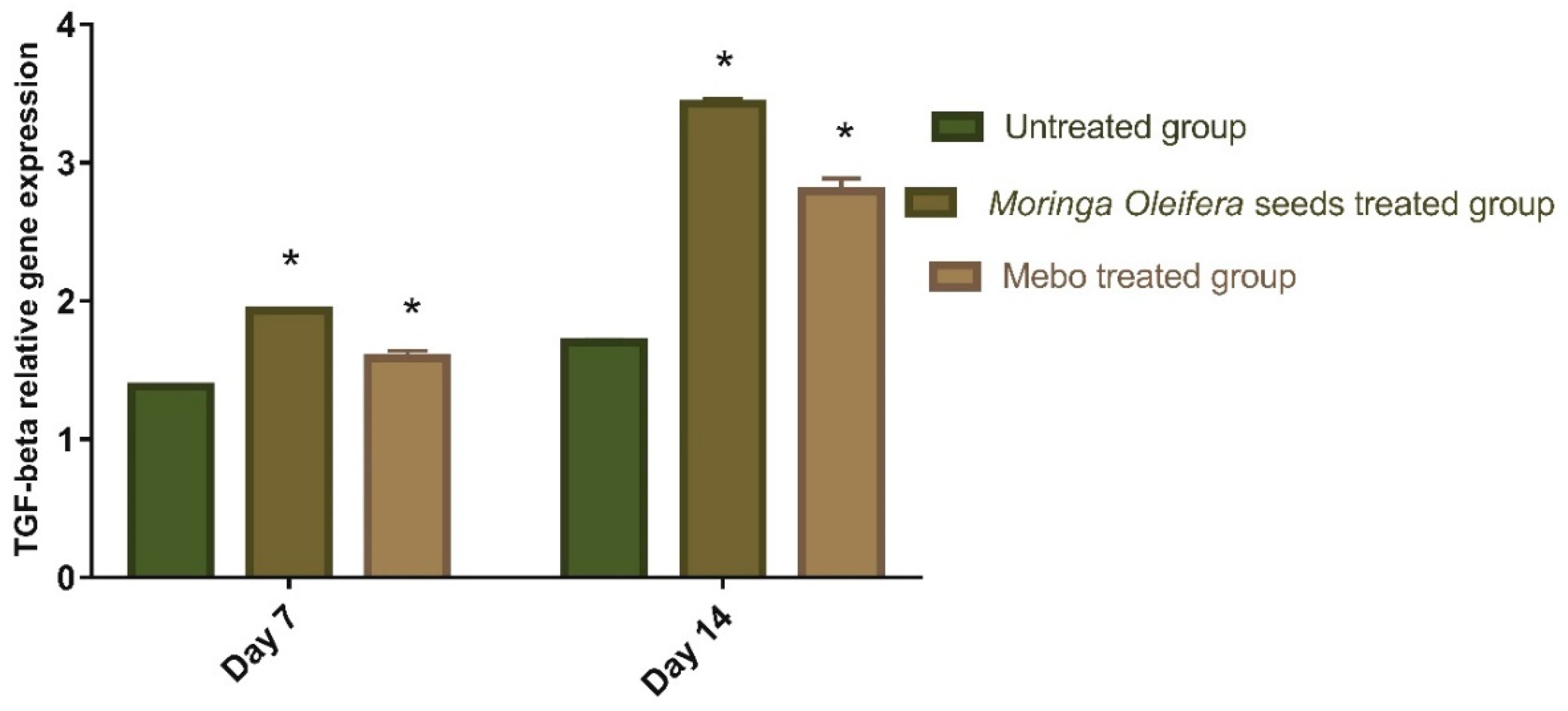
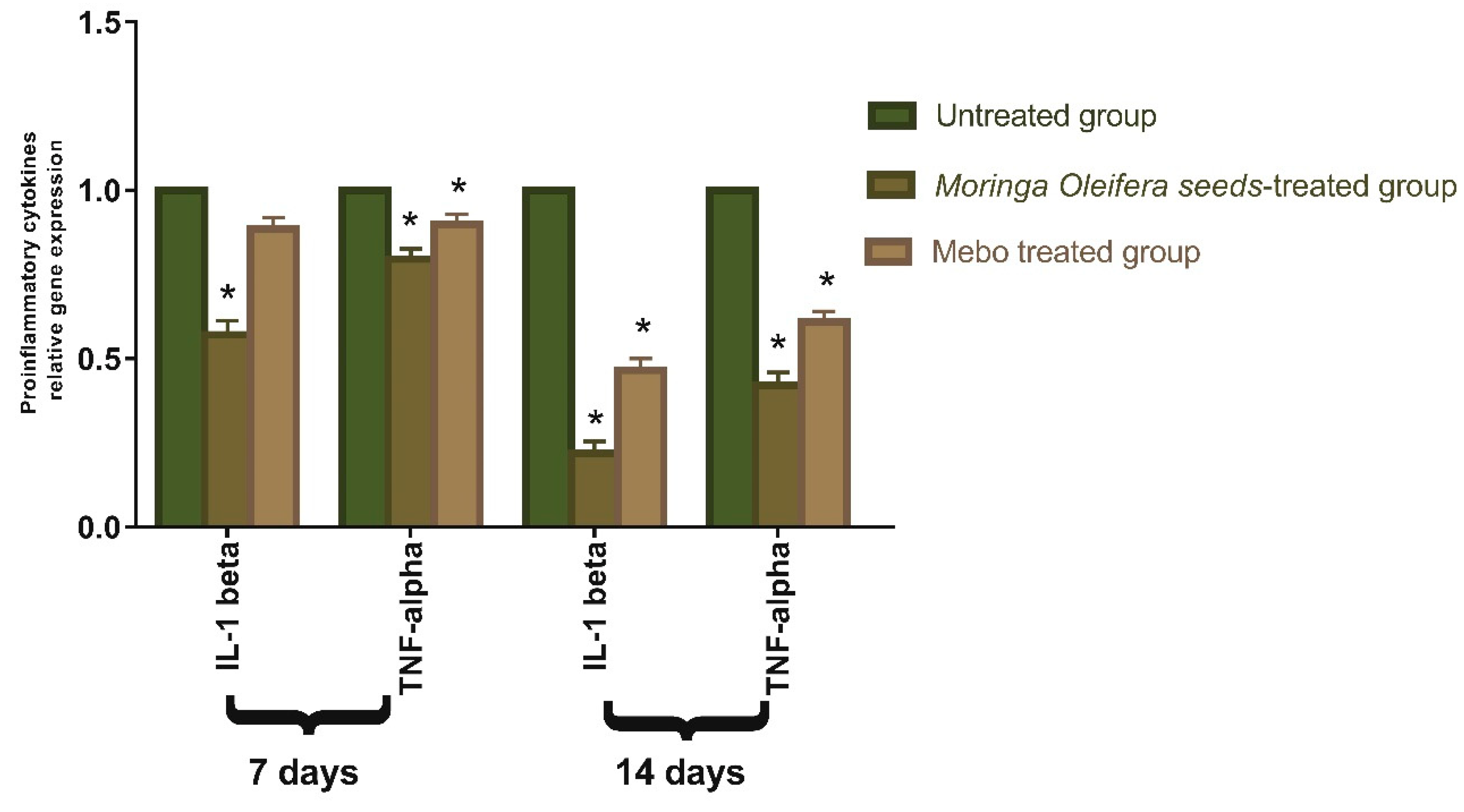
3.3. Effect of Moringa oleifera Seeds Extract on the Expression of TGF-β, TNF-α, and IL-1β
3.4. In Vitro Antioxidant Assessment of Moringa oleifera Seeds Extract
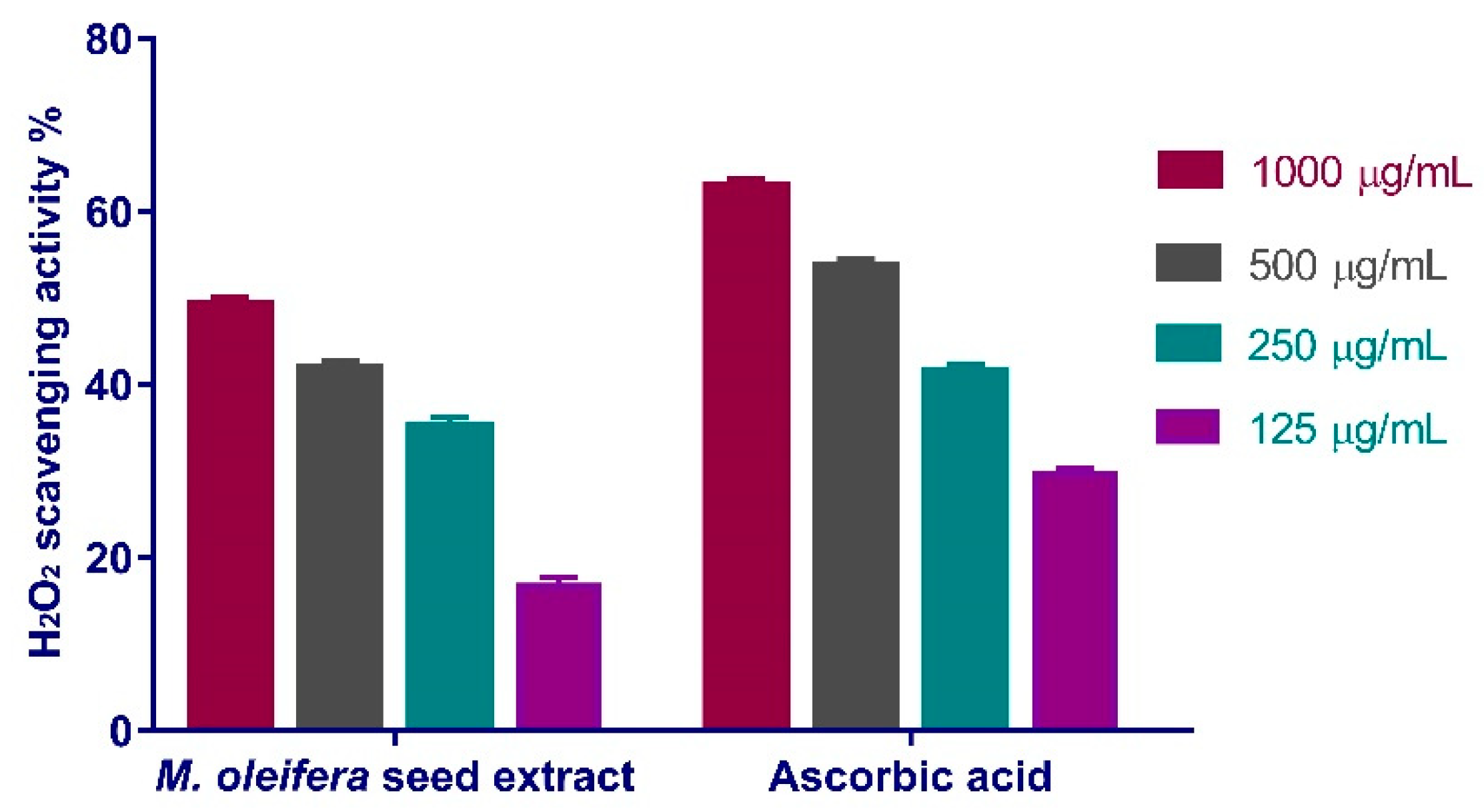
3.4.1. Hydrogen Peroxide Scavenging Activity
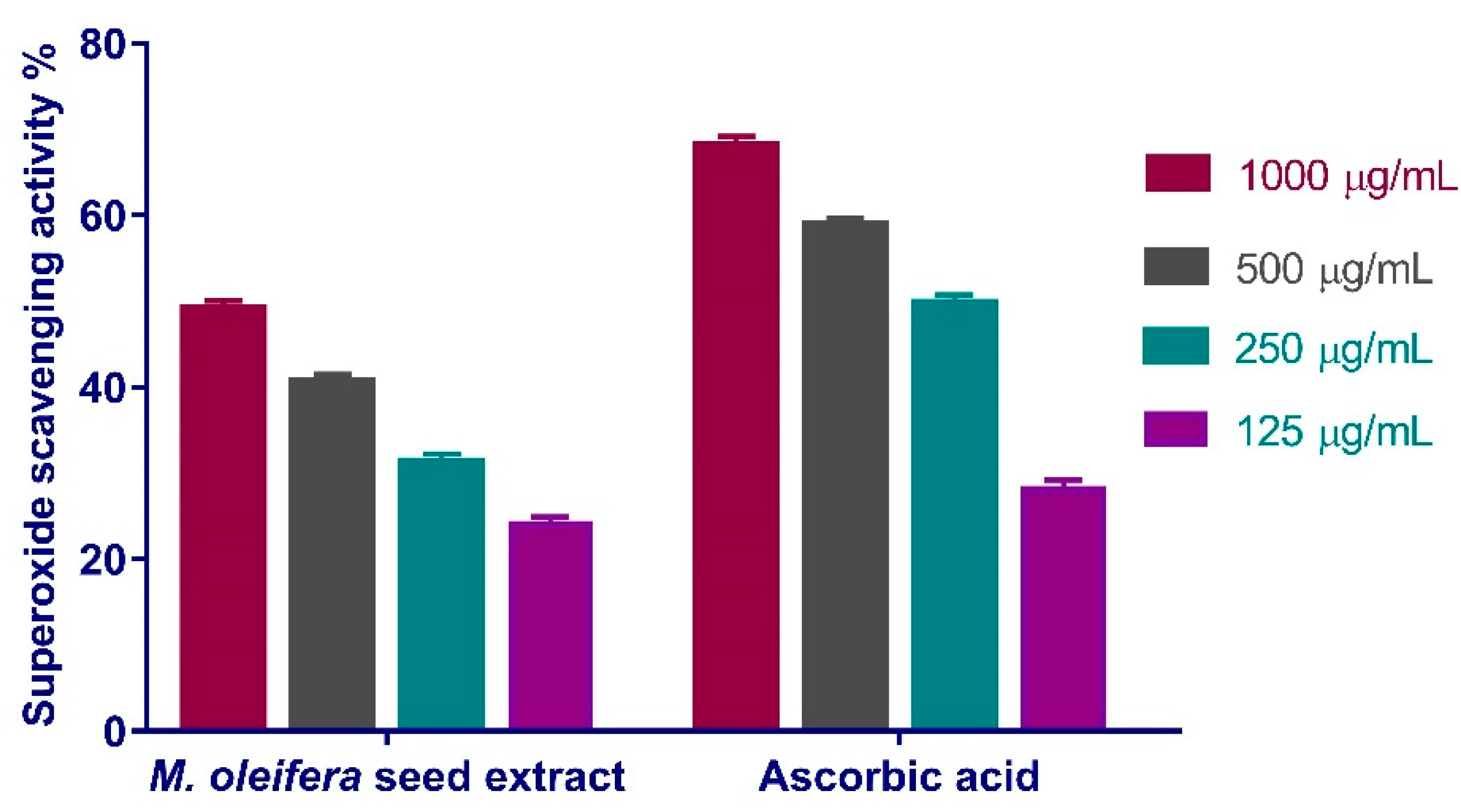
3.4.2. Superoxide Radical Scavenging Activity
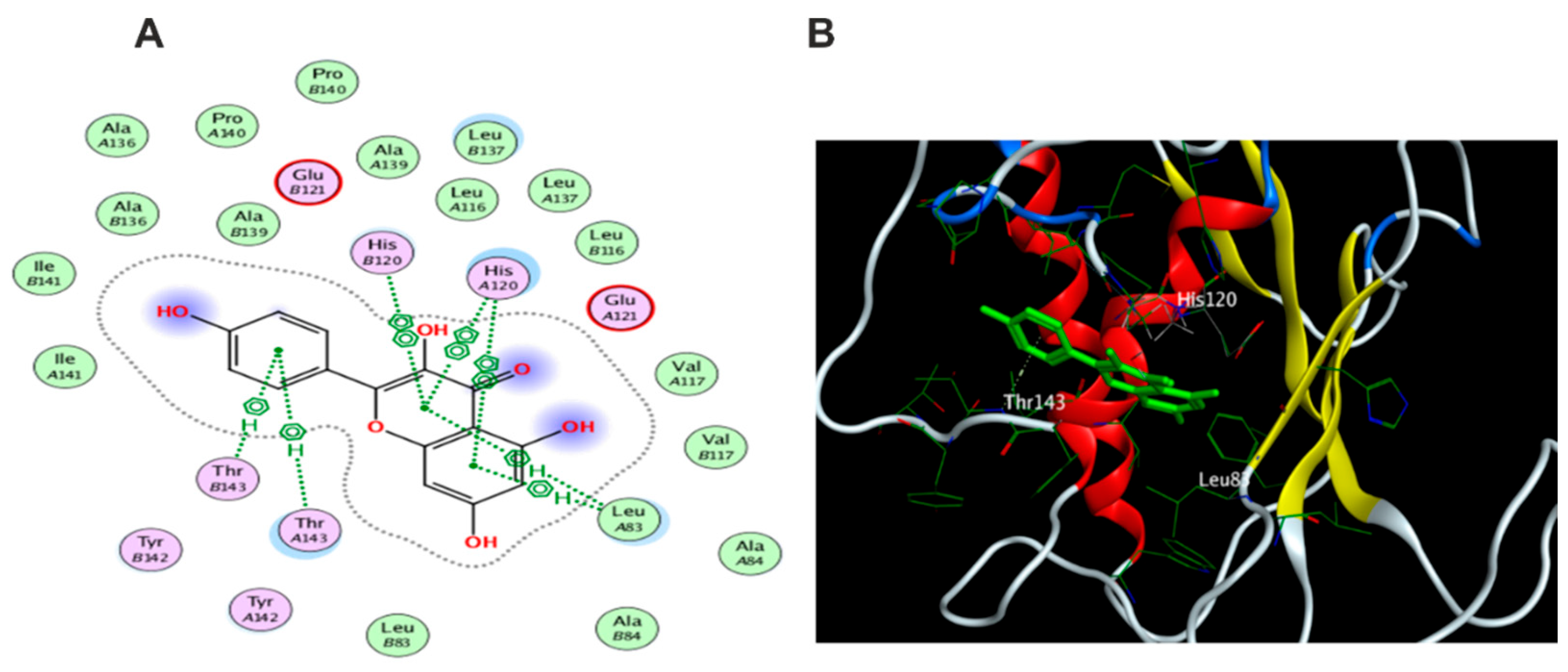
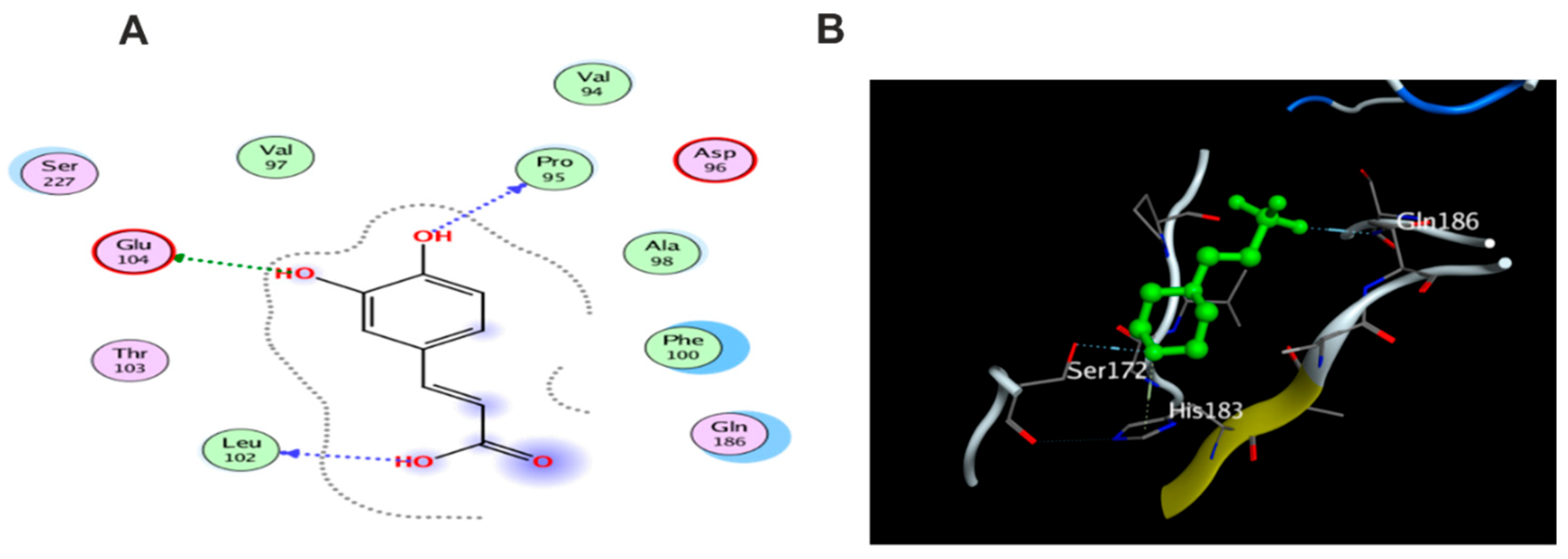
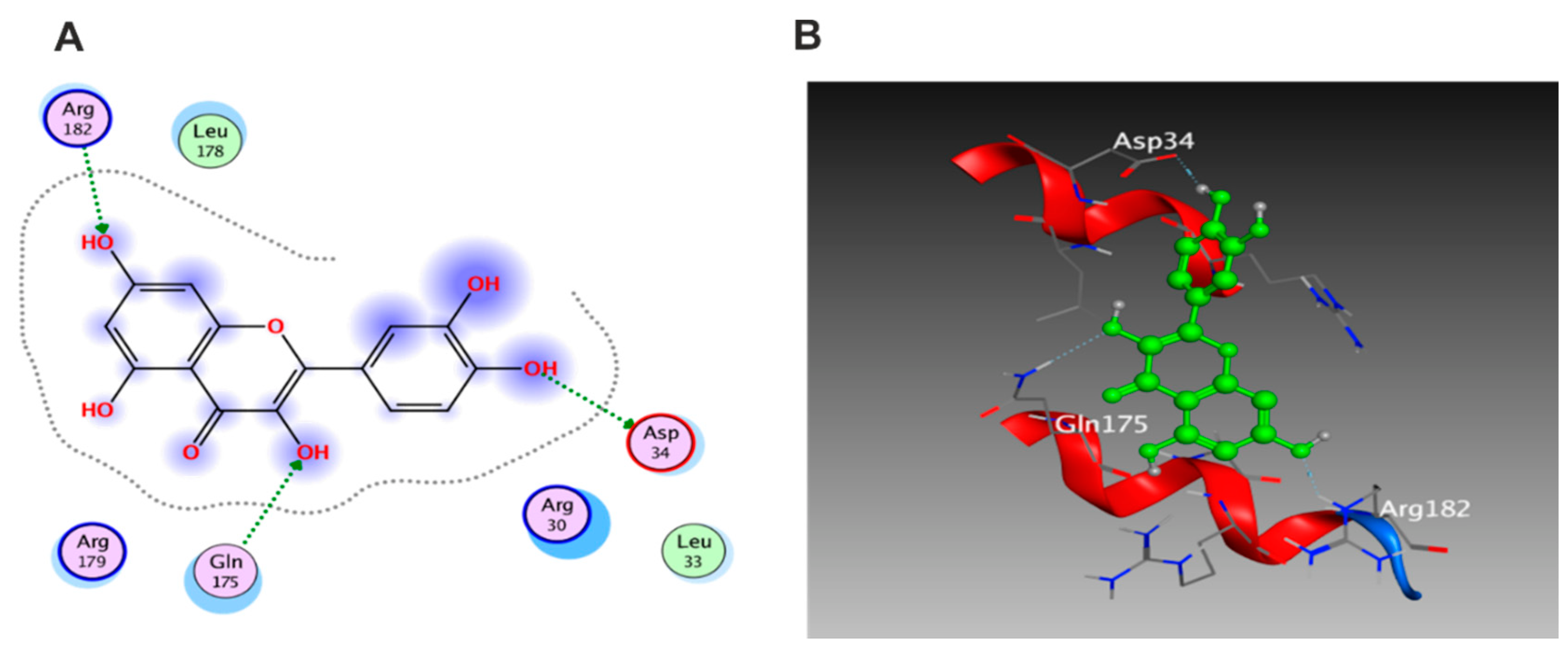
3.5. Molecular Docking Study
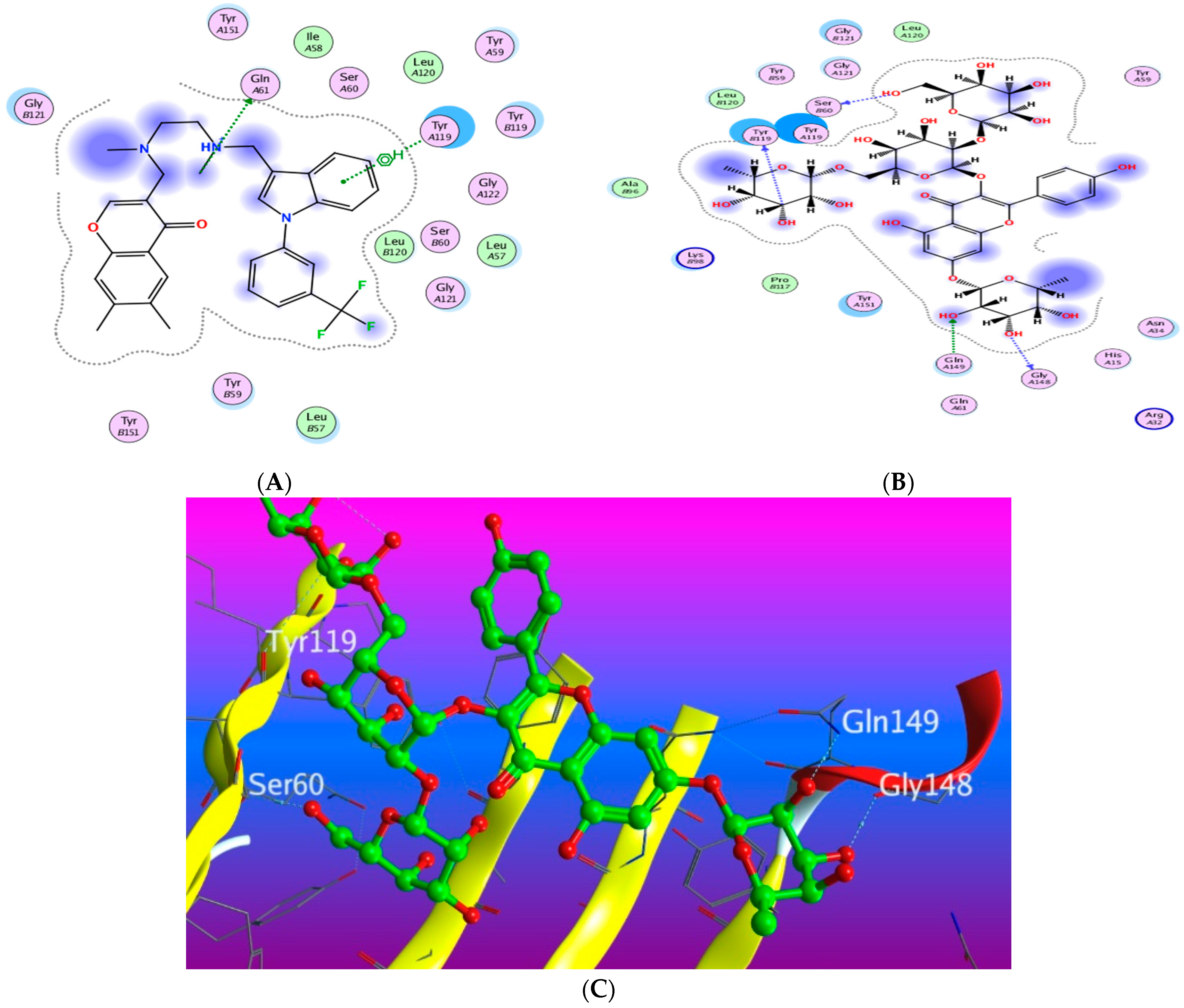
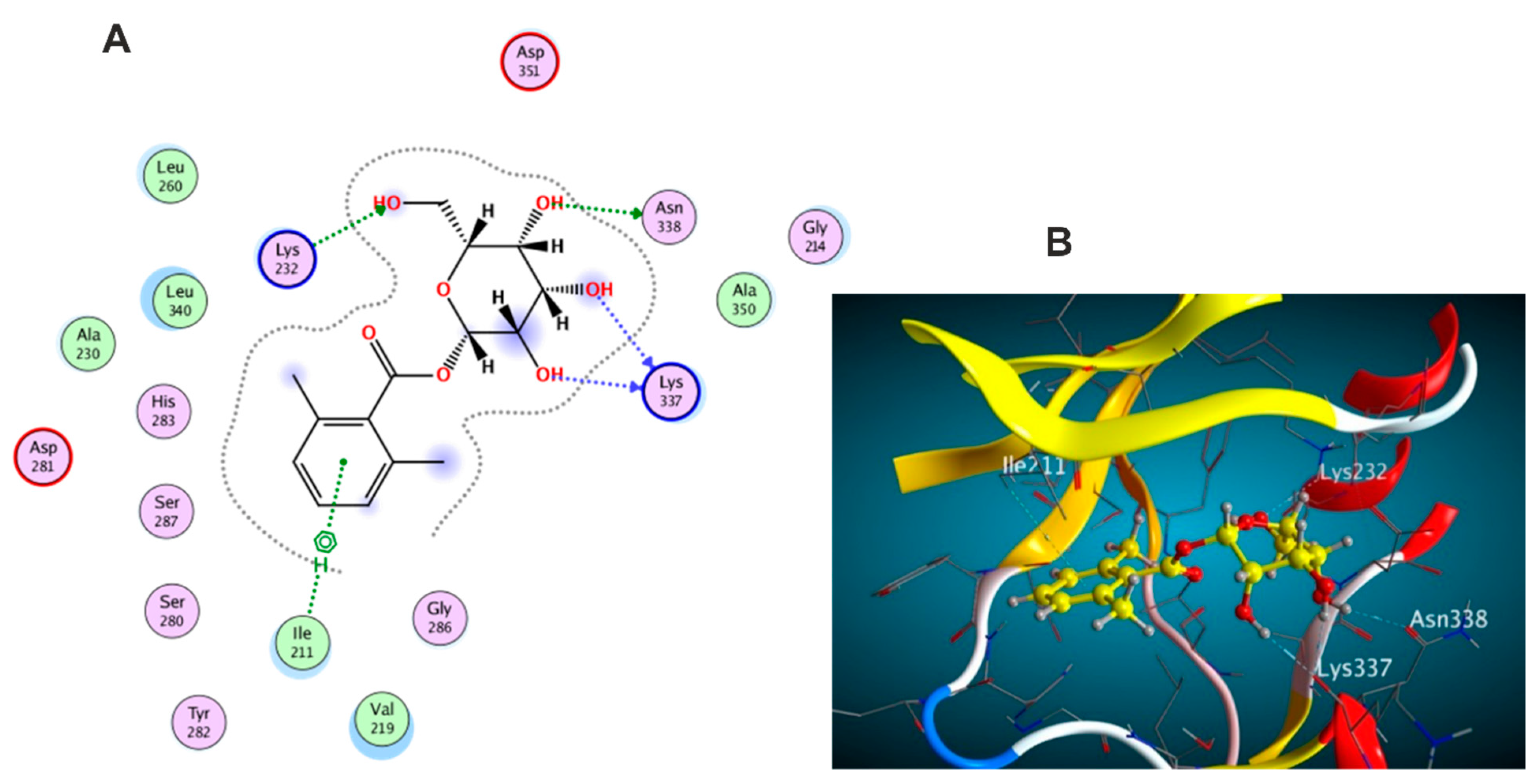
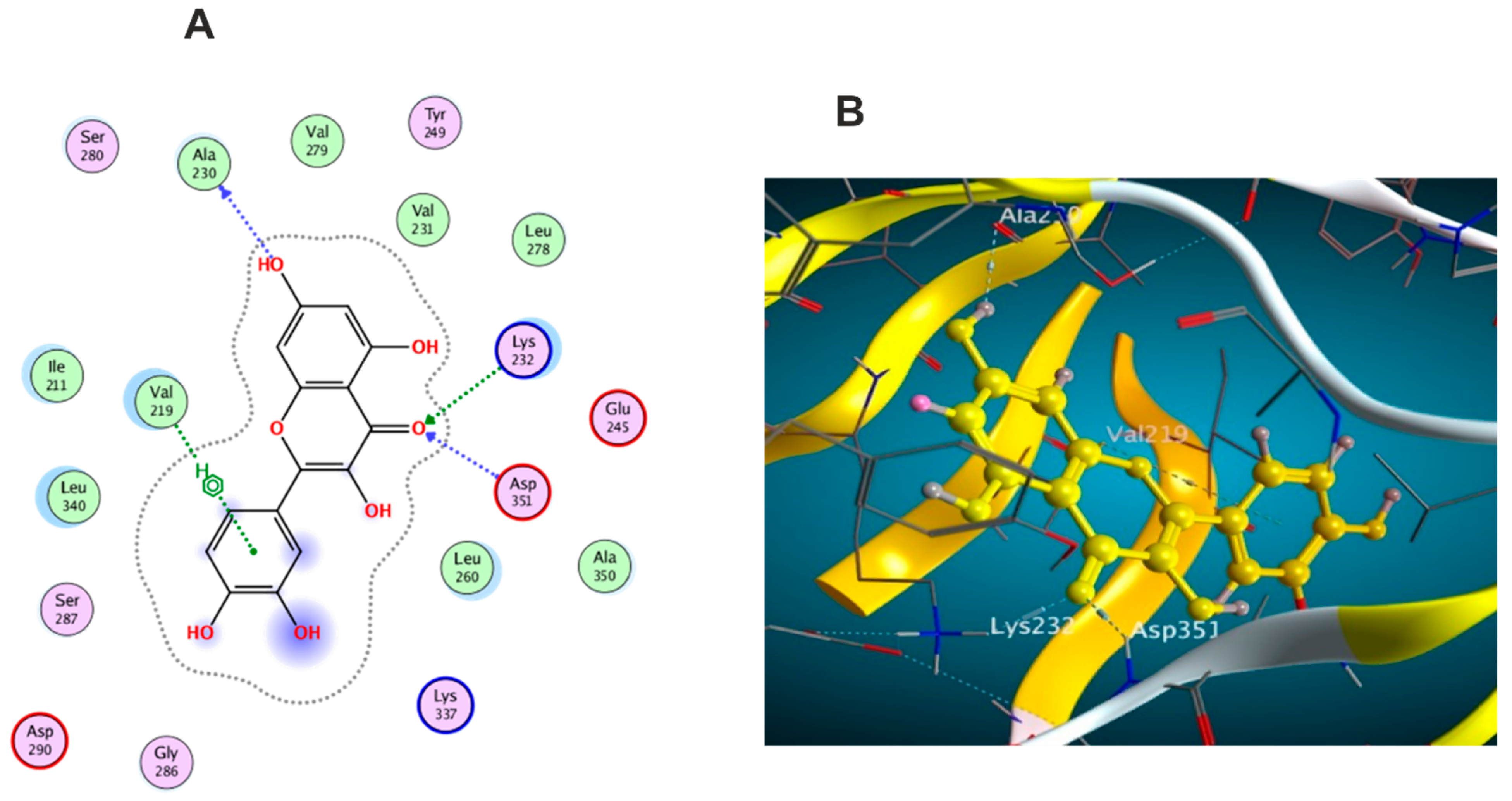
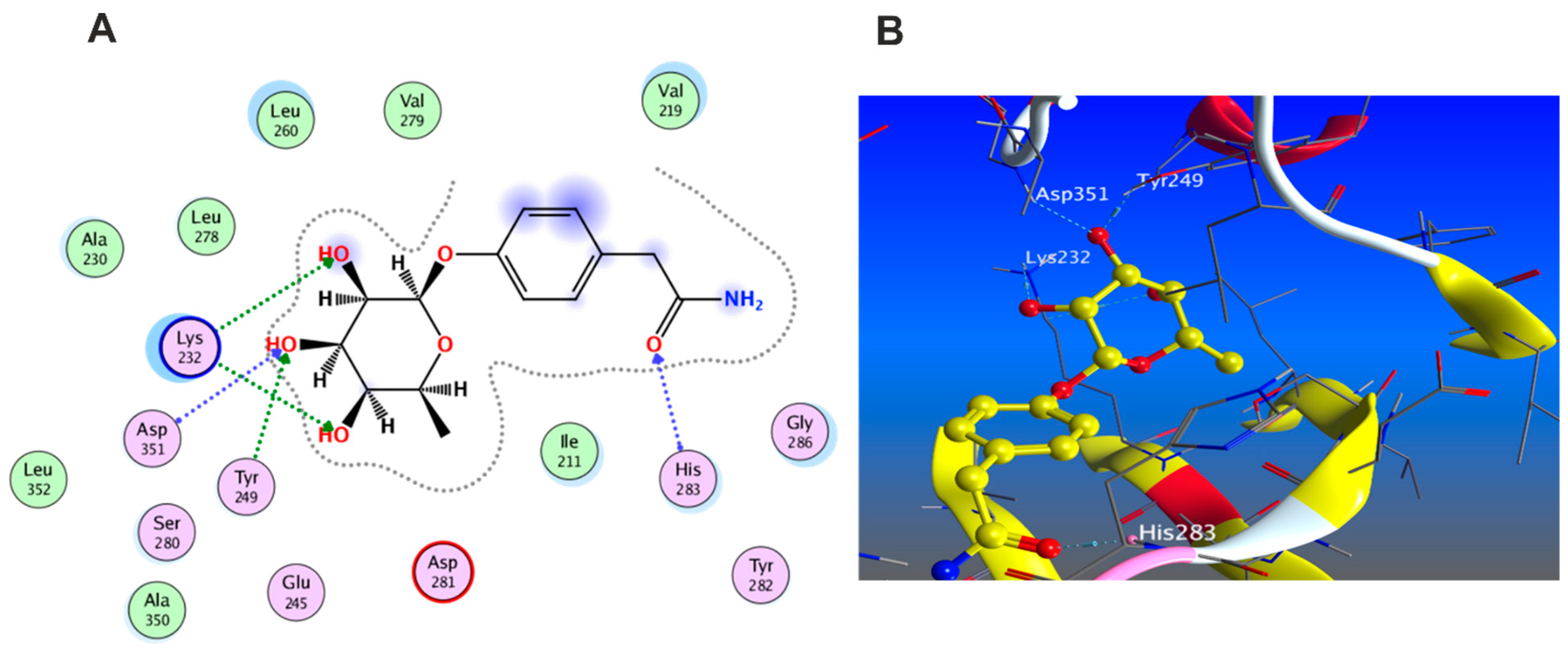
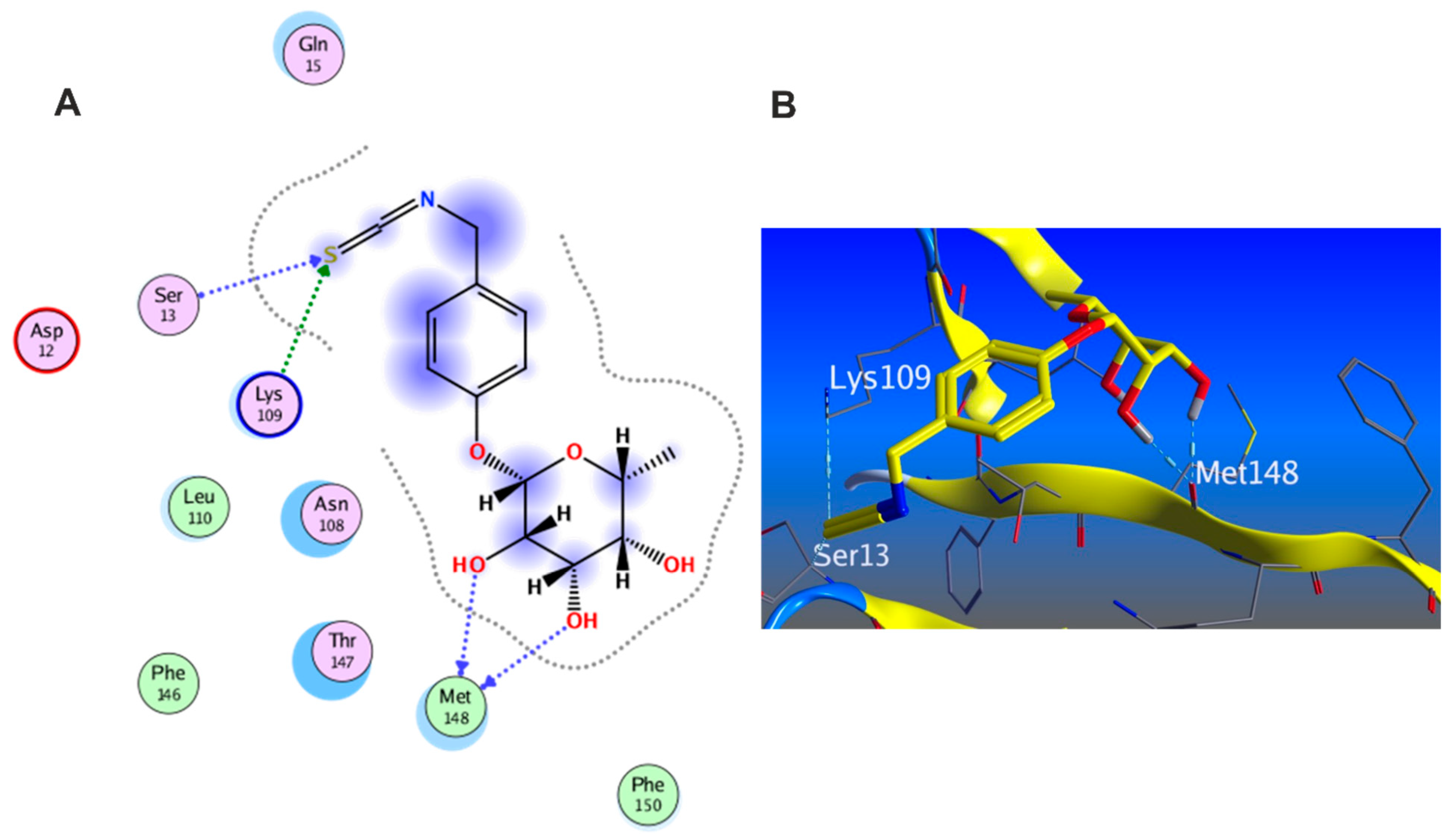
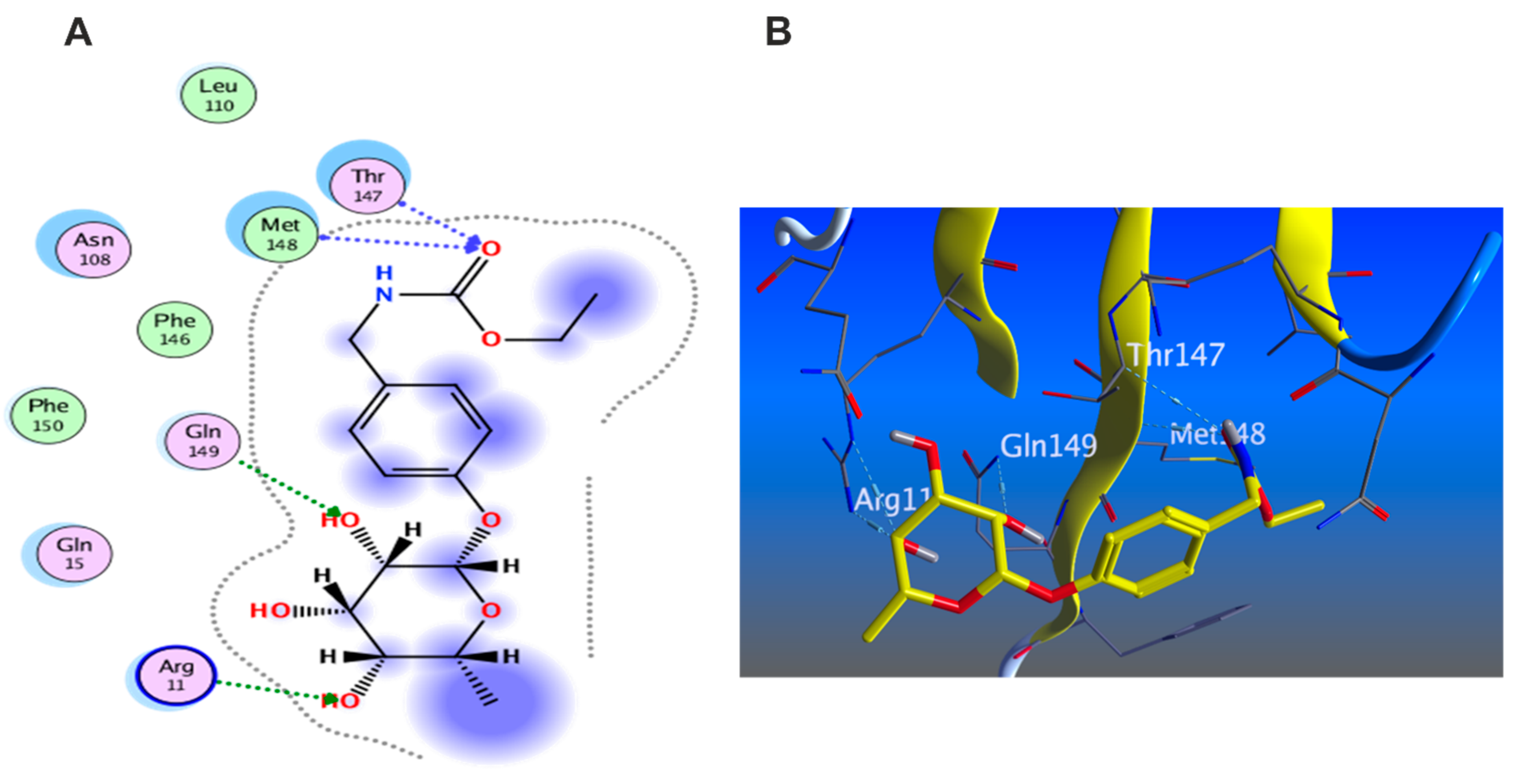
3.5.1. Docking with PDB ID: 2AZ5
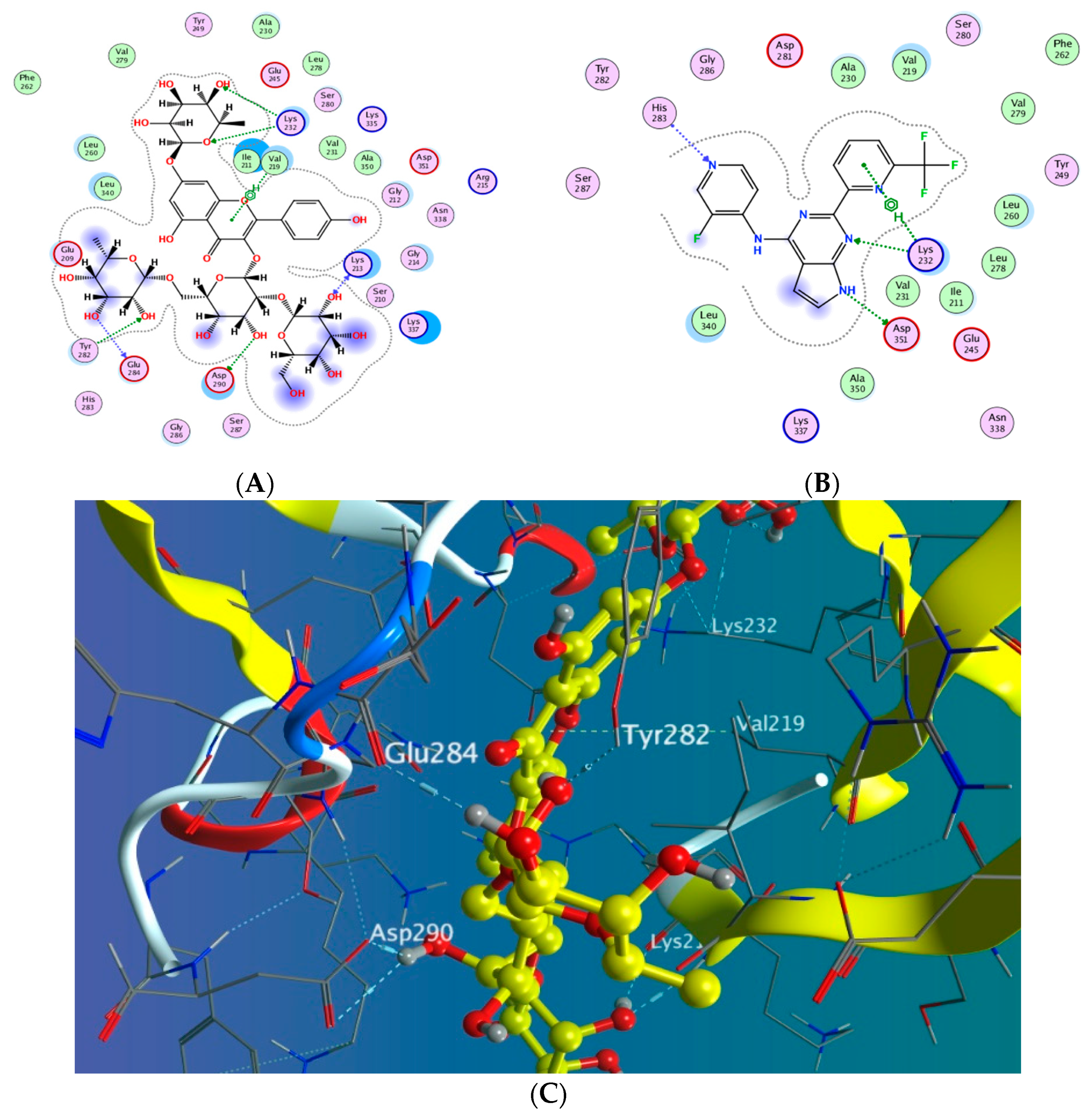
3.5.2. Docking with PDB ID:6B8Y
3.5.3. Docking with PDB ID:6Y8M
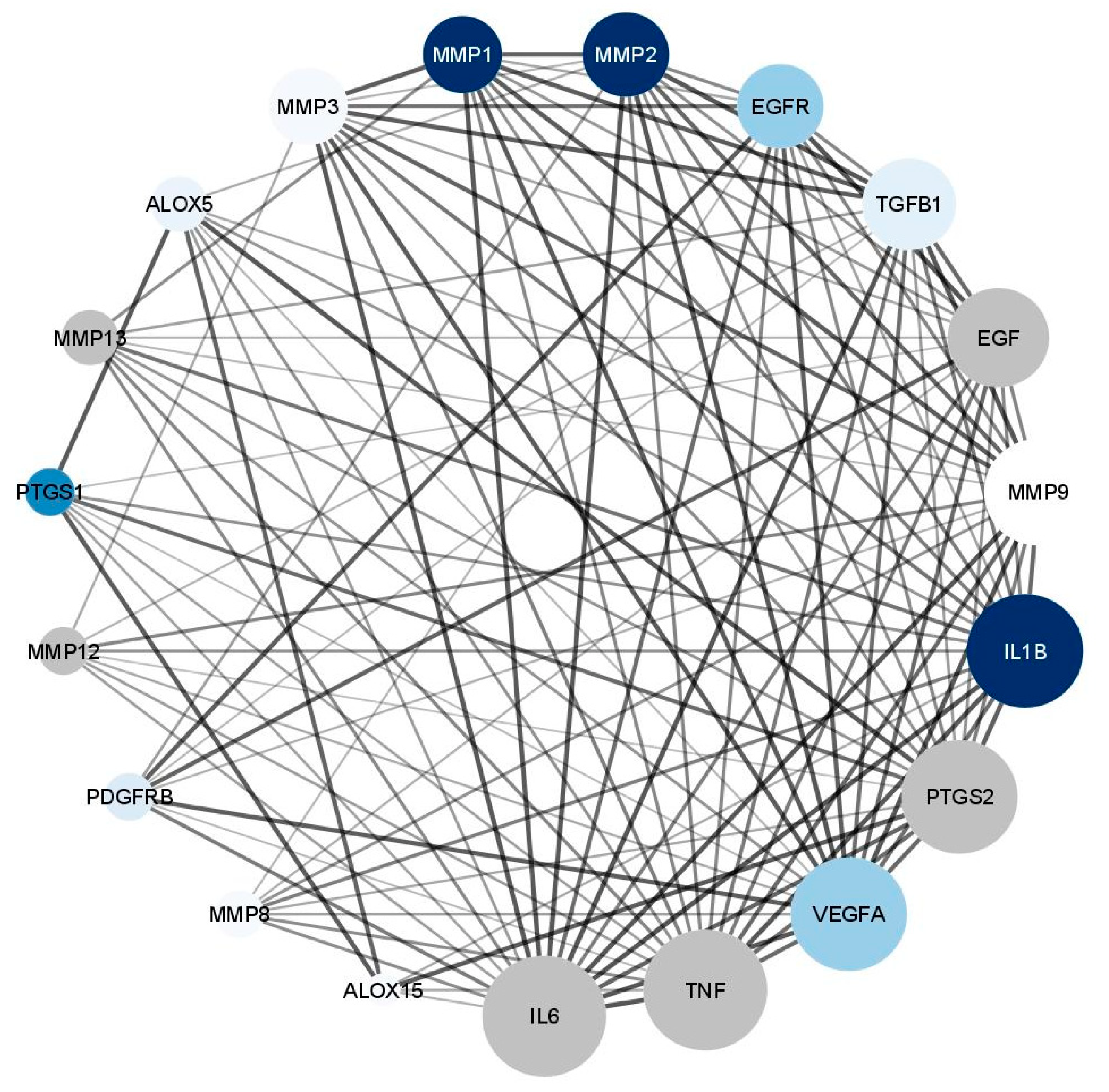
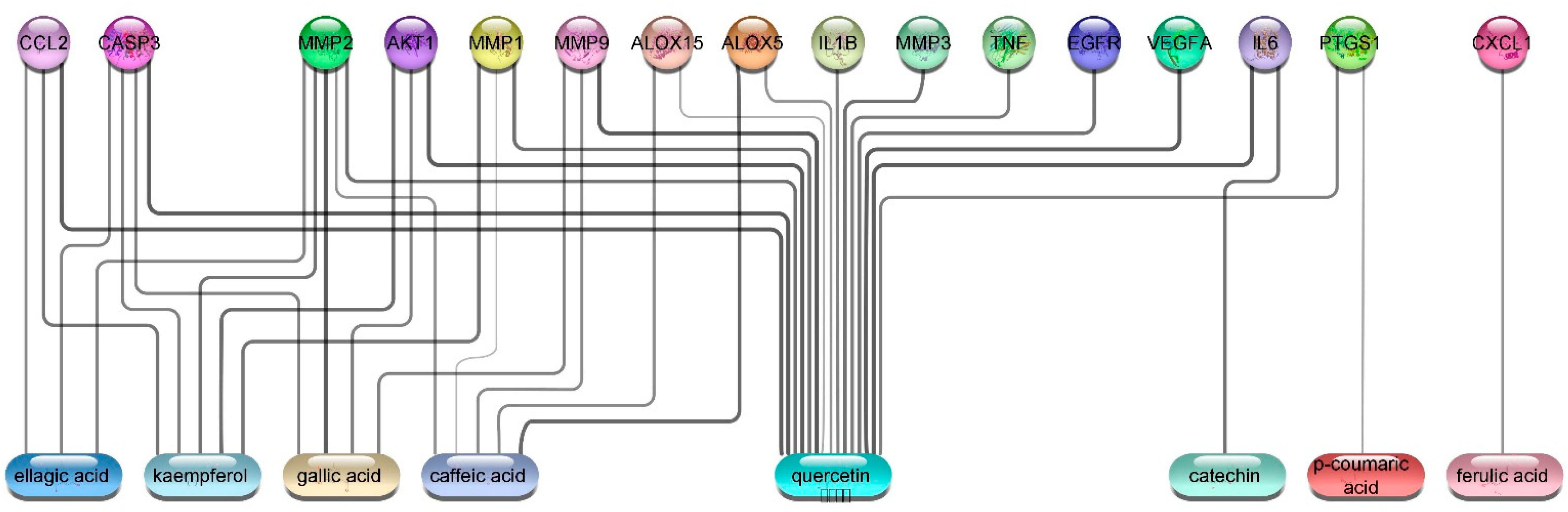
3.5.4. Wound Healing Network Design
Collection of Potential Targets for Wound Healing
Network Construction
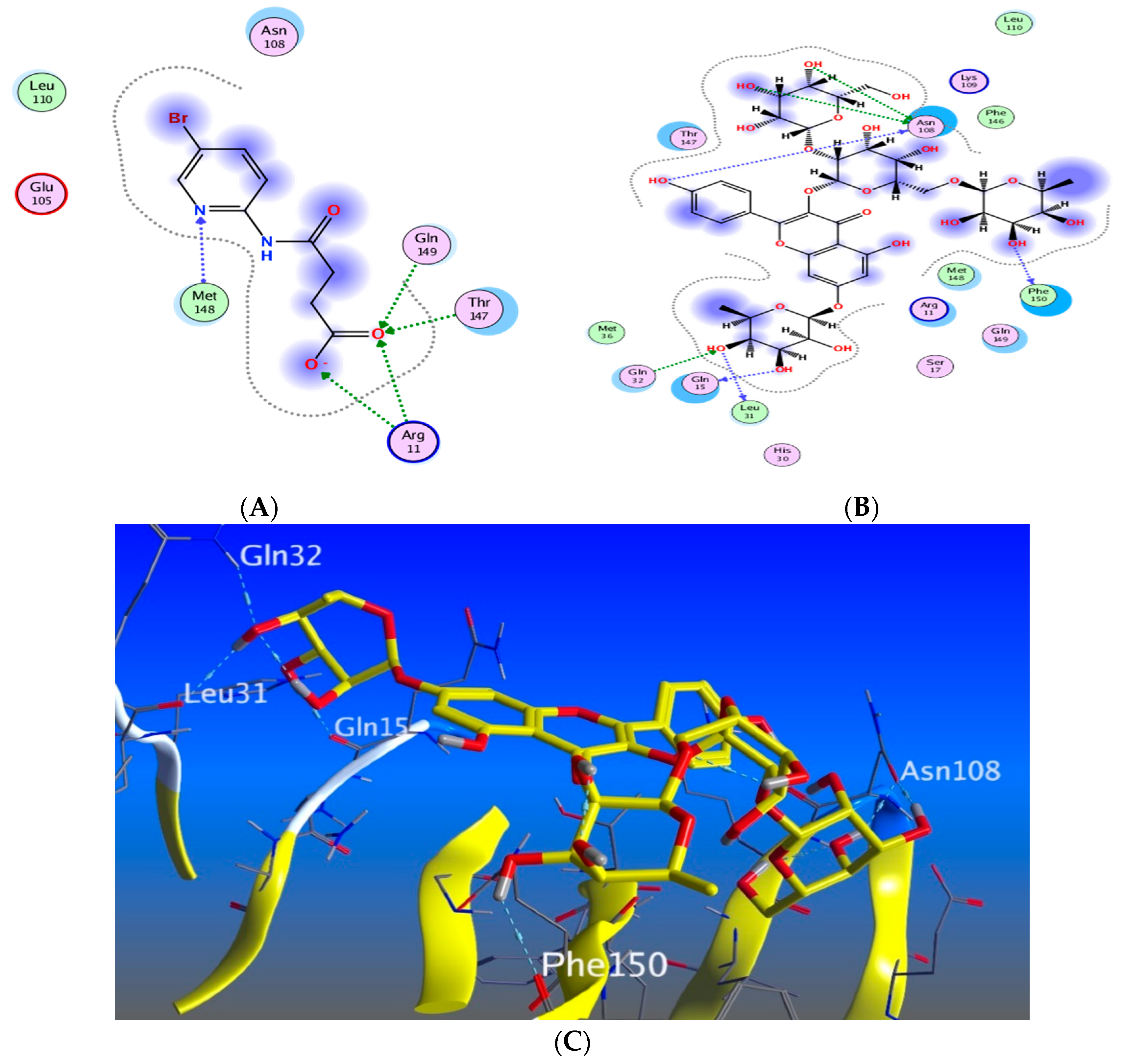
3.5.5. In Silico Molecular Docking
3.6. The Effects of the Major Metabolites in M. oleifera Seed Extract on the Inhibition of the Proinflammatory Cytokine Interleukin-6
3.7. The Effects of the Major Metabolites in Moringa oleifera Seed Extract on the Inhibition of the Endopeptidases, Matrix Metalloproteinases (MMP) 1 and 2
3.8. In Silico Drug Likeness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edmond, M.P.; Mostafa, N.M.; El-Shazly, M.; Singab, A.N.B. Two clerodane diterpenes isolated from Polyalthia longifolia leaves: Comparative structural features, anti-histaminic and anti-Helicobacter pylori activities. Nat. Prod. Res. 2021, 35, 5282–5286. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Mostafa, N.M.; El-Badry, M.A.; Eldahshan, O.A.; Singab, A.N.B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 2021, 35, 5369–5372. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Abdallah, S.H.; Mostafa, N.M.; Mohamed, M.; Nada, A.S.; Singab, A.N.B. UPLC-ESI-MS/MS profiling and hepatoprotective activities of Stevia leaves extract, butanol fraction and stevioside against radiation-induced toxicity in rats. Nat. Prod. Res. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.Y.; Mostafa, N.M.; Singab, A.N.B. Pulchranin A: First report of isolation from an endophytic fungus and its inhibitory activity on cyclin dependent kinases. Nat. Prod. Res. 2020, 34, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Singab, A.N.B. A new antidiabetic and anti-inflammatory biflavonoid from Schinus polygama (Cav.) Cabrera leaves. Nat. Prod. Res. 2022, 36, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Khoomrung, S.; Wanichthanarak, K.; Nookaew, I.; Thamsermsang, O.; Seubnooch, P.; Laohapand, T.; Akarasereenont, P. Metabolomics and Integrative Omics for the Development of Thai Traditional Medicine. Front. Pharm. 2017, 8, 474. [Google Scholar] [CrossRef]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Desamparados Soriano, M. Moringa oleifera: An Unknown Crop in Developed Countries with Great Potential for Industry and Adapted to Climate Change. Foods 2020, 10, 31. [Google Scholar] [CrossRef]
- Meireles, D.; Gomes, J.; Lopes, L.; Hinzmann, M.; Machado, J. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: Integrative approach on conventional and traditional Asian medicine. Adv. Tradit. Med. 2020, 20, 495–515. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef] [PubMed]
- Matic, I.; Guidi, A.; Kenzo, M.; Mattei, M.; Galgani, A. Investigation of medicinal plants traditionally used as dietary supplements: A review on Moringa oleifera. J. Public Health Afr. 2018, 9, 841. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera seeds and oil: Characteristics and uses for human health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [PubMed]
- Milla, P.G.; Peñalver, R.; Nieto, G. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Kujath, P.; Michelsen, A. Wounds—From physiology to wound dressing. Dtsch. Arztebl. Int. 2008, 105, 239–248. [Google Scholar] [CrossRef]
- Gothai, S.; Arulselvan, P.; Tan, W.S.; Fakurazi, S. Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts. J. Intercult. Ethnopharmacol. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In Vitro Wound Healing Potential and Identification of Bioactive Compounds from Moringa oleifera Lam. BioMed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef]
- Mehwish, H.M.; Liu, G.; Rajoka, M.S.R.; Cai, H.; Zhong, J.; Song, X.; Xia, L.; Wang, M.; Aadil, R.M.; Inam-Ur-Raheem, M.; et al. Therapeutic potential of Moringa oleifera seed polysaccharide embedded silver nanoparticles in wound healing. Int. J. Biol. Macromol. 2021, 184, 144–158. [Google Scholar] [CrossRef]
- Memon, G.M.; Memon, S.A.; Memon, A.R. Isolation and structure elucidation of moringyne—A new glycoside from seeds of Moringa oleifera Lam. Pak. J. Sci. Ind. Res. 1985, 28, 7–9. [Google Scholar]
- Govardhan Singh, R.S.; Negi, P.S.; Radha, C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J. Funct. Foods 2013, 5, 1883–1891. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Ferlazzo, N.; Gugliandolo, A.; Musumeci, L.; Mazzon, E.; Bramanti, A.; Navarra, M. Moringin from Moringa oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-κB and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef]
- Marzouk, M.M.; Al-Nowaihi, A.-S.M.; Kawashty, S.A.; Saleh, N.A.M. Chemosystematic studies on certain species of the family Brassicaceae (Cruciferae) in Egypt. Biochem. Syst. Ecol. 2010, 38, 680–685. [Google Scholar] [CrossRef]
- Jiang, M.-Y.; Lu, H.; Pu, X.-Y.; Li, Y.-H.; Tian, K.; Xiong, Y.; Wang, W.; Huang, X.-Z. Laxative Metabolites from the Leaves of Moringa oleifera. J. Agric. Food Chem. 2020, 68, 7850–7860. [Google Scholar] [CrossRef]
- Song, L.; Morrison, J.J.; Botting, N.P.; Thornalley, P.J. Analysis of glucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC–MS/MS. Anal. Biochem. 2005, 347, 234–243. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Lu, C.-H.; Liu, S.-S.; Wang, J.-Y.; Wang, M.-Z.; Shen, Y.-M. Characterization of Eight New Secondary Metabolites from the Mutant Strain G-444 of Tubercularia sp. TF5. Helv. Chim. Acta 2014, 97, 334–344. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Al-Madhagy, S.A.; Mostafa, N.M.; Youssef, F.S.; Awad, G.E.A.; Eldahshan, O.A.; Singab, A.N.B. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019, 10, 6267–6275. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Pachuau, L. Recent developments in novel drug delivery systems for wound healing. Expert Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Suguna, L.; Singh, S.; Sivakumar, P.; Sampath, P.; Chandrakasan, G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother. Res. 2002, 16, 227–231. [Google Scholar] [CrossRef]
- Tang, T.; Yin, L.; Yang, J.; Shan, G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur. J. Pharmacol. 2007, 567, 177–185. [Google Scholar] [CrossRef]
- Wankell, M.; Munz, B.; Hübner, G.; Hans, W.; Wolf, E.; Goppelt, A.; Werner, S. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 2001, 20, 5361–5372. [Google Scholar] [CrossRef]
- Beer, H.-D.; Gassmann, M.G.; Munz, B.; Steiling, H.; Engelhardt, F.; Bleuel, K.; Werner, S. Expression and function of keratinocyte growth factor and activin in skin morphogenesis and cutaneous wound repair. J. Investig. Dermatol. Symp. Proc. 2000, 5, 34–39. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Edmond, M.P.; El-Shazly, M.; Fahmy, H.A.; Sherif, N.H.; Singab, A.N.B. Phytoconstituents and renoprotective effect of Polyalthia longifolia leaves extract on radiation-induced nephritis in rats via TGF-β/smad pathway. Nat. Prod. Res. 2021, 36, 4187–4192. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haroon, Z.A.; Amin, K.; Saito, W.; Wilson, W.; Greenberg, C.S.; Dewhirst, M.W. SU5416 delays wound healing through inhibition of TGF-β activation. Cancer Biol. Ther. 2002, 1, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, R.A.; Kim, I.S.; Hokama, L.; De Ruyter, K.; Keen, C. Operational determinants of caller satisfaction in the call center. Int. J. Serv. Ind. Manag. 2000, 11, 131–141. [Google Scholar] [CrossRef]
- Schultz, G.S.; Ladwig, G.; Wysocki, A. Extracellular matrix: Review of its roles in acute and chronic wounds. World Wide Wounds 2005, 2005, 1–18. [Google Scholar]
- Sasaki, M.; Kashima, M.; Ito, T.; Watanabe, A.; Izumiyama, N.; Sano, M.; Kagaya, M.; Shioya, T.; Miura, M. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1β and TNF-α. Mediat. Inflamm. 2000, 9, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Sano, C.; Shimizu, T.; Tomioka, H. Effects of secretory leukocyte protease inhibitor on the tumor necrosis factor-alpha production and NF-κB activation of lipopolysaccharide-stimulated macrophages. Cytokine 2003, 21, 38–42. [Google Scholar] [CrossRef]
- Ashmawy, A.; Mostafa, N.; Eldahshan, O. GC/MS Analysis and Molecular Profiling of Lemon Volatile Oil against Breast Cancer. J. Essent. Oil Bear. Plants 2019, 22, 903–916. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Mostafa, A.M.; Ashour, M.L.; Elhady, S.S. Neuroprotective Effects of Black Pepper Cold-Pressed Oil on Scopolamine-Induced Oxidative Stress and Memory Impairment in Rats. Antioxidants 2021, 10, 1993. [Google Scholar] [CrossRef]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C. Small-molecule inhibition of TNF-α. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef]
- Harikrishnan, L.S.; Warrier, J.; Tebben, A.J.; Tonukunuru, G.; Madduri, S.R.; Baligar, V.; Mannoori, R.; Seshadri, B.; Rahaman, H.; Arunachalam, P. Heterobicyclic inhibitors of transforming growth factor beta receptor I (TGFβRI). Bioorg. Med. Chem. 2018, 26, 1026–1034. [Google Scholar] [CrossRef]
- Rebhan, M. GeneCards: Encyclopedia for Genes, Proteins and Diseases. 1997. Available online: https://www.genecards.org/ (accessed on 25 August 2022).
- Vitali, F.; Cohen, L.D.; Demartini, A.; Amato, A.; Eterno, V.; Zambelli, A.; Bellazzi, R. Correction: A Network-Based Data Integration Approach to Support Drug Repurposing and Multi-Target Therapies in Triple Negative Breast Cancer. PLoS ONE 2017, 12, e0170363. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Marini, S.; Balli, M.; Grosemans, H.; Sampaolesi, M.; Lussier, Y.A.; Cusella De Angelis, M.G.; Bellazzi, R. Exploring wound-healing genomic machinery with a network-based approach. Pharmaceuticals 2017, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- El-Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-derived targeted therapy for wound healing: Beyond classical strategies. Pharmaceuticals 2021, 170, 105749. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Petricoin, E.F.; Ward, K.R.; Goldberg, S.R.; Duane, T.M.; Bonchev, D.; Arodz, T.; Diegelmann, R.F. Network proteomics of human dermal wound healing. Physiol. Meas. 2018, 39, 124002. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2016, 45, D362–D368. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Balli, M.; Vitali, F.; Janiszewski, A.; Caluwé, E.; Cortés-Calabuig, A.; Carpentier, S.; Duelen, R.; Ronzoni, F.; Marcelis, L.; Bosisio, F.M.; et al. Autologous micrograft accelerates endogenous wound healing response through ERK-induced cell migration. Cell Death Differ. 2020, 27, 1520–1538. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Issa, M.Y.; Ebrahim, H.S.; Alaaeldin, R.; Elrehany, M.A.; Abd El-Kadder, E.M.; Abdelmohsen, U.R. Anti-Alzheimer chemical constituents of Morus macroura Miq.: Chemical profiling, in silico and in vitro investigations. Food Funct. 2021, 12, 8078–8089. [Google Scholar] [CrossRef]
- Alzhrani, Z.M.M.; Alam, M.M.; Neamatallah, T.; Nazreen, S. Design, synthesis and in vitro antiproliferative activity of new thiazolidinedione-1, 3, 4-oxadiazole hybrids as thymidylate synthase inhibitors. J. Enzyme. Inhib. Med. Chem. 2020, 35, 1116–1123. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.; Tang, Y. A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. ACS Publ. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef] [PubMed]




| Peak No. | Identified Metabolite | Chemical Structure | Exact Mass | Phytochemical Class | Ref. |
|---|---|---|---|---|---|
| 1 | Moringyne | 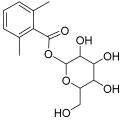 | 312.12090 | Phenolic acid derivative | [21] |
| 2 | Catechin |  | 290.07904 | Flavan-3-ol | [22] |
| 3 | Quercetin | 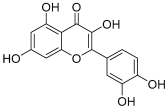 | 302.042655 | Flavonol | [22] |
| 4 | Kaempferol | 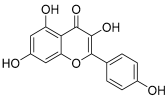 | 286.04774 | [23] | |
| 5 | Gallic acid | 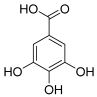 | 170.021525 | Benzoic acid derivative | [24] |
| 6 | p-Coumaric acid |  | 164.047345 | Cinnamic acid derivative | [22] |
| 7 | Ferulic acid | 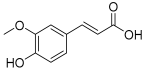 | 194.058 | Cinnamic acid derivative | [22] |
| 8 | Caffeic acid | 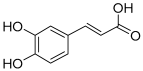 | 180.04226 | [22] | |
| 9 | Protocatechuic acid | 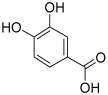 | 154.02661 | Benzoic acid derivative | [22] |
| 10 | Cinnamic acid |  | 148.05243 | Organic acid | [22] |
| 11 | Ellagic acid | 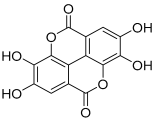 | 302.00627 | Polyphenol | [23] |
| 12 | Vanillic acid | 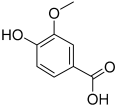 | 168.04226 | Benzoic acid derivative | [22] |
| 13 | Benzylamine | 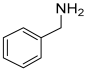 | 107.073499 | Organic amine | [25] |
| 14 | Kaempferol 3,7-diglycosides 3-O-[β-D-Glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside], 7-O-α-L-rhamnopyranoside |  | 902.26921 | Flavonol glycoside | [26] |
| 15 | 4-Hydroxybenzyl isothiocyanate O-α-L-rhamnopyranoside | 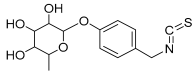 | 311.082744 | Isothiocyanate derivative | [27] |
| 16 | (4-Hydroxybenzyl) carbamic acid ester, O-α-L-rhamnopyranoside | 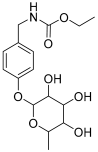 | 341.147454 | Carbamate derivative | [28] |
| 17 | Rhamnose; α-L-pyranose-form, Phenolic glycoside |  | 240.0997 | Phenolic glycoside | [29] |
| 18 | 4-Hydroxyphenylacetic acid amide, O-α-L-rhamnopyranoside | 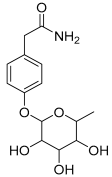 | 297.121239 | Phenolic glycoside | [29] |
| 19 | 3,4-Dihydro-4,8-dihydroxy-3-methyl-1H-2-benzopyran-1-one; (3R,4S)-form | 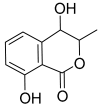 | 194.05791 | Isocoumarin | [30] |
| No | Name | Target | Degree | Betweenness | Closeness | Tissue/Skin |
|---|---|---|---|---|---|---|
| 1 | Tumor necrosis factor | TNF | 18 | 0.058613445 | 1 | 0.72563 |
| 2 | Interleukin 6 | IL6 | 18 | 0.058613445 | 1 | 0.88975 |
| 3 | Vascular endothelial growth factor A | VEGFA | 17 | 0.03889667 | 0.947368421 | 1.449221 |
| 4 | Prostaglandin-endoperoxide synthase 2 | PTGS2 | 17 | 0.045650482 | 0.947368421 | 0.745856 |
| 5 | Interleukin 1B | IL1B | 17 | 0.045650482 | 0.947368421 | 4 |
| 6 | Matrix metallopeptidase 9 | MMP9 | 16 | 0.027552132 | 0.9 | 0.166331 |
| 7 | Epidermal growth factor | EGF | 15 | 0.021864301 | 0.857142857 | 0.698752 |
| 8 | Transforming growth factor beta 1 | TGFβ1 | 14 | 0.012044818 | 0.818181818 | 0.94118 |
| 9 | Epidermal growth factor receptor | EGFR | 13 | 0.007625272 | 0.782608696 | 1.459392 |
| 10 | Matrix metallopeptidase 2 | MMP2 | 13 | 0.009126984 | 0.782608696 | 2.805839 |
| 11 | Matrix metallopeptidase 1 | MMP1 | 12 | 0.002178649 | 0.75 | 4.357237 |
| 12 | Matrix metallopeptidase 3 | MMP3 | 12 | 0.003267974 | 0.75 | 0.475876 |
| 13 | Matrix metallopeptidase 13 | MMP13 | 9 | 0 | 0.666666667 | 0.413252 |
| 14 | Arachidonate 5-Lipoxygenase | ALOX5 | 9 | 0.005571117 | 0.666666667 | 0.753328 |
| 15 | platelet-derived growth factor receptor beta | PDGFRβ | 8 | 0 | 0.642857143 | 1.185637 |
| 16 | Matrix metallopeptidase 12 | MMP12 | 8 | 0 | 0.642857143 | 0.658791 |
| 17 | Matrix metallopeptidase 8 | MMP8 | 8 | 0 | 0.642857143 | 0.44169 |
| 18 | Prostaglandin-endoperoxide synthase 1 | PTGS1 | 8 | 0.003213508 | 0.642857143 | 2.071426 |
| 19 | Arachidonate 15-Lipoxygenase | ALOX15 | 6 | 0 | 0.6 | 0.102662 |
| Median | 13 | 0.007625272 | 0.782608696 | 0.753328 |
| Metabolite Name | IL-6 Concentration (pg/mL) | Approximate % of IL-6 Relative to LPS |
|---|---|---|
| Quercetin | 71.51 ± 1.56 | 43.4% |
| Kaempferol | 39.65 ± 1.19 | 24% |
| Caffeic acid | 77.41 ± 1.79 | 47% |
| LPS (as control) | 164.7 ± 13.7 | 100% |
| Compound Name | MMP-1 (pg/mL) | Approximate % of MMP-1 Relative to LPS | MMP-2 (pg/mL) | Approximate % of MMP-2 Relative to LPS |
|---|---|---|---|---|
| Quercetin | 2160 ± 30.7 | 67.8% | 170.3 ± 2.49 | 59.7% |
| Kaempferol | 1276 ± 52.2 | 40.1% | 92.59 ± 3.36 | 32.4% |
| Caffeic acid | 1926 ± 49.1 | 60.5% | 199.3 ± 1.56 | 69.9% |
| LPS (as control) | 3183 ± 78.8 | 100% | 285 ± 10.9 | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shady, N.H.; Mostafa, N.M.; Fayez, S.; Abdel-Rahman, I.M.; Maher, S.A.; Zayed, A.; Saber, E.A.; Khowdiary, M.M.; Elrehany, M.A.; Alzubaidi, M.A.; et al. Mechanistic Wound Healing and Antioxidant Potential of Moringa oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies. Antioxidants 2022, 11, 1743. https://doi.org/10.3390/antiox11091743
Shady NH, Mostafa NM, Fayez S, Abdel-Rahman IM, Maher SA, Zayed A, Saber EA, Khowdiary MM, Elrehany MA, Alzubaidi MA, et al. Mechanistic Wound Healing and Antioxidant Potential of Moringa oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies. Antioxidants. 2022; 11(9):1743. https://doi.org/10.3390/antiox11091743
Chicago/Turabian StyleShady, Nourhan Hisham, Nada M. Mostafa, Shaimaa Fayez, Islam M. Abdel-Rahman, Sherif A. Maher, Ahmed Zayed, Entesar Ali Saber, Manal M. Khowdiary, Mahmoud A. Elrehany, Mubarak A. Alzubaidi, and et al. 2022. "Mechanistic Wound Healing and Antioxidant Potential of Moringa oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies" Antioxidants 11, no. 9: 1743. https://doi.org/10.3390/antiox11091743
APA StyleShady, N. H., Mostafa, N. M., Fayez, S., Abdel-Rahman, I. M., Maher, S. A., Zayed, A., Saber, E. A., Khowdiary, M. M., Elrehany, M. A., Alzubaidi, M. A., Altemani, F. H., Shawky, A. M., & Abdelmohsen, U. R. (2022). Mechanistic Wound Healing and Antioxidant Potential of Moringa oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies. Antioxidants, 11(9), 1743. https://doi.org/10.3390/antiox11091743










