Chitooligosaccharide Maintained Cell Membrane Integrity by Regulating Reactive Oxygen Species Homeostasis at Wounds of Potato Tubers during Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Artificial Wound of Tubers and COS Treatment
2.2.2. Determination of Cell Membrane Permeability
2.2.3. Sampling
2.2.4. Determination of MDA Content
2.2.5. Determination of O2●− and H2O2 Contents
2.2.6. Total RNA Extraction and qRT-PCR Analysis
2.2.7. Enzyme Activities
2.2.8. Determination of Substrates and Product Contents of the AsA–GSH Cycle
2.2.9. Determination of the Scavenging Ability of ABTS+, DPPH and FRAP
2.3. Statistical Analysis
3. Results
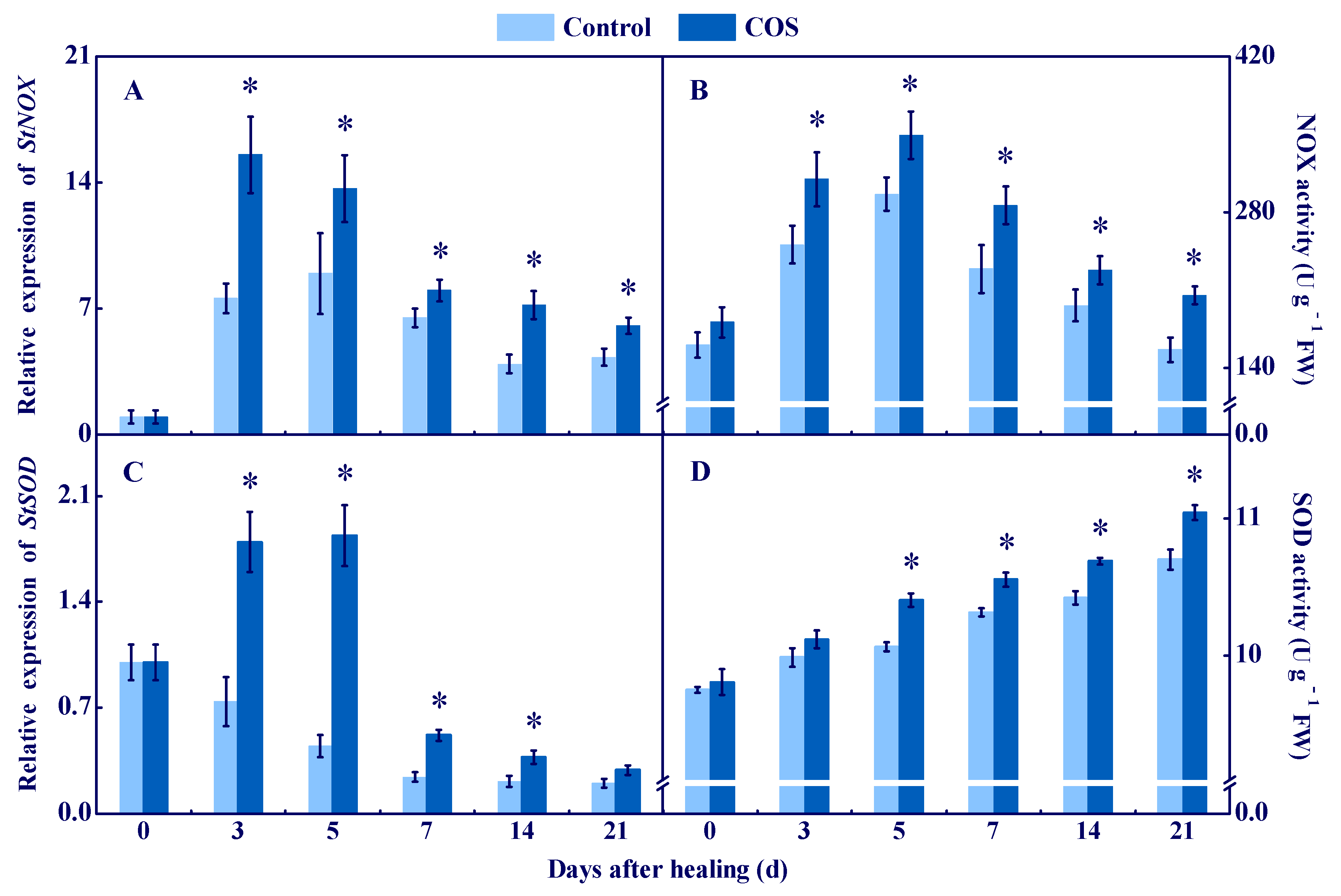
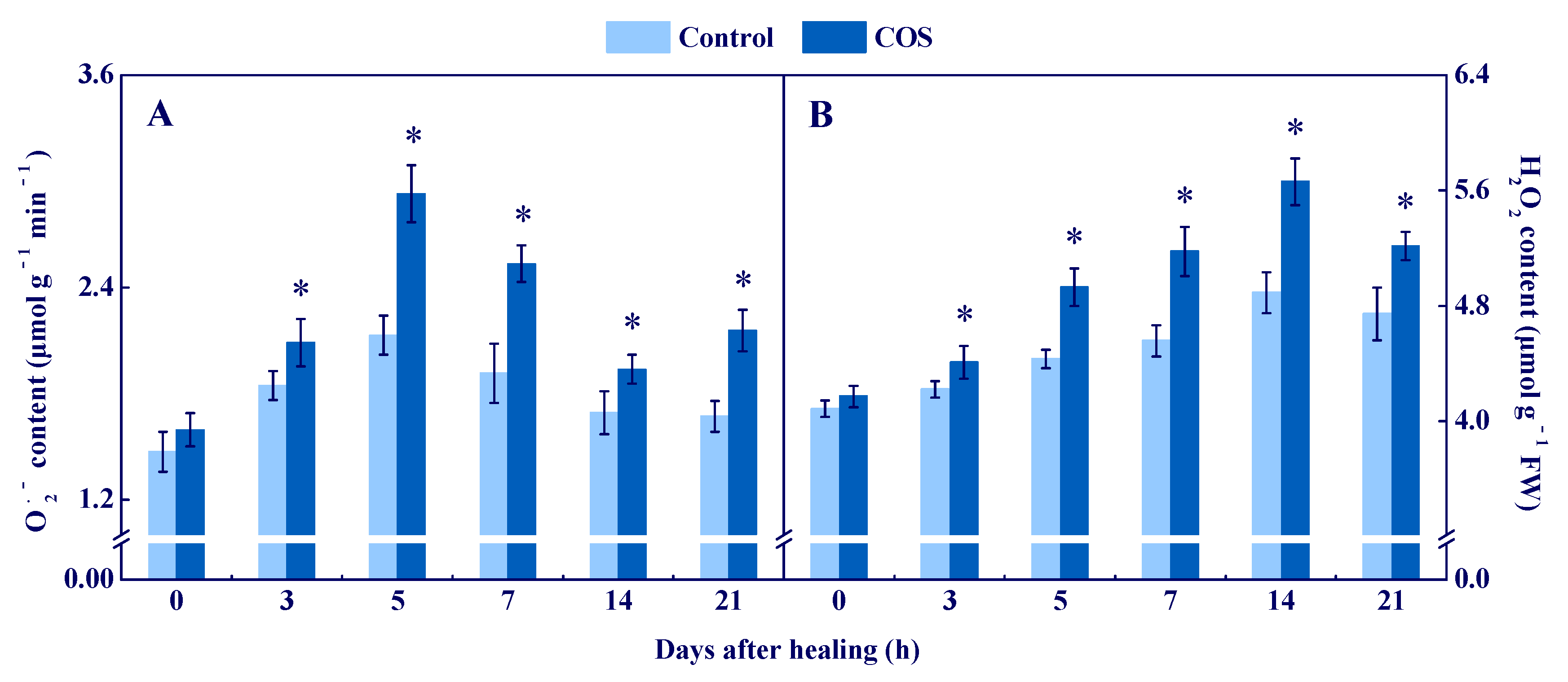
3.1. COS Activated NOX and SOD and Enhanced O2●− and H2O2 Contents at Tuber Wounds
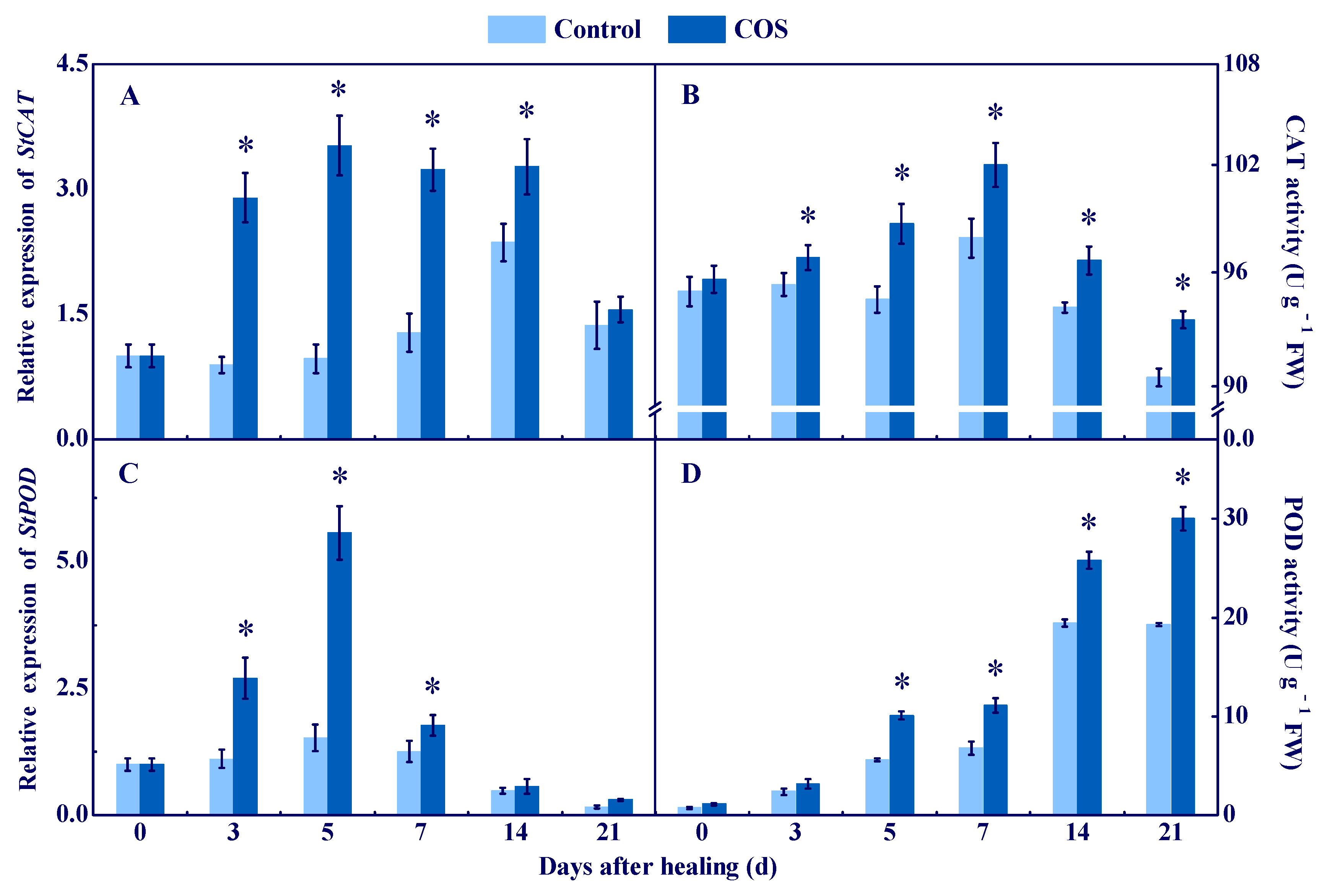
3.2. COS Elicited CAT and POD at Tuber Wounds
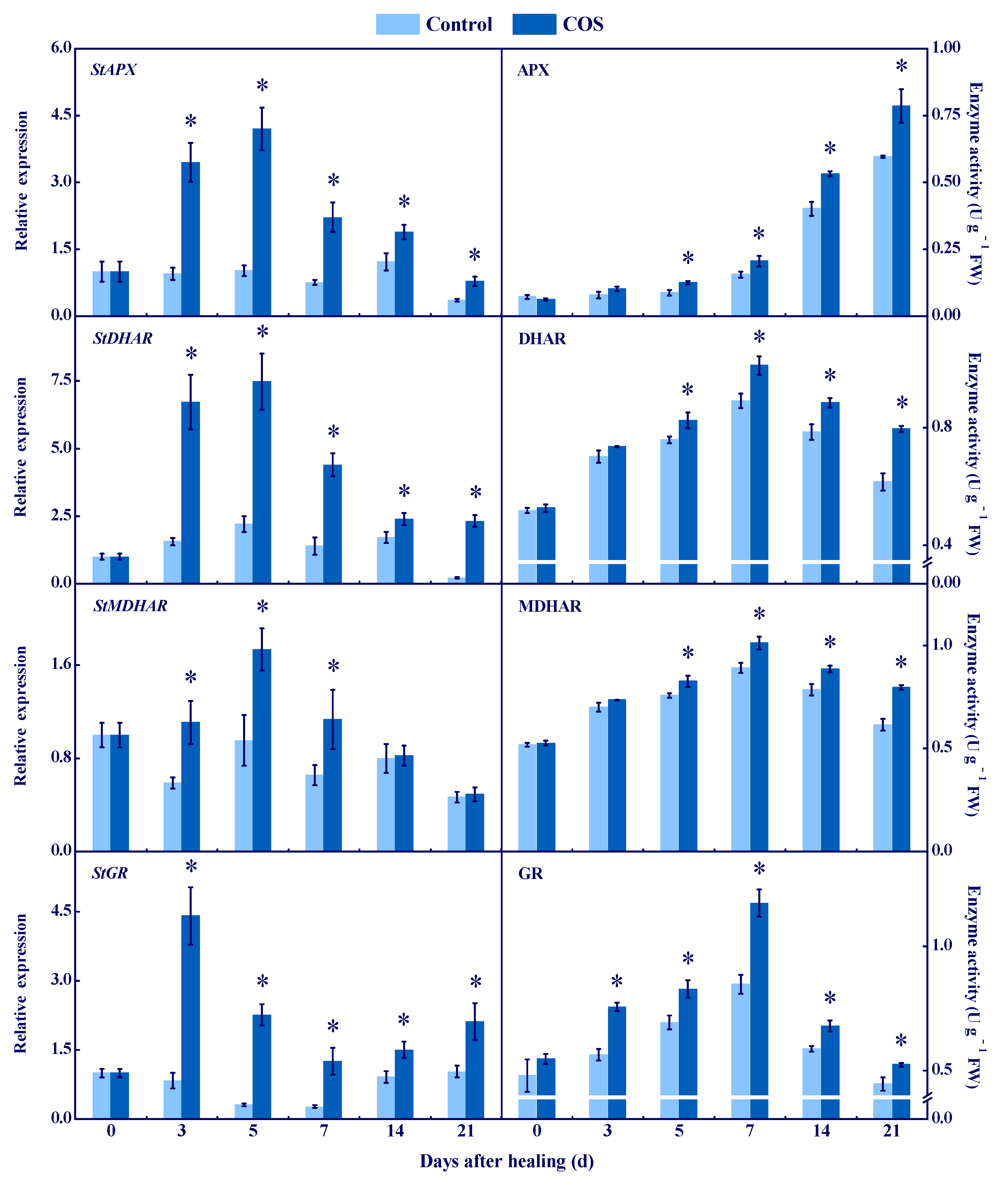
3.3. COS Activated the AsA–GSH Cycle at Tuber Wounds
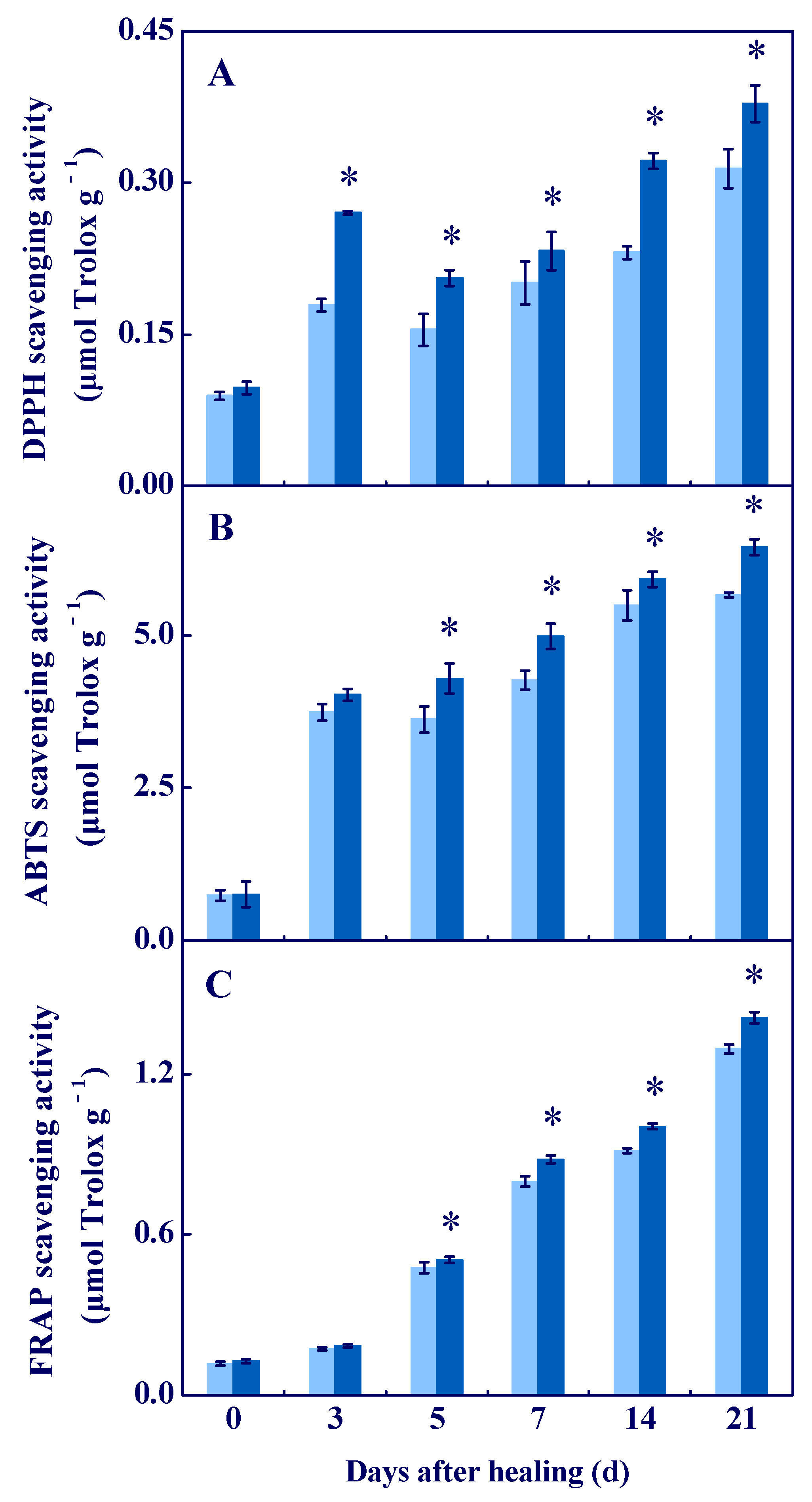
3.4. COS Enhanced the Antioxidant Capacity In Vitro at Tuber Wounds
3.5. COS Reduced Cell Membrane Permeability and MDA Content at Tuber Wounds
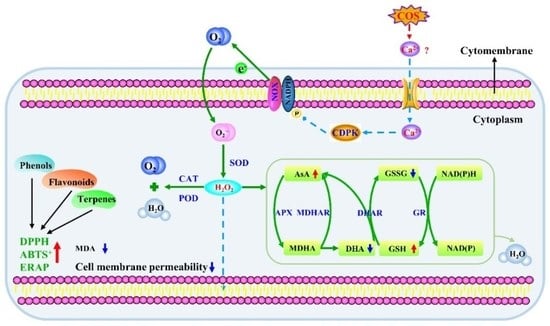
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, H.; Wang, Y.; Li, C.J.; Wang, B.; Ma, L.; Ren, Y.Y.; Bi, Y.; Li, Y.C.; Xue, H.L.; Prusky, D. The effect of benzo-(1, 2, 3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH) treatment on regulation of reactive oxygen species metabolism involved in wound healing of potato tubers during postharvest. Food Chem. 2019, 309, 125608. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Y.; Jiang, H.; Ma, L.; Zheng, X.Y.; Prusky, D.; Eshel, D.; Bi, Y. Diphenyleneiodonium delays the deposition of suberin polyphenolic and lignin at wounds of potato tubers by inhibiting Strbohs transcription and NADPH oxidase activity. Postharvest Biol. Technol. 2021, 185, 111798. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, H.; Bi, Y.; Li, Y.C.; Yang, G.W.; Si, H.J.; Ren, Y.Y.; Pyusky, D. The Interaction Between StCDPK14 and StRbohB contributes to benzo-(1, 2, 3)-thiadiazole-7-carbothioic Acid S-methyl ester-induced wound healing of potato tubers by regulating reactive oxygen species generation. Front. Plant Sci. 2021, 12, 737524. [Google Scholar] [CrossRef]
- Guan, G.; Azad, M.A.K.; Lin, Y.S.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H.B. Biological effects and applications of chitosan and chito-oligosaccharides. Front. Physiol. 2019, 10, 516–526. [Google Scholar] [CrossRef]
- Yin, N.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Characterization of antibacterial bacterial cellulose composite membranes modified with chitosan or chitooligosaccharide. Carbohydr. Polym. 2019, 229, 115520. [Google Scholar] [CrossRef]
- Huang, Y.; Ming, J.; Deng, Y.Y.; Deng, L.L.; Zhang, Z.Q.; Zeng, K.F. Induction of Colletotrichum gloeosporioides Penz. resistance through oligochitosan treatment and active oxygen change in citrus fruits. Food Sci. 2009, 30, 344–349. [Google Scholar] [CrossRef]
- Chen, C.; Hu, W.Z.; Zhang, R.D.; Jiang, A.L. Effects of postharvest oligochitosan treatment on fungal diseases and defence responses in Dongxue peach fruit. Food Sci. Technol. Int. 2017, 24, 161–171. [Google Scholar] [CrossRef]
- Liu, J.; Kennedy, J.F.; Zhang, X.F.; Heng, Y.; Chen, W.; Chen, Z.; Wu, X.; Wu, X.H. Preparation of alginate oligosaccharide and its effects on decay control and quality maintenance of harvested kiwifruit. Carbohydr. Polym. 2020, 242, 116462. [Google Scholar] [CrossRef]
- Ru, L.; Jiang, L.F.; Wills, R.B.H.; Golding, J.B.; Huo, Y.R.; Yang, H.Q.; Li, Y.X. Chitosan oligosaccharides induced chilling resistance in cucumber fruit and associated stimulation of antioxidant and HSP gene expression. Sci. Hortic. 2020, 264, 109187. [Google Scholar] [CrossRef]
- Yan, J.Q.; Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Effects of preharvest oligochitosan sprays on postharvest fungal diseases, storage quality, and defense responses in jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. Sci. Hortic. 2012, 142, 196–204. [Google Scholar] [CrossRef]
- Cheplick, S.; Sarkar, D.; Bhowmik, P.C.; Shetty, K. Improved resilience and m etabolic response of transplanted blackberry plugs using chitosan oligosaccharide elicitor treatment. Can. J. Plant Sci. 2018, 98, 717–731. [Google Scholar] [CrossRef]
- He, Y.Q.; Bose, S.K.; Wang, W.X.; Jia, X.C.; Lu, H.; Yin, H. Pre-harvest treatment of chitosan oligosaccharides improved strawberry fruit quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.L.; Li, B.Q.; Tian, S.P. Alginate oligosaccharide improves resistance to postharvest decay and quality in kiwifruit (Actinidia deliciosa cv. Bruno). Hortic. Plant J. 2022, 8, 44–52. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Zong, Y.Y.; Liang, W.; Kong, R.; Gong, D.; Han, Y.; Li, Y.C.; Bi, Y.; Prusky, D. Sorbitol immersion accelerates the deposition of suberin polyphenolic and lignin at wounds of potato tubers by activating phenylpropanoid metabolism. Sci. Hortic. 2022, 297, 110971. [Google Scholar] [CrossRef]
- Yang, R.R.; Han, Y.; Han, Z.H.; Ackah, S.; Li, Z.C.; Bi, Y.; Yang, Q.; Prusky, D. Hot water dipping stimulated wound healing of potato tubers. Postharvest Biol. Technol. 2020, 167, 111245. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Zheng, X.L.; Tian, S.P.; Gidley, M.J.; Yue, H.; Li, B.Q.; Xu, Y.; Zhou, Z.W. Slowing the deterioration of mango fruit during cold storage by pre-storage application of oxalic acid. J. Hortic. Sci. Biotechnol. 2007, 82, 707–714. [Google Scholar] [CrossRef]
- Kundu, A.; Patel, A.; Pal, A. Defining reference genes for qPCR normalization to study biotic and abiotic stress responses in Vigna mungo. Plant Cell Rep. 2013, 32, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 2001, 126, 1281–1290. [Google Scholar] [CrossRef]
- Bai, J.P.; Gao, H.J.; Yang, H.Y.; Lou, Y.; Zhang, J.L.; Wang, D.; Zhang, J.L. Comparison of ultrastructural and physiological changes of potato (Solanum tuberosum L.) plantlets subjected to salt and modeling drought stresses. Acta Physiol. Plant. 2016, 38, 182. [Google Scholar] [CrossRef]
- Venisse, J.S.; Malnoy, M.; Faize, M.; Paulin, J.P.; Brisset, M.N. Modulation of defense responses of Malus spp. during compatible and incompatible interactions with Erwinia amylovora. Mol. Plant Microbe Interact. 2002, 15, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.H.; Bi, Y.; Li, Y.C.; Kou, Z.H.; Hu, L.G.; Ge, Y.H.; Wang, Y.; Wang, D. Overproduction of reactive oxygen species involved in the pathogenicity of Fusarium in potato tubers. Physiol. Mol. Plant Pathol. 2014, 86, 35–42. [Google Scholar] [CrossRef]
- Queirós, F.; Rodrigues, J.A.; Almeida, J.M.; Almeida, D.P.F.; Fidalgo, F. Differential responses of the antioxidant defence system and ultrastructure in a salt-adapted potato cell line. Plant Physiol. Biochem. 2011, 49, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Turcsányi, E.; Lyons, T.; Plöchl, M.; Barnes, J. Does ascorbate in the mesophyll cell walls form the first line of defence against ozone? testing the concept using broad bean (Vicia faba L.). J. Exp. Bot. 2000, 51, 901–910. [Google Scholar] [CrossRef]
- Imahori, Y.; Bai, J.; Baldwin, E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016, 198, 398–406. [Google Scholar] [CrossRef]
- Ma, Y.H.; Ma, F.W.; Zhang, J.K.; Li, M.Y.; Wang, Y.H.; Liang, D. Effects of high temperature on activities and gene expression of enzymes involved in ascorbate-glutathione cycle in apple leaves. Plant Sci. 2008, 175, 761–766. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Jun-Cheol, M.; Changsoo, K.; Manoharan, K.; Wook, K. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanism. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar] [CrossRef]
- Guo, X.Y.; Yu, Z.M.; Zhang, M.H.; Tang, W.Z.; Sun, Y.M.; Li, X.Z. Enhancing the production of phenolic compounds during barley germination by using chitooligosaccharides to improve the antioxidant capacity of malt. Biotechnol. Lett. 2018, 40, 1335–1341. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Tao, Y.; Wu, X.Z.; Cui, Z.B. Influence of γ-irradiation on the reactive-oxygen metabolism of blueberry fruit during cold storage. Innov. Food Sci. Emerg. Technol. 2017, 41, 397–403. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 372, 1331–1341. [Google Scholar] [CrossRef]
- Ning, W.; Chen, F.; Mao, B.; Li, Q.; Liu, Z.; Guo, Z.; He, Z.H. N-acetylchitooligosaccharides elicit rice defence responses including hypersensitive response-like cell death, oxidative burst and defence gene expression. Physiol. Mol. Plant Pathol. 2004, 64, 263–271. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.M.; Wang, W.X.; Yin, H.; Xu, J.G.; Bai, X.F.; Du, Y.G. Inhibition effect on tobacco mosaic virus and regulation effect on calreticulin of oligochitosan in tobacco by induced Ca2+ influx. Carbohydr. Polym. 2010, 82, 136–142. [Google Scholar] [CrossRef]
- Yin, H.; Li, S.; Zhao, X.; Du, Y.; Ma, X. cDNA microarray analysis of gene expression in Brassica napus treated with oligochitosan elicitor. Plant Physiol. Biochem. 2006, 44, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.N.; Zhang, Q.Q.; Ji, D.Z.; Li, Y.Y.; Zhou, X.; Jin, L.H. Physiological, transcriptomic investigation on the tea plant growth and yield motivation by chitosan oligosaccharides. Horticulturae 2022, 8, 68. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, J.J.; Xiao, X.; Zhang, M.F.; Yun, Z.; Zeng, Y.L.; Xu, J.; Cheng, Y.J.; Deng, X.X. Salicylic acid treatment reduces the rot of postharvest citrus fruit by inducing the accumulation of H2O2, primary metabolites and lipophilic poly-methoxylated flavones. Food Chem. 2016, 207, 68–74. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Ma, Z.X.; Yang, L.Y.; Yan, H.X.; Kennedy, J.F.; Meng, X.H. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef]
- Pu, F.; Ren, X.L. Ascorbate levels and activities of enzymes related to the glutathione-ascorbate cycle in fruits of Chinese persimmon cultivars. Hortic. Environ. Biotechnol. 2014, 55, 315–321. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Chumyam, A.; Shank, L.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Effect of chlorine dioxide fumigation on redox balancing potential of antioxidative ascorbate-glutathione cycle in ‘Daw’ longan fruit during storage. Sci. Hortic. 2017, 222, 76–83. [Google Scholar] [CrossRef]
- Andrys, D.; Kulpa, D.; Grzeszczuk, M.; Bihun, M.; Dobrowolska, A. Antioxidant and antimicrobial activities of Lavandula angustifolia Mill. field-grown and propagated in vitro. Folia Hortic. 2017, 29, 161–180. [Google Scholar] [CrossRef]
- Gupta, M.; Karmakar, N.; Sasmal, S.; Chowdhury, S.; Biswas, S. Free radical scavenging activity of aqueous and alcoholic extracts of Glycyrrhiza glabra Linn. measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical antioxidant assay. Int. J. Pharmacol. Toxicol. 2016, 4, 235–240. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Ma, L.; Ren, Y.Y.; Bi, Y.; Prusky, D. Transcriptome sequencing and differential expression analysis of natural and BTH-treated wound healing in potato tubers (Solanum tuberosum L.). BMC Genom. 2022, 23, 263. [Google Scholar] [CrossRef] [PubMed]
- Mondy, N.I.; Leja, M. Effect of mechanical injury on the ascorbic acid content of potatoes. J. Food Sci. 1986, 51, 355–357. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Kallash, L.; Wang, I.; Phan, V.C.; Huang, W.L.; Serra, O.; Stark, R.E. Defensive armor of potato tubers: Nonpolar metabolite profiling, antioxidant assessment, and solid-state NMR compositional analysis of suberin-enriched wound-healing tissues. J. Agric. Food Chem. 2015, 63, 6810–6822. [Google Scholar] [CrossRef]
- Kerch, G.; Sabovics, M.; Kruma, Z.; Kampuse, S.; Straumite, E. Effect of chitosan and chitooligosaccharide on vitamin C and polyphenols contents in cherries and strawberries during refrigerated storage. Eur. Food Res. Technol. 2011, 233, 351–358. [Google Scholar] [CrossRef]
- Cao, S.F.; Hu, Z.C.; Zheng, Y.H.; Yang, Z.F.; Lu, B.H. Effect of BTH on antioxidant enzymes, radical-scavenging activity and decay in strawberry fruit. Food Chem. 2011, 125, 145–149. [Google Scholar] [CrossRef]
- Lulai, E.C. The Canon of Potato Science: 43. Skin-set and Wound-healing/Suberization. Potato Res. 2007, 50, 387–390. [Google Scholar] [CrossRef]
- Schreiber, L.; Franke, R.; Harmann, K. Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpirations. Planta 2005, 220, 520–530. [Google Scholar] [CrossRef]


| Gene | Gene Accession Number | Primer Sequences (52032–3′) |
|---|---|---|
| StNOX | NM_001288375.1 | Forward Primer: GTT TAC CTG GGC ATG AAC GC |
| Reverse Primer: CTC CAC CAA TAC CGA CTC C | ||
| StSOD | AF354748 | Forward Primer: GTT TGT GGC ACC ATC CTC TT |
| Reverse Primer: GTG GTC CTG TTG ACA TGC AG | ||
| StPOD | M21334.1 | Forward Primer: CAG CAA CCA AGG TAT AAT GTT T |
| Reverse Primer: CGC GGA TGG AGG CAA GTC T | ||
| StRCAT | AY442179 | Forward Primer: TGC CCT TCT ATT GTG GTT CC |
| Reverse Primer: GAT GAG CAC ACT TTG GAG GA | ||
| StAPX | AB041343.1 | Forward Primer: ACC AAT TGG CTG GTG TTG TT |
| Reverse Primer: TCA CAA ACA CGT CCC TCA AA | ||
| StDHAR | DQ512964 | Forward Primer: AGG TGA ACC CAG AAG GGA AA |
| Pr Reverse imer: TAT TTT CGA GCC CAC AGA GG | ||
| StMDHAR | NM_001247084.2 | Forward Primer: GCT GAT CCC AAC TCT GCA ACT |
| Reverse Primer: CAC TCT CGA GGA ATG CAC CAA | ||
| StGR | X76533 | Forward Primer: GGA TCC TCA TAC GGT GGA TG |
| Reverse Primer: TTA GGC TTC GTT GGC AAA TC | ||
| Efla | AB061263.1 | Forward Primer: CAA GGA TGA CCC AGC CAA G |
| Reverse Primer: TTC CTT ACC TGA ACG CCT GT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; Yang, Y.; Gong, D.; Yu, L.; Han, Y.; Zong, Y.; Li, Y.; Prusky, D.; Bi, Y. Chitooligosaccharide Maintained Cell Membrane Integrity by Regulating Reactive Oxygen Species Homeostasis at Wounds of Potato Tubers during Healing. Antioxidants 2022, 11, 1791. https://doi.org/10.3390/antiox11091791
Xie P, Yang Y, Gong D, Yu L, Han Y, Zong Y, Li Y, Prusky D, Bi Y. Chitooligosaccharide Maintained Cell Membrane Integrity by Regulating Reactive Oxygen Species Homeostasis at Wounds of Potato Tubers during Healing. Antioxidants. 2022; 11(9):1791. https://doi.org/10.3390/antiox11091791
Chicago/Turabian StyleXie, Pengdong, Yangyang Yang, Di Gong, Lirong Yu, Ye Han, Yuanyuan Zong, Yongcai Li, Dov Prusky, and Yang Bi. 2022. "Chitooligosaccharide Maintained Cell Membrane Integrity by Regulating Reactive Oxygen Species Homeostasis at Wounds of Potato Tubers during Healing" Antioxidants 11, no. 9: 1791. https://doi.org/10.3390/antiox11091791
APA StyleXie, P., Yang, Y., Gong, D., Yu, L., Han, Y., Zong, Y., Li, Y., Prusky, D., & Bi, Y. (2022). Chitooligosaccharide Maintained Cell Membrane Integrity by Regulating Reactive Oxygen Species Homeostasis at Wounds of Potato Tubers during Healing. Antioxidants, 11(9), 1791. https://doi.org/10.3390/antiox11091791