Modified Montmorillonite Improved Growth Performance of Broilers by Modulating Intestinal Microbiota and Enhancing Intestinal Barriers, Anti-Inflammatory Response, and Antioxidative Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatment and Experimental Design
2.2. Sample Collection and Treatment
2.3. Measurement of the Growth Performance
2.4. Serum-Biochemical Indexes
2.5. Transmission Electron Microscopy (TEM)
2.6. RNA Extraction and RT-qPCR
2.7. Western Blot Analysis
2.8. Intestinal Microbial DNA Extraction and High-Throughput Sequencing
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Cecum Microbiota Analysis
3.2.1. Microbiota Diversity in Intestinal Contents
3.2.2. Cluster Analysis
3.2.3. Overall Structure Modulation of Gut Microbiota
3.2.4. Predicted Metabolic Functions in the Gut Microbiota
3.3. Intestinal Physical Barrier Function
3.4. Immune Responses and Inflammation
3.5. Antioxidant Status
3.6. Apoptosis-Related Genes in the Jejunum
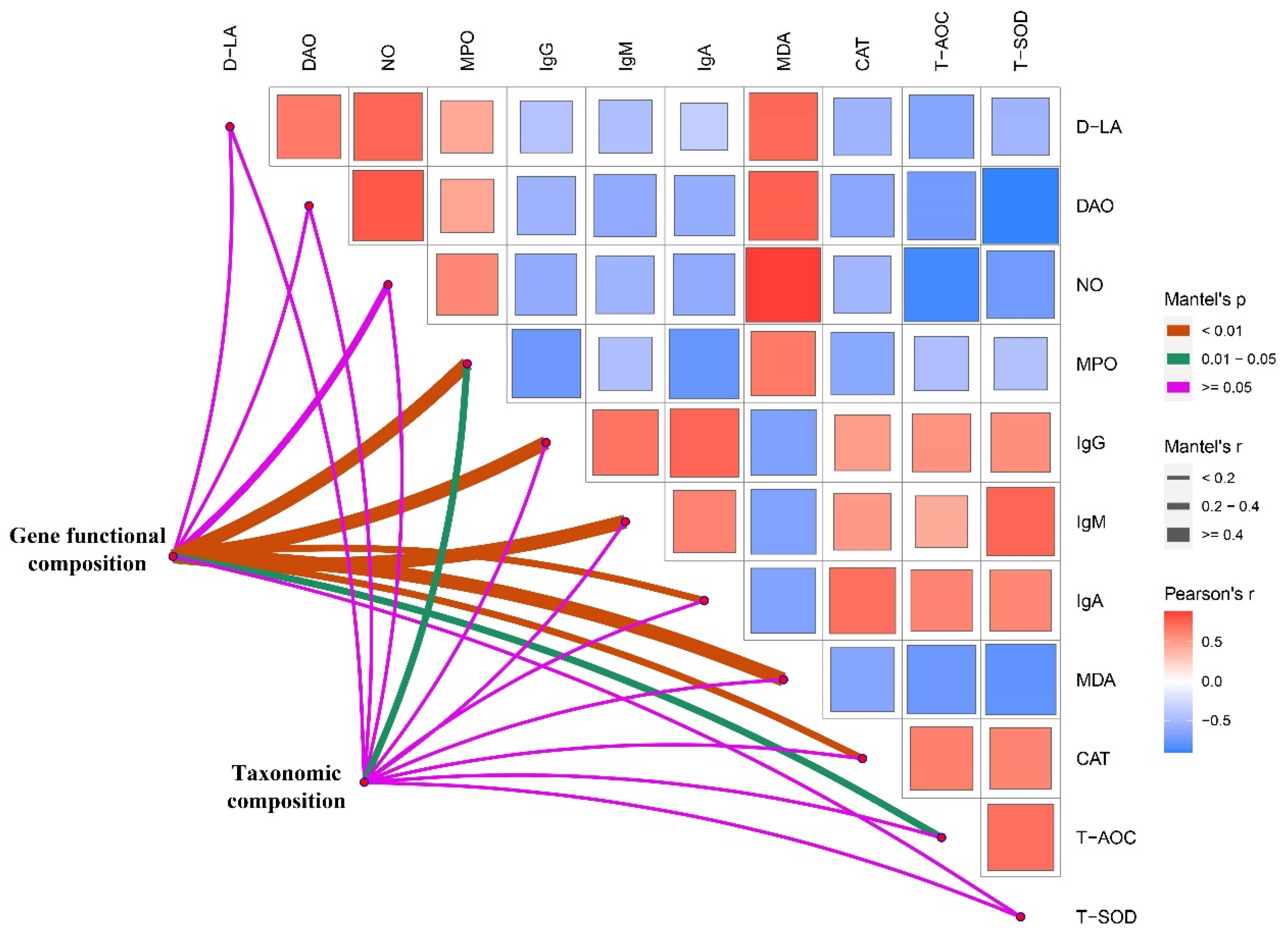
3.7. Correlation Heat Map
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Z.; Deng, H.; Deng, Y.; Liang, Z.; Deng, J.; Zuo, Z.; Hu, Y.; Shen, L.; Yu, S.; Cao, S. Combined effects of deoxynivalenol and zearalenone on oxidative injury and apoptosis in porcine splenic lymphocytes in vitro. Exp. Toxicol. Pathol. 2017, 69, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Jerab, J.; Jansen, W.; Blackwell, J.; van Hout, J.; Palzer, A.; Lister, S.; Chantziaras, I.; Dewulf, J.; De Briyne, N. Real-World Data on Antibiotic Group Treatment in European Livestock: Drivers, Conditions, and Alternatives. Antibiotics 2022, 11, 1046. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017, 96, 3104–3108. [Google Scholar] [CrossRef] [PubMed]
- Lesne, J.; Baron, S. Antibiotic resistance: Moving from individual health norms to social norms in One Health and Global Health. Environ. Risques Sante 2022, 21, 303–309. [Google Scholar]
- Wang, B.; Gong, L.; Zhou, Y.; Tang, L.; Zeng, Z.; Wang, Q.; Zou, P.; Yu, D.; Li, W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021, 7, 829–840. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Al Sulaiman, A.R.; Aljumaah, R.S.; Alabdullatif, A.A.; Ferronato, G.; Alqhtani, A.H.; Al-Garadi, M.A.; Al-sornokh, H.; Abudabos, A.M. The efficacy of bentonite and zeolite in reducing aflatoxin B1 toxicity on production performance and intestinal and hepatic health of broiler chickens. Ital. J. Anim. Sci. 2022, 21, 1181–1189. [Google Scholar] [CrossRef]
- Ates, M.B.; Ortatatli, M.; Oguz, H.; Ozdemir, O.; Terzi, F.; Ciftci, M.K.; Hatipoglu, F. The ameliorative effects of Nigella sativa, thymoquinone, and bentonite against aflatoxicosis in broilers via AFAR and Nrf2 signalling pathways, and down-regulation of caspase-3. Br. Poult. Sci. 2022, 63, 332–339. [Google Scholar] [CrossRef]
- Ghazalah, A.A.; Abd-Elsamee, M.O.; Moustafa, K.; Khattab, M.A.; Rehan, A. Effect of Nanosilica and Bentonite as Mycotoxins Adsorbent Agent in Broiler Chickens’ Diet on Growth Performance and Hepatic Histopathology. Animals 2021, 11, 2129. [Google Scholar] [CrossRef]
- Szczerba, M.; McCarty, D.K.; Derkowski, A.; Kowalik, M. Molecular dynamics simulations of interactions of organic molecules found in oil with smectite: Influence of brine chemistry on oil recovery. J. Pet. Sci. Eng. 2020, 191, 107148. [Google Scholar] [CrossRef]
- Pour, A.R.A.; Kermanshahi, H.; Golian, A. Effects of conditioning time and activated sodium bentonite on pellet quality, performance, intestinal morphology, and nutrients retention in growing broilers fed wheat-soybean meal diets. Anim. Feed. Sci. Technol. 2021, 277, 114955. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Huang, W.; Wang, H.; Qin, S.; Pei, W.; Yang, M.; Shi, Z. Effects of montmorillonite on the growth performance, immunity, intestinal morphology and caecal microflora of broilers. Anim. Prod. Sci. 2021, 61, 1546–1552. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, Y.; Wei, J.T.; Zhu, M.X.; Zhang, L.; Zhang, J.C.; Karrow, N.A.; Han, Y.M.; Wu, Y.Y.; Guo, Y.M.; et al. Mitigation Effects of Bentonite and Yeast Cell Wall Binders on AFB(1), DON, and OTA Induced Changes in Laying Hen Performance, Egg Quality, and Health. Toxins 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Yan, H.L.; Cao, S.C.; Hu, Y.D.; Zhang, H.F. Effects of absorbents on growth performance, blood profiles and liver gene expression in broilers fed diets naturally contaminated with aflatoxin. Asian-Australas. J. Anim. Sci. 2020, 33, 294–304. [Google Scholar] [CrossRef]
- Chen, J.F.; Xu, M.M.; Kang, K.L.; Tang, S.G.; He, C.Q.; Qu, X.Y.; Guo, S.C. The effects and combinational effects of Bacillus subtilis and montmorillonite on the intestinal health status in laying hens. Poult. Sci. 2020, 99, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M.; Alhouri, H.A.A.; Alhidary, I.A.; Nassan, M.A.; Swelum, A.A. Ameliorative effect of Bacillus subtilis, Saccharomyces boulardii, oregano, and calcium montmorillonite on growth, intestinal histology, and blood metabolites on Salmonella-infected broiler chicken. Environ. Sci. Pollut. Res. 2019, 26, 16274–16278. [Google Scholar] [CrossRef]
- Deng, S.; Xing, T.; Li, C.; Xu, X.; Zhou, G. The Effect of Breed and Age on the Growth Performance, Carcass Traits and Metabolic Profile in Breast Muscle of Chinese Indigenous Chickens. Foods 2022, 11, 483. [Google Scholar] [CrossRef]
- Song, W.J.; Song, Q.L.; Chen, X.L.; Liu, G.H.; Zou, Z.H.; Tan, J.; Liu, L.X.; Zeng, Y.B. Effects of honeycomb extract on the growth performance, carcass traits, immunity, antioxidant function and intestinal microorganisms of yellow bantam broilers. Poult. Sci. 2022, 101, 101811. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Zhong, Y.; Huang, C.; Deng, Z.; Cui, Y.; Liu, J.; Wang, H.L. L. reuteri ZJ617 inhibits inflammatory and autophagy signaling pathways in gut-liver axis in piglet induced by lipopolysaccharide. J. Anim. Sci. Biotechnol. 2021, 12, 110. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Soares, J.A.; Che, T.M.; Osuna, O.; Maddox, C.W.; Pettigrew, J.E. Dietary clays alleviate diarrhea of weaned pigs. J. Anim. Sci. 2012, 90, 345–360. [Google Scholar] [CrossRef]
- Li, X.; Wen, J.; Jiao, L.; Wang, C.; Hong, Q.; Feng, J.; Hu, C. Dietary copper/zinc-loaded montmorillonite improved growth performance and intestinal barrier and changed gut microbiota in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 678–686. [Google Scholar] [CrossRef]
- Jiao, L.F.; Zhang, Q.H.; Wu, H.; Wang, C.C.; Cao, S.T.; Feng, J.; Hu, C.H. Influences of Copper/Zinc-Loaded Montmorillonite on Growth Performance, Mineral Retention, Intestinal Morphology, Mucosa Antioxidant Capacity, and Cytokine Contents in Weaned Piglets. Biol. Trace Elem. Res. 2018, 185, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Z.; Deng, Y.; Zhou, Z.; Hou, J. Toxicity induced by F. Toxicity induced by F. poae-contaminated feed and the protective effect of Montmorillonite supplementation in broilers. Food Chem. Toxicol. 2014, 74, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Perez, W.; Barquero-Calvo, E.; Chaves, A.J. Effect of the use of probiotic Bacillus subtilis (QST 713) as a growth promoter in broilers: An alternative to bacitracin methylene disalicylate. Poult. Sci. 2021, 100, 101372. [Google Scholar] [CrossRef] [PubMed]
- Trckova, M.; Vondruskova, H.; Zraly, Z.; Alexa, P.; Hamrik, J.; Kummer, V.; Maskova, J.; Mrlik, V.; Krizova, K.; Slana, I.; et al. The effect of kaolin feeding on efficiency, health status and course of diarrhoeal infections caused by enterotoxigenic Escherichia coli strains in weaned piglets. Vet. Med. 2009, 54, 47–63. [Google Scholar] [CrossRef]
- Clays as dietary supplements for swine: A review. J. Anim. Sci. Biotechnol. 2016, 7, 41–49.
- Almeida, J.A.S.; Liu, Y.; Song, M.; Lee, J.J.; Gaskins, H.R.; Maddox, C.W.; Osuna, O.; Pettigrew, J.E. Escherichia coli challenge and one type of smectite alter intestinal barrier of pigs. J. Anim. Sci. Biotechnol. 2013, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.W.; Li, J.T.; Gong, L.M.; Wu, H.; Zhang, L.Y. Effects of Graded Levels of Montmorillonite on Performance, Hematological Parameters and Bone Mineralization in Weaned Pigs. Asian-Australas. J. Anim. Sci. 2013, 26, 1614–1621. [Google Scholar] [CrossRef]
- Jiao, L.; Lin, F.; Cao, S.; Wang, C.; Wu, H.; Shu, M.; Hu, C. Preparation, characterization, antimicrobial and cytotoxicity studies of copper/zinc-loaded montmorillonite. J. Anim. Sci. Biotechnol. 2017, 8, 27. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Chen, J.F.; Kuang, Y.H.; Qu, X.Y.; Guo, S.C.; Kang, K.L.; He, C.Q. The effects and combinational effects of Bacillus subtilis and montmorillonite supplementation on performance, egg quality, oxidation status, and immune response in laying hens. Livest. Sci. 2019, 227, 114–119. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, G.; Zhang, H.; Lan, J.; Yang, C. Effects of rhamnolipids on growth performance and intestinal health parameters in Linnan yellow broilers. Poultry Sci. 2021, 100, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, H.; Liu, J.; Zeng, X.; Wu, Y.; Yang, C. Rhamnolipids enhance growth performance by improving the immunity, intestinal barrier function, and metabolome composition in broilers. J. Sci. Food Agric. 2022, 102, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Huang, Z.; Lv, L.; Liu, X.; Yin, C.; Yan, H.; Yuan, J. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016, 120, 195–204. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 2022, 36, 27–37. [Google Scholar] [CrossRef]
- Takahashi, K.; Andoh, A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. J. Gastroenterol. Hepatol. 2016, 31, 178. [Google Scholar] [CrossRef]
- Obata, M.; Ohtsuji, M.; Iida, Y.; Shirai, T.; Hirose, S.; Nishimura, H. Genome-Wide Genetic Study in Autoimmune Disease-Prone Mice. In Arthritis Research: Methods and Protocols, 2nd ed.; Shiozawa, S., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NY, USA, 2014; Volume 1142, pp. 111–141. [Google Scholar]
- Schulz-Weidner, N.; Weigel, M.; Turujlija, F.; Komma, K.; Mengel, J.P.; Schlenz, M.A.; Bulski, J.C.; Kraemer, N.; Hain, T. Microbiome Analysis of Carious Lesions in Pre-School Children with Early Childhood Caries and Congenital Heart Disease. Microorganisms 2021, 9, 1904. [Google Scholar] [CrossRef]
- Chen, T.T.; Long, W.M.; Zhang, C.H.; Liu, S.; Zhao, L.P.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides- dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- Hemsworth, G.R.; Dejean, G.; Davies, G.J.; Brumer, H. Learning from microbial strategies for polysaccharide degradation. Biochem. Soc. Trans. 2016, 44, 94–108. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Gu, X.; Zhao, J.; Nie, C.; Zhang, W.; Ma, X. Dietary Montmorillonite Improves the Intestinal Mucosal Barrier and Optimizes the Intestinal Microbial Community of Weaned Piglets. Front. Microbiol. 2020, 11, 593056. [Google Scholar] [CrossRef] [PubMed]
- Prakatur, I.; Miskulin, M.; Pavic, M.; Marjanovic, K.; Blazicevic, V.; Miskulin, I.; Domacinovic, M. Intestinal Morphology in Broiler Chickens Supplemented with Propolis and Bee Pollen. Animals 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Xie, Q.; Evivie, S.E.; Liu, D.; Dong, J.; Ping, L.; Liu, F.; Li, B.; Huo, G. Bifidobacterium dentium N8 with potential probiotic characteristics prevents LPS-induced intestinal barrier injury by alleviating the inflammatory response and regulating the tight junction in Caco-2 cell monolayers. Food Funct. 2021, 12, 7171–7184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, Y.; Ma, W.; Yu, H.; Lu, Y.; Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Xiao, R. 27-Hydroxycholesterol contributes to cognitive deficits in APP/PS1 transgenic mice through microbiota dysbiosis and intestinal barrier dysfunction. J. Neuroinflamm. 2020, 17, 199. [Google Scholar] [CrossRef]
- Wu, Q.J.; Liu, N.; Wu, X.H.; Wang, G.Y.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 2019, 24, 513. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lai, Y.-H.; Hsiao, F.S.-H.; Cheng, Y.-H. Effects of Deoxynivalenol and Mycotoxin Adsorbent Agents on Mitogen-Activated Protein Kinase Signaling Pathways and Inflammation-Associated Gene Expression in Porcine Intestinal Epithelial Cells. Toxins 2021, 13, 301. [Google Scholar] [CrossRef]
- Li, X.H.; Chen, Y.P.; Cheng, Y.F.; Yang, W.L.; Wen, C.; Zhou, Y.M. Effect of yeast cell wall powder with different particle sizes on the growth performance, serum metabolites, immunity and oxidative status of broilers. Anim. Feed. Sci. Technol. 2016, 212, 81–89. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Chen, Y.; Liu, J.; Wu, Y.; Xu, Y. Multi-Omics Revealed the Protective Effects of Rhamnolipids in Lipopolysaccharide Challenged Broilers. Front. Immunol. 2022, 13, 824664. [Google Scholar] [CrossRef]
- Mucksova, J.; Chalupsky, K.; Plachy, J.; Kalina, J.; Rachacova, P.; Stanek, O.; Trefil, P. Simultaneous detection of chicken cytokines in plasma samples using the Bio-Plex assay. Poult. Sci. 2018, 97, 1127–1133. [Google Scholar] [CrossRef]
- Afroz, R.; Tanvir, E.M.; Tania, M.; Fu, J.; Kamal, M.A.; Khan, M.A. LPS/TLR4 pathways in breast cancer: Insights into cell signalling. Curr. Med. Chem. 2021, 29, 2274–2289. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Nabil, M.; Abdo, W.; Abdelfattah, M.A.O.; El-Shazly, A.M.; El Kharrassi, Y.; Sobeh, M. Syzygium samarangense leaf extract mitigates indomethacin-induced gastropathy via the NF-B-K signaling pathway in rats. Biomed. Pharmacother. 2021, 139, 111675. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ezeorba, T.P.C.; Aham, E.C.; Aguchem, R.N.; Nechi, R.N. Recent findings on the cellular and molecular mechanisms of action of novel food-derived antihypertensive peptides. Food Chem. Mol. Sci. 2022, 4, 100078. [Google Scholar] [CrossRef] [PubMed]
- Xuliang, Z.; Qi, W.; Jian, Z.; Miao, S.; Bing, S.; Yanfei, H.; Xu, Y.; Yanfei, L. The protective effect of selenium on T-2-induced nephrotoxicity is related to the inhibition of ROS-mediated apoptosis in mice kidney. Biol. Trace Elem. Res. 2022, 200, 206–216. [Google Scholar]
- Aghemo, A.; Alekseeva, O.P.; Angelico, F.; Bakulin, I.G.; Bakulina, N.V.; Bordin, D.; Bueverov, A.O.; Drapkina, O.M.; Gillessen, A.; Kagarmanova, E.M.; et al. Role of silymarin as antioxidant in clinical management of chronic liver diseases: A narrative review. Ann. Med. 2022, 54, 1548–1560. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Nogales, F.; Gallego-Lopez, M.d.C.; Carreras, O. Binge drinking during the adolescence period causes oxidative damage-induced cardiometabolic disorders: A possible ameliorative approach with selenium supplementation. Life Sci. 2022, 301, 120618. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; El-Kady, A.A.; Hassan, A.M.; Abd El-Moneim, O.M.; Abdel-Aziem, S.H. Effectiveness of activated carbon and Egyptian montmorillonite in the protection against deoxynivalenol-induced cytotoxicity and genotoxicity in rats. Food Chem. Toxicol. 2015, 83, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Wang, J.; Ishfaq, M.; Li, J. Baicalin ameliorates Mycoplasma gallisepticum-induced inflammatory injury in the chicken lung through regulating the intestinal microbiota and phenylalanine metabolism. Food Funct. 2021, 12, 4092–4104. [Google Scholar] [CrossRef]
- Pineda-Quiroga, C.; Borda-Molina, D.; Chaves-Moreno, D.; Ruiz, R.; Atxaerandio, R.; Camarinha-Silva, A.; Garcia-Rodriguez, A. Microbial and Functional Profile of the Ceca from Laying Hens Affected by Feeding Prebiotics, Probiotics, and Synbiotics. Microorganisms 2019, 7, 123. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Liu, J.; Liu, Y.; Xu, Y.; Zhang, R.; Yu, Y.; Wang, Y.; Yang, C. Integrating Serum Metabolome and Gut Microbiome to Evaluate the Benefits of Lauric Acid on Lipopolysaccharide- Challenged Broilers. Front. Immunol. 2021, 12, 759323. [Google Scholar] [CrossRef] [PubMed]












| Item (%, Unless Otherwise Indicated) | Amount |
|---|---|
| Ingredients | |
| Corn | 60.00 |
| Soybean meal | 28.50 |
| Fish meal | 2.00 |
| Wheat middling | 4.50 |
| Dicalcium phosphate | 1.30 |
| Limestone | 2.25 |
| 50% choline chloride | 0.15 |
| Salt | 0.30 |
| Vitamin and mineral premix 1 | 1.00 |
| Total | 100.00 |
| Calculated Nutrient Level | |
| CP (crude protein) | 22.39 |
| Total P | 0.70 |
| Ca (calcium) | 1.00 |
| Lys (Lysine) | 1.17 |
| Met + Cys (Methionine + Cysteine) | 0.65 |
| Met (Methionine) | 0.48 |
| ME (MJ/kg) | 12.22 |
| Gene Name | Accession Number | Sequence (5′-3′) |
|---|---|---|
| β-actin | NM_205518.1 | F: CATTGTCCACCGCAAATGCT |
| R: AAGCCATGCCAATCTCGTCT | ||
| TLR2 | NM_001161650.2 | F: GGATTTCTCGCACTTTCGCC |
| R: AAAGGGACAAAAAGGCAATGGA | ||
| TLR4 | NM_001030693.1 | F: GGCTCAACCTCACGTTGGTA |
| R: AGTCCGTTCTGAAATGCCGT | ||
| TRAF6 | XM_046942060.1 | F: CGTCGTGCATTCCTACCGAT |
| R: GACAGCATAGGGCAACGAGT | ||
| Myd88 | NM_010851.3 | F: AGGCATCACCACCCTTGATG |
| R: CGAAAAGTTCCGGCGTTTGT | ||
| NF-κB | NM_205134.1 | F: TGGAGAAGGCTATGCAGCTT |
| R: CATCCTGGACAGCAGTGAGA | ||
| iNOS | NM_204961.2 | F: GAACAGCCAGCTCATCCGATA |
| R: CCCAAGCTCAATGCACAACTT | ||
| IL-1β | NM_204524.1 | F: TCTGCCTGCAGAAGAAGCC |
| R: CCTCACTTTCTGGCTGGAGG | ||
| IL-6 | NM_204628.1 | F: CCTTTCAGACCTACCTGGAATT |
| R: ACTTCATCGGGATTTATCACCA | ||
| TNF-α | NM_205427.1 | F: CAACGACACCATCCTGGACA |
| R: ATCCGGTTGAGGAGGCTTTG | ||
| IL-4 | NM_001007079.1 | F: GTGCCCACGCTGTGCTTAC |
| R: AGGAAACCTCTCCCTGGATGTC | ||
| IL-10 | NM_001004414.4 | F: CGCTGTCACCGCTTCTTCA |
| R: TCCCGTTCTCATCCATCTTCTC | ||
| Occludin | XM_025144247.1 | F: CGGAGCCCAGACTACCAAAG |
| R: TTACACAGCTTCAGCCTTACA | ||
| Claudin-1 | NM_001013611.2 | F: GGTATGGCAACAGAGTGGCT |
| R: CAGCCAATGAAGAGGGCTGA | ||
| ZO-1 | NM_204918.1 | F: TAAAATGGACAGGCGCTGACA |
| R: TTGGGCGTGACGTATAGCTG | ||
| CAT | NM_214301.2 | F: TCCAGCCAGTGACCAGATGA |
| R: CTCTCCCGGTCAAAGTGAGC | ||
| SOD1 | NM_001190422.1 | F: CAGGGCACCATCTACTTCGAGC |
| R: ACGTGCCTCTCTTGATCCTTTG | ||
| GPX4 | NM_214407.1 | F: TGGATGAAAGTCCAGCCCAAG |
| R: CTAGAGGTAGCACGGCAGGT | ||
| Nrf2 | NM_001004027 | F: CGCTCCCGAATGAACAC |
| R: GCTCCTGCACCTCCTC | ||
| HO1 | XM_003133500.5 | F: GCCCCTGGAAGCGTTAAAC |
| R: GGACTGTATCCCCAGAAGGTTGT | ||
| Keap1 | NM_001114671.1 | F: GCGTTCCGCACCTCCC |
| R: GACAAGGGACAACACCACCA | ||
| Bax | XM_015274882.1 | F: GTGATGGCATGGGACATAGCTC |
| R: TGGCGTAGACCTTGCGGATAA | ||
| Bcl2 | NM_205339.2 | F: GATGACCGAGTACCTGAACC |
| R: CAGGAGAAATCGAACAAAGGC | ||
| Caspase-3 | NM_204725.1 | F: ACTCTGGAAATTCTGCCTGATGAC |
| R: CATCTGCATCCGTGCCTGA | ||
| Caspase-9 | XM_424580.6 | F: TCAGACATCGTATCCTCCA |
| R: AAGTCACAGCAGGGACA |
| Stage | Indicator | CTR | ANTI | MMT | SEM | p-Value |
|---|---|---|---|---|---|---|
| BW | 1d | 37.974 | 37.908 | 38.434 | 0.337 | 0.285 |
| 21d | 411.842 b | 429.883 a | 434.000 a | 5.087 | 0.004 | |
| 38d | 1083.445 b | 1141.136 a | 1125.938 ab | 19.987 | 0.045 | |
| 63d | 1928.834 b | 2036.510 a | 2026.644 a | 33.738 | 0.020 | |
| 1-21d | ADG | 17.803 b | 18.666 a | 18.837 a | 0.238 | 0.004 |
| ADFI | 30.502 | 30.712 | 31.169 | 0.590 | 0.537 | |
| F/G | 1.714 | 1.647 | 1.655 | 0.047 | 0.346 | |
| 22-38d | ADG | 39.506 | 41.838 | 40.702 | 1.146 | 0.182 |
| ADFI | 81.376 | 80.519 | 82.792 | 1.746 | 0.453 | |
| F/G | 2.060 a | 1.927 b | 2.034 a | 0.043 | 0.030 | |
| 38-63d | ADG | 33.816 | 35.815 | 36.028 | 1.784 | 0.427 |
| ADFI | 93.329 b | 91.096 b | 98.466 a | 2.399 | 0.041 | |
| F/G | 2.760 a | 2.552 b | 2.741 a | 0.085 | 0.067 | |
| 1-63d | ADG | 30.014 b | 31.724 a | 31.559 a | 0.536 | 0.020 |
| ADFI | 71.736 | 71.609 | 74.142 | 1.383 | 0.175 | |
| F/G | 2.390 a | 2.258 b | 2.350 ab | 0.045 | 0.041 |
| Item | CTR | ANTI | MMT | SEM | p-Value |
|---|---|---|---|---|---|
| Serum | |||||
| T-AOC (U/mL) | 7.16 b | 9.01 a | 9.57 a | 0.488 | 0.001 |
| T-SOD (U/mL) | 129.17 b | 148.42 a | 148.89 a | 3.875 | 0.001 |
| CAT (U/mL) | 6.09 b | 7.54 a | 7.78 a | 0.462 | 0.005 |
| GSH-Px (U/mL) | 2045.56 | 2138.04 | 2035.74 | 107.451 | 0.587 |
| MDA (nmol/mL) | 7.94 a | 6.84 b | 6.78 b | 0.177 | <0.001 |
| Jejunal mucosa | |||||
| T-AOC (U/mg prot) | 2.64 b | 3.48 a | 3.61 a | 0.152 | 0.001 |
| T-SOD (U/mg prot) | 130.6 b | 154.03 a | 155.49 a | 3.291 | 0.001 |
| CAT (U/mg prot) | 13.33 | 13.4 | 13.14 | 0.346 | 0.743 |
| GSH-Px (U/mg prot) | 1404.92 | 1407.07 | 1408.67 | 7.509 | 0.883 |
| MDA (nmol/mg prot) | 3.29 a | 2.66 b | 2.61 b | 0.11 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhan, X.; Wang, B.; Wang, F.; Zhou, Y.; Xu, S.; Li, X.; Tang, L.; Jin, Q.; Li, W.; et al. Modified Montmorillonite Improved Growth Performance of Broilers by Modulating Intestinal Microbiota and Enhancing Intestinal Barriers, Anti-Inflammatory Response, and Antioxidative Capacity. Antioxidants 2022, 11, 1799. https://doi.org/10.3390/antiox11091799
Wang Q, Zhan X, Wang B, Wang F, Zhou Y, Xu S, Li X, Tang L, Jin Q, Li W, et al. Modified Montmorillonite Improved Growth Performance of Broilers by Modulating Intestinal Microbiota and Enhancing Intestinal Barriers, Anti-Inflammatory Response, and Antioxidative Capacity. Antioxidants. 2022; 11(9):1799. https://doi.org/10.3390/antiox11091799
Chicago/Turabian StyleWang, Qi, Xiaoli Zhan, Baikui Wang, Fei Wang, Yuanhao Zhou, Shujie Xu, Xiang Li, Li Tang, Qian Jin, Weifen Li, and et al. 2022. "Modified Montmorillonite Improved Growth Performance of Broilers by Modulating Intestinal Microbiota and Enhancing Intestinal Barriers, Anti-Inflammatory Response, and Antioxidative Capacity" Antioxidants 11, no. 9: 1799. https://doi.org/10.3390/antiox11091799





