Dietary Supplementation with Eucommia ulmoides Leaf Extract Improved the Intestinal Antioxidant Capacity, Immune Response, and Disease Resistance against Streptococcus agalactiae in Genetically Improved Farmed Tilapia (GIFT; Oreochromis niloticus)
Abstract
:1. Introduction
2. Materials and methods
2.1. Diet Preparation
2.2. Experimental Fish and Procedures
2.3. Sample Collection
2.4. Proximate Composition and Chemical Analysis
2.5. Histology
2.6. Real-Time PCR Analysis
2.7. Streptococcus Agalactiae Challenge Test
2.8. Statistical Analysis
3. Results
3.1. Growth Performance and Whole-Body Composition
3.2. Plasma Parameters
3.3. Intestinal Antioxidant Enzyme Activities
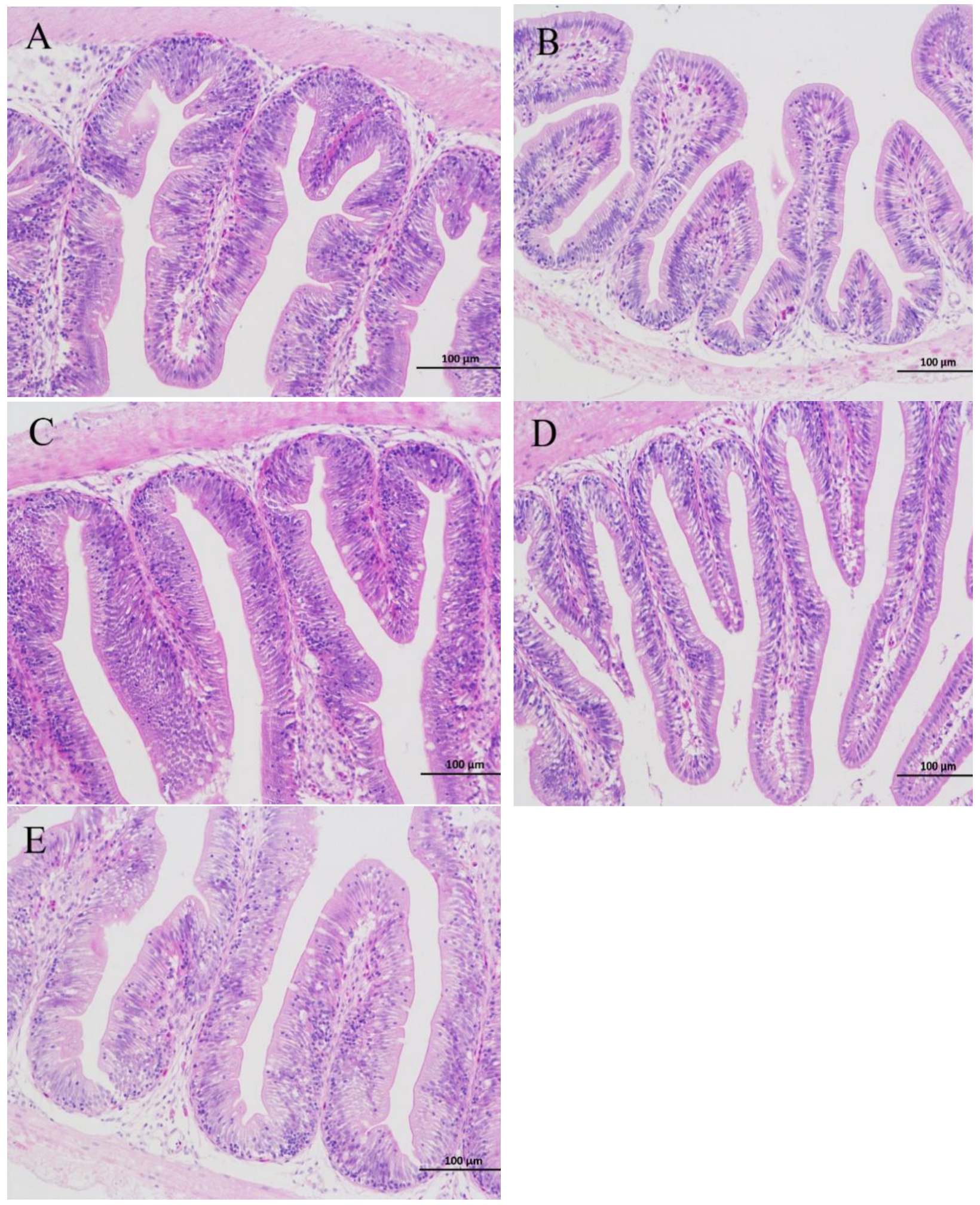
3.4. Histopathological Examination
3.5. Nrf2 Signaling Pathway and Hsp70
3.6. TLR2-MyD88 Signaling Pathway
3.7. Relative Expression of the Genes in the NF-κB Signaling Pathway
3.8. Relative Expression of the Genes in the Apoptosis Signaling Pathway
3.9. Streptococcus Agalactiae Challenge Test
4. Discussion
4.1. Effects of ELE Supplementation on Growth Performance and Whole-Body Composition
4.2. Effects of ELE Supplementation on Intestinal Morphology
4.3. Effects of ELE Supplementation on Antioxidant Status
4.4. Effects of ELE Supplementation on Immunocompetence
4.5. Effects of ELE Supplementation on Apoptosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisheries and Aquaculture Software. FishStatJ-software for fishery and aquaculture statistical time series. In FAO Fisheries and Aquaculture Department; FAO: Rome, Italy, 2020; Available online: https://www.fao.org/home/en/ (accessed on 28 December 2020).
- Zhang, Z. Research advances on tilapia Streptococcosis. Pathogens 2021, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Ndong, D.; Chen, Y.Y.; Lin, Y.H.; Vaseeharan, B.; Chen, J.C. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 2007, 22, 686–694. [Google Scholar] [CrossRef]
- Chen, J.Z.; Zang, X.L.; Qu, J.H.; Hu, G.D.; Meng, S.L.; Song, C. The immune response of tilapia (GIFT Oreochromis niloticus) and its susceptibility to Streptococcus iniae under temperatures stress. J. Agro-Environ. Sci. 2011, 9, 1896–1901. Available online: https://doi.org/CNKI:SUN:NHBH.0.2011-09-038 (accessed on 3 August 2022).
- Srisapoome, P.; Areechon, N. Efficacy of viable Bacillus pumilus isolated from farmed fish on immune responses and increased disease resistance in Nile tilapia (Oreochromis niloticus): Laboratory and on-farm trials. Fish Shellfish Immunol. 2017, 67, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Eweedah, N.M.; El-Sharawy, M.E.; Awad, S.S.; Van Doan, H.; Paray, B.A. Dietary white button mushroom improved the growth, immunity, antioxidative status and resistance against heat stress in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 523, 735229. [Google Scholar] [CrossRef]
- He, X.R.; Wang, J.H.; Li, M.X.; Hao, D.J.; Yang, Y.; Zhang, C.L.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2013, 151, 78–92. [Google Scholar] [CrossRef]
- Peng, M.J.; Wang, Z.H.; Peng, S.; Zhang, M.L.; Duan, Y.H.; Li, F.N.; Shi, S.Y.; Yang, Q.L.; Zhang, C.W. Dietary supplementation with the extract from Eucommia ulmoides leaves changed epithelial restitution and gut microbial community and composition of weanling piglets. PLoS ONE 2019, 14, e0223002. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, Y.Q.; Yang, F.X.; Peng, J.N.; Li, X.H.; Sun, R.C. Antioxidative activity of water extracts from leaf, male flower, raw cortex and fruit of Eucommia ulmoides Oliv. For. Prod. J. 2007, 57, 74–79. [Google Scholar]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G. Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef]
- Luo, X.M.; Wu, J.S.; Li, Z.Z.; Jin, W.Y.; Zhang, F.Q.; Sun, H.; Shi, Y. Safety evaluation of Eucommia ulmoides extract. Regul. Toxicol. Pharmacol. 2020, 118, 104811. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.E.; Liu, G.; Oladele, O.A.; Rahu, N.; Tossou, M.C.; Yin, Y. Health-promoting properties of Eucommia ulmoides: A review. Evid. Based Complementary Altern. Med. 2016, 2016, 5202908. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, Y.; Yu, B.; Chen, D.W.; Mao, X.B.; Zheng, P.; Luo, J.Q.; He, J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018, 96, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.S.; Deng, W.; Liu, H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019, 98, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Kim, H.Y.; Song, Y.M.; Jung, H.J.; Ji, S.Y.; Jang, H.D.; Ryu, J.W.; Park, J.C.; Moon, H.K.; Kim, I.C. The effect of Eucommia ulmoides leaf supplementation on the growth performance, blood and meat quality parameters in growing and finishing pigs. Anim. Sci. J. 2009, 80, 41–45. [Google Scholar] [CrossRef]
- Liu, H.W.; Li, K.; Zhao, J.S.; Deng, W. Effects of polyphenolic extract from Eucommia ulmoides Oliver leaf on growth performance, digestibility, rumen fermentation and antioxidant status of fattening lambs. Anim. Sci. J. 2018, 89, 888–894. [Google Scholar] [CrossRef]
- Huang, W.X.; Yao, C.W.; Liu, Y.T.; Xu, N.; Yin, Z.Y.; Xu, W.X.; Miao, Y.Q.; Mai, K.S.; Ai, Q.H. Effects of dietary eucommia ulmoides leaf extract (ELE) on growth performance, expression of feeding-related genes, activities of digestive enzymes, antioxidant capacity, immunity and cytokines expression of large yellow croaker (Larimichthys crocea) larvae. Br. J. Nutr. 2021, 1–29. [Google Scholar] [CrossRef]
- Zhang, F.L.; Hao, Q.; Zhang, Q.S.; Lv, H.Y.; Yang, Y.L.; Zhang, Z.; Zhou, Z.G. Influences of dietary Eucommia ulmoides leaf extract on the hepatic lipid metabolism, inflammation response, intestinal antioxidant capacity, intestinal microbiota, and disease resistance of the channel catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2022, 123, 75–84. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Fischer, M.; Ehlers, M. Toll-like receptors in autoimmunity. Ann. N. Y. Acad. Sci. 2008, 1143, 21–34. [Google Scholar] [CrossRef]
- Thompson, A.J.; Locarnini, S.A. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol. Cell Biol. 2007, 85, 435–445. [Google Scholar] [CrossRef]
- Ruckdeschel, K.; Pfaffinger, G.; Haase, R.; Sing, A.; Weighardt, H.; Häcker, G.; Holzmann, B.; Heesemann, J. Signaling of apoptosis through TLRs critically involves Toll/IL-1 receptor domain-containing adapter inducing IFN-β, but not MyD88, in bacteria-infected murine macrophages. J. Immunol. 2004, 173, 3320–3328. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Jia, Q.J.; Yao, C.L. Characterization and expression analysis of Toll-like receptor 2 gene in large yellow croaker, Larimichthys crocea. Fish Shellfish Immunol. 2015, 44, 129–137. [Google Scholar] [CrossRef]
- Liu, F.; Su, B.; Gao, C.; Zhou, S.; Song, L.; Tan, F.; Dong, X.; Ren, Y.; Li, C. Identification and expression analysis of TLR2 in mucosal tissues of turbot (Scophthalmus maximus L.) following bacterial challenge. Fish Shellfish Immunol. 2016, 55, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.E.; Regoli, F. Identification of the Nrf2–Keap1 pathway in the European eel Anguilla anguilla: Role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat. Toxicol. 2014, 150, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.R.; Islam, M.A.; Wahab, M.A.; Hoq, M.E.; Rahman, M.M.; Azim, M.E. Evaluation of production performance and profitability of hybrid red tilapia and genetically improved farmed tilapia (GIFT) strains in the carbon/nitrogen controlled periphyton-based (C/N-CP) on-farm prawn culture system in Bangladesh. Aquac. Rep. 2016, 4, 101–111. [Google Scholar] [CrossRef]
- Ren, M.C.; Liao, Y.J.; Xie, J.; Liu, B.; Zhou, Q.L.; Ge, X.P.; Cui, H.H.; Pan, L.K.; Chen, R.L. Dietary arginine requirement of juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2013, 414, 229–234. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, TX, USA, 2003. [Google Scholar]
- Liang, H.L.; Ji, K.; Ge, X.P.; Xi, B.W.; Ren, M.C.; Chen, X.R. Tributyrin plays an important role in regulating the growth and health status of juvenile blunt snout bream (Megalobrama amblycephala), as evidenced by pathological examination. Front. Immunol. 2021, 12, 1160. [Google Scholar] [CrossRef]
- Sun, W.T.; Li, X.Q.; Xu, H.B.; Chen, J.N.; Xu, X.Y.; Leng, X.J. Effects of dietary chlorogenic acid on growth, flesh quality and serum biochemical indices of grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2017, 23, 1254–1263. [Google Scholar] [CrossRef]
- Zhang, B.L.; Li, C.Q.; Wang, X.; Zhou, H.H.; Mai, K.S.; He, G. The effects of dietary Eucommia ulmoides Oliver on growth, feed utilization, antioxidant activity and immune responses of turbot (Scophthalmus maximus L.). Aquac. Nutr. 2019, 25, 367–376. [Google Scholar] [CrossRef]
- Rašković, B.S.; Stanković, M.B.; Marković, Z.Z.; Poleksić, V.D. Histological methods in the assessment of different feed effects on liver and intestine of fish. J. Agric. Sci. 2011, 56, 87–100. [Google Scholar] [CrossRef]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992, 55, 299S–308S. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin. Eye Res. 2016, 54, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiharto, S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 2016, 15, 99–111. [Google Scholar] [CrossRef]
- Jiang, W.D.; Zhou, X.Q.; Zhang, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Vitamin A deficiency impairs intestinal physical barrier function of fish. Fish Shellfish Immunol. 2019, 87, 546–558. [Google Scholar] [CrossRef]
- Feng, L.; Xiao, W.W.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.D.; Li, S.H.; Zhou, X.Q. Methionine hydroxy analogue prevents oxidative damage and improves antioxidant status of intestine and hepatopancreas for juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2011, 17, 595–604. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401. [Google Scholar] [CrossRef]
- Fontagné-Dicharry, S.; Lataillade, E.; Surget, A.; Larroquet, L.; Cluzeaud, M.; Kaushik, S. Antioxidant defense system is altered by dietary oxidized lipid in first-feeding rainbow trout (Oncorhynchus mykiss). Aquaculture 2014, 424, 220–227. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Basu, N.; Todgham, A.E.; Ackerman, P.A.; Bibeau, M.R.; Nakano, K.; Schulte, P.M.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Abarike, E.D.; Jian, J.; Tang, J.; Cai, J.; Sakyi, E.M.; Kuebutornye, F.K. A mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100438. [Google Scholar] [CrossRef]
- Yilmaz, S. Effects of dietary blackberry syrup supplement on growth performance, antioxidant, and immunological responses, and resistance of Nile tilapia, Oreochromis niloticus to Plesiomonas shigelloides. Fish Shellfish Immunol. 2019, 84, 1125–1133. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.Y.; Lian, X.Q.; Wang, Y.; Luo, C.Z.; Tao, S.Q.; Liao, Y.L.; Yang, J.M.; Chen, A.J.; Yang, Y.H. Dietary yeast culture modulates immune response related to TLR2-MyD88-NF-kβ signaling pathway, antioxidant capability and disease resistance against Aeromonas hydrophila for Ussuri catfish (Pseudobagrus ussuriensis). Fish Shellfish Immunol. 2019, 84, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Karumuthil-Melethil, S.; Perez, N.; Li, R.; Vasu, C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J. Immunol. 2008, 181, 8323–8334. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, D.S.; Kim, S.J.; Park, J.; Kim, H.L.; Kim, S.Y.; Ahn, K.S.; Jang, H.J.; Lee, S.G.; Lee, K.M.; et al. Eucommiae cortex inhibits TNF-α and IL-6 through the suppression of caspase-1 in lipopolysaccharide-stimulated mouse peritoneal macrophages. Am. J. Chin. Med. 2012, 40, 135–149. [Google Scholar] [CrossRef]
- Liang, H.L.; Mokrani, A.; Ji, K.; Ge, X.P.; Ren, M.C.; Xie, J.; Liu, B.; Xi, B.W.; Zhou, Q.L. Dietary leucine modulates growth performance, Nrf2 antioxidant signaling pathway and immune response of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2018, 73, 57–65. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Sheikh, Z.A.; Ahmed, I. Impact of environmental changes on plasma biochemistry and hematological parameters of Himalayan snow trout, Schizothorax plagiostomus. Comp. Clin. Pathol. 2019, 28, 793–804. [Google Scholar] [CrossRef]
- Ismail, H.T.H.; Mahboub, H.H.H. Effect of acute exposure to nonylphenol on biochemical, hormonal, and hematological parameters and muscle tissues residues of Nile tilapia; Oreochromis niloticus. Vet. World 2016, 9, 616. [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Yang, R.B.; Weiss, D.S.; Godowski, P.; Zychlinsky, A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000, 19, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Yang, R.B.; Mark, M.R.; Suggett, S.; Devaux, B.; Radolf, J.D.; Klimpel, G.R.; Godowski, P.; Zychlinsky, A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 1999, 285, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.S.; Li, M.L.; Wang, K.Z.; Wang, S.; Lu, Q.; Yan, J.H.; Mossman, K.L.; Lin, R.T.; Zheng, C.F. The herpes simplex virus 1-encoded envelope glycoprotein B activates NF-κB through the Toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLoS ONE 2013, 8, e54586. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Ingredients | Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 |
|---|---|---|---|---|---|
| Fish meal a | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Rapeseed meal a | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Soybean meal a | 26.00 | 26.00 | 26.00 | 26.00 | 26.00 |
| Cottonseed meal a | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 |
| Wheat flour a | 12.01 | 12.01 | 12.01 | 12.01 | 12.01 |
| Soybean oil | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin C (35%) | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamins premix b | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Calcium dihydrogen phosphate | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Mineral premix c | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Rice bran | 14.05 | 14.05 | 14.05 | 14.05 | 14.05 |
| Ethoxy quinoline | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Bentonite | 2.00 | 1.98 | 1.96 | 1.94 | 1.92 |
| Methionine d | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 |
| ELE | 0 | 0.02 | 0.04 | 0.06 | 0.08 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Analyzed proximate composition | |||||
| Crude protein (%) | 31.55 | 31.74 | 31.73 | 31.88 | 31.78 |
| Crude lipid (%) | 7.04 | 7.06 | 7.12 | 7.19 | 7.13 |
| Crude ash (%) | 10.67 | 10.59 | 10.81 | 10.55 | 10.84 |
| Items | Methods | Assay Kits/Testing Equipment |
|---|---|---|
| Composition of diets/whole body | ||
| Moisture | Drying method (ID 920.36) | Electric blast drying oven (Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China) |
| Protein | Kjeldahl (ID 984.13) | Auto kieldahl apparatus: Hanon K1100 (Jinan Hanon Instruments Co., Ltd., Jinan, China) |
| Lipid | Soxhlet (ID 991.36) | Auto fat analyzer: Hanon SOX606 (Jinan Hanon Instruments Co., Ltd., Jinan, China) |
| Ash | Combustion (ID 923.03) | Muffle: XL-2A (Hangzhou Zhuochi Instrument Co., Ltd., Hangzhou, China) |
| Plasma parameters | ||
| TP | International Federation of Clinical Chemistry recommended | Assay kits (TP: 105-000451-00. ALB: 105-000450-00. ALT: 105-000442-00. AST: 105-000443-00.) purchased from Mindray Medical International Ltd. (Shenzhen, China); Mindray BS-400 automatic biochemical analyzer (Mindray Medical International Ltd., Shenzhen, China). |
| ALB | ||
| ALT | ||
| AST | ||
| Intestinal parameters related antioxidant capacity | ||
| MDA | TBA method | Assay kits (MDA: A003-1-2. CAT: A007-1-1. SOD: A001-3-2. GSH: A006-1-1. GPx: A005-1-2.) purchased from Jian Cheng Bioengineering Institute (Nanjing, China); Spectrophotometer (Thermo Fisher Multiskan GO, Shanghai, China). |
| CAT | Ammonium molybdenum acid method | |
| SOD | WST-1 method | |
| GSH | Microplate method | |
| GPx | Colorimetric method | |
| Genes a | Forward primer (5′-3′) | Reverse primer (5′-3′) | Length | Accession No. |
|---|---|---|---|---|
| TLR2 | GCAGCCGCTTCAAAACTCAT | GAACAAAGCCCTCAAAGCGG | 105 | NP_997977 |
| MyD88 | GTTGCGCTAAACATGAGCGT | GTCTTCTCTGTCCAGCTCCG | 237 | A8QMS7 |
| FADD | GGCAGAAGATAACACGGCCT | ATTTGCGGCCTAGTTTTCGC | 200 | NP_001373289 |
| NF-κB | TCACAGGGTCCTCGATGTCT | CTGGCTGTTTGGAGACAGGT | 78 | NP_001001839 |
| TGF-β | CGTCTTCCAGCAAGCTCAGA | TCCGAAGACGCAATTCTGCT | 116 | NP_878293 |
| IL-10 | CACAACCCCAATCGACTCCA | GAGCAAATCAAGCTCCCCCA | 175 | NP_001018621 |
| IL-8 | GGAAGACCTGCCTCAATCCC | GGGGCGGAGGTAGAATTTGG | 118 | XP_001342606 |
| TNF-α | GCAATCCGCTCAATCTGCAC | GCAGCGCCGAGGTAAATAGT | 74 | NP_998024 |
| Caspase8 | ACCAGGACCTGCTGTCATTG | TATCTGGAGATGCGCTGCTG | 160 | XP_685430 |
| Bcl-2 | GCGCTTCAACGCAGTCATAG | GCAGCTAGACCAAAGACCGT | 291 | XP_001341214 |
| Bcl-xl | CAAGGAGGATGGGAACGCTT | TTCTGTGCAATGAGTCCCCC | 146 | NP_571882 |
| AP-1 | CGTGAGTGTCACCTCGACTC | GTCCTCATAAACCGGCGACT | 127 | NP_956281 |
| Nrf2 | CTGCCGTAAACGCAAGATGG | ATCCGTTGACTGCTGAAGGG | 287 | NM_182889.1 |
| Keap1 | GGAAGTCACCCTTCGAGACG | AGAGGACGTGAAGAACGCAG | 107 | NM_182864.2 |
| CAT | GGAAGAGGATGACGAAGAG | GTTACGGCGAGATGATGT | 232 | NP_570987 |
| SOD | ACAGAAGAGAAGTATCAGGAG | CACCGTAACAGCAGACAT | 228 | NP_956270 |
| Hsp70 | TCCATCACAAGGGCACGTTT | CAGGGCTTTCTCAACTGGGT | 78 | Q91233.1 |
| β-actin | ACCCCATTGAGCACGGTATT | GCTCCTCAGGGGCAACTCTC | 96 | KJ126772.1 |
| Eucommia ulmoides Leaf Extract (%) | IBW (g) a | FBW (g) b | FCR c | WGR (%) d | SGR (% Day−1) e | SR (%) f |
|---|---|---|---|---|---|---|
| 0 | 12.08 ± 0.04 | 73.75 ± 4.39 | 0.59 ± 0.04 | 510.4 ± 37.00 | 3.47 ± 0.12 | 93.3 ± 6.67 |
| 0.02 | 12.08 ± 0.04 | 72.32 ± 2.43 | 0.59 ± 0.02 | 498.5 ± 20.43 | 3.44 ± 0.07 | 100.0 ± 0.00 |
| 0.04 | 12.02 ± 0.03 | 74.22 ± 3.33 | 0.59 ± 0.03 | 517.7 ± 28.94 | 3.50 ± 0.09 | 100.0 ± 0.00 |
| 0.06 | 12.03 ± 0.02 | 71.93 ± 2.19 | 0.62 ± 0.02 | 497.7 ± 17.53 | 3.44 ± 0.06 | 100.0 ± 0.00 |
| 0.08 | 12.03 ± 0.04 | 69.38 ± 0.43 | 0.63 ± 0.00 | 506.7 ± 26.46 | 3.46 ± 0.08 | 100.0 ± 0.00 |
| p-value | ||||||
| Linear trend | 0.241 | 0.339 | 0.278 | 0.393 | 0.429 | 0.188 |
| Quadratic trend | 0.572 | 0.564 | 0.661 | 0.540 | 0.516 | 0.260 |
| Eucommia ulmoides Leaf Extract (%) | Moisture (%) | Protein (%) | Lipid (%) | Ash (%) |
|---|---|---|---|---|
| 0 | 74.53 ± 0.57 | 14.47 ± 0.76 | 5.20 ± 0.23 | 4.04 ± 0.13 |
| 0.02 | 75.14 ± 0.37 | 14.36 ± 0.20 | 4.45 ± 0.25 | 3.82 ± 0.11 |
| 0.04 | 74.00 ± 0.84 | 14.69 ± 0.32 | 5.34 ± 0.93 | 4.10 ± 0.29 |
| 0.06 | 73.98 ± 0.59 | 15.02 ± 0.43 | 4.98 ± 0.45 | 3.86 ± 0.06 |
| 0.08 | 73.55 ± 0.31 | 14.99 ± 0.19 | 5.95 ± 0.27 | 3.73 ± 0.05 |
| p-value | ||||
| Linear trend | 0.112 | 0.289 | 0.200 | 0.086 |
| Quadratic trend | 0.656 | 0.937 | 0.297 | 0.330 |
| Eucommia ulmoides Leaf Extract (%) | TP (g/L) | ALB (g/L) | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|
| 0 | 31.25 ± 0.99 | 14.87 ± 0.53 a | 36.91 ± 2.62 b | 90.12 ± 8.74 b |
| 0.02 | 30.05 ± 1.22 | 14.71 ± 0.29 a | 35.16 ± 3.34 ab | 81.67 ± 8.00 b |
| 0.04 | 29.56 ± 1.15 | 14.96 ± 0.59 ab | 30.52 ± 4.92 ab | 73.60 ± 8.96 ab |
| 0.06 | 33.84 ± 1.96 | 16.61 ± 0.71 b | 31.73 ± 3.40 ab | 64.60 ± 5.27 a |
| 0.08 | 33.77 ± 1.50 | 16.19 ± 0.50 ab | 25.14 ± 2.26 a | 62.57 ± 7.68 a |
| p-value | ||||
| Linear trend | 0.054 | 0.009 | 0.000 | 0.003 |
| Quadratic trend | 0.188 | 0.528 | 0.644 | 0.656 |
| Eucommia ulmoides leaf extract (%) | CAT (U/mgprot) | SOD (U/mgprot) | MDA (nmol/mL) | GSH (umol/gprot) | GSH-Px (U/mgprot) |
|---|---|---|---|---|---|
| 0 | 1.60 ± 0.11 a | 0.34 ± 0.04 a | 0.97 ± 0.09 | 56.88 ± 2.59 | 2.37 ± 0.32 a |
| 0.02 | 1.68 ± 0.09 ab | 0.37 ± 0.04 ab | 0.84 ± 0.07 | 59.90 ± 3.16 | 2.82 ± 0.41 ab |
| 0.04 | 2.03 ± 0.08 c | 0.41 ± 0.04 ab | 0.91 ± 0.06 | 69.59 ± 5.71 | 3.71 ± 0.39 bc |
| 0.06 | 1.94 ± 0.10 bc | 0.48 ± 0.03 b | 0.88 ± 0.08 | 67.59 ± 4.17 | 4.26 ± 0.58 c |
| 0.08 | 1.77 ± 0.12 abc | 0.38 ± 0.04 ab | 0.92 ± 0.10 | 58.95 ± 5.87 | 3.68 ± 0.42 bc |
| p-value | |||||
| Linear trend | 0.066 | 0.142 | 0.886 | 0.411 | 0.005 |
| Quadratic trend | 0.018 | 0.047 | 0.441 | 0.044 | 0.147 |
| Parameters | Eucommia ulmoides Leaf Extract (%) | ||||
|---|---|---|---|---|---|
| 0 | 0.02 | 0.04 | 0.06 | 0.08 | |
| Number of goblet cells | 9.6 ± 0.7 a | 9.4 ± 1.3 a | 13.6 ± 1.4 ab | 15.4 ± 2.2 b | 12.2 ± 2.4 ab |
| Villus length (mm) | 0.72 ± 0.07 ab | 0.60 ± 0.01 a | 0.80 ± 0.07 b | 0.81 ± 0.04 b | 0.78 ± 0.07 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Zhu, J.; Zhang, L.; Ge, X.; Ren, M.; Liang, H. Dietary Supplementation with Eucommia ulmoides Leaf Extract Improved the Intestinal Antioxidant Capacity, Immune Response, and Disease Resistance against Streptococcus agalactiae in Genetically Improved Farmed Tilapia (GIFT; Oreochromis niloticus). Antioxidants 2022, 11, 1800. https://doi.org/10.3390/antiox11091800
Huang D, Zhu J, Zhang L, Ge X, Ren M, Liang H. Dietary Supplementation with Eucommia ulmoides Leaf Extract Improved the Intestinal Antioxidant Capacity, Immune Response, and Disease Resistance against Streptococcus agalactiae in Genetically Improved Farmed Tilapia (GIFT; Oreochromis niloticus). Antioxidants. 2022; 11(9):1800. https://doi.org/10.3390/antiox11091800
Chicago/Turabian StyleHuang, Dongyu, Jian Zhu, Lu Zhang, Xianping Ge, Mingchun Ren, and Hualiang Liang. 2022. "Dietary Supplementation with Eucommia ulmoides Leaf Extract Improved the Intestinal Antioxidant Capacity, Immune Response, and Disease Resistance against Streptococcus agalactiae in Genetically Improved Farmed Tilapia (GIFT; Oreochromis niloticus)" Antioxidants 11, no. 9: 1800. https://doi.org/10.3390/antiox11091800
APA StyleHuang, D., Zhu, J., Zhang, L., Ge, X., Ren, M., & Liang, H. (2022). Dietary Supplementation with Eucommia ulmoides Leaf Extract Improved the Intestinal Antioxidant Capacity, Immune Response, and Disease Resistance against Streptococcus agalactiae in Genetically Improved Farmed Tilapia (GIFT; Oreochromis niloticus). Antioxidants, 11(9), 1800. https://doi.org/10.3390/antiox11091800











