Carob Extract Supplementation Together with Caloric Restriction and Aerobic Training Accelerates the Recovery of Cardiometabolic Health in Mice with Metabolic Syndrome
Abstract
:1. Introduction
2. Material and Methods
2.1. Carob Extract (CSAT+®)
2.2. Animals
2.3. Quantification of Triglyceride Content in Hepatic Tissue
2.4. Protein Quantification by Western Blot
2.5. Adipocyte Size
2.6. RNA Preparation and Quantitative Real-Time PCR
2.7. Mean Arterial Pressure (MAP) Measurement by the Tail-Cuff System
2.8. Experiments of Vascular Reactivity
2.9. Vascular O2*− Content
2.10. Statistical Analysis
3. Results
3.1. Body Weight Gain and Organ Weights
3.2. Food and Caloric Intake
3.3. Serum Parameters, Adiposity Index and HOMA-IR
3.4. Mean Arterial Pressure (MAP)
3.5. Activation of PI3K/Akt Pathway in Liver, Gastrocnemius and Retroperitoneal Adipose Explants in Response to Insulin
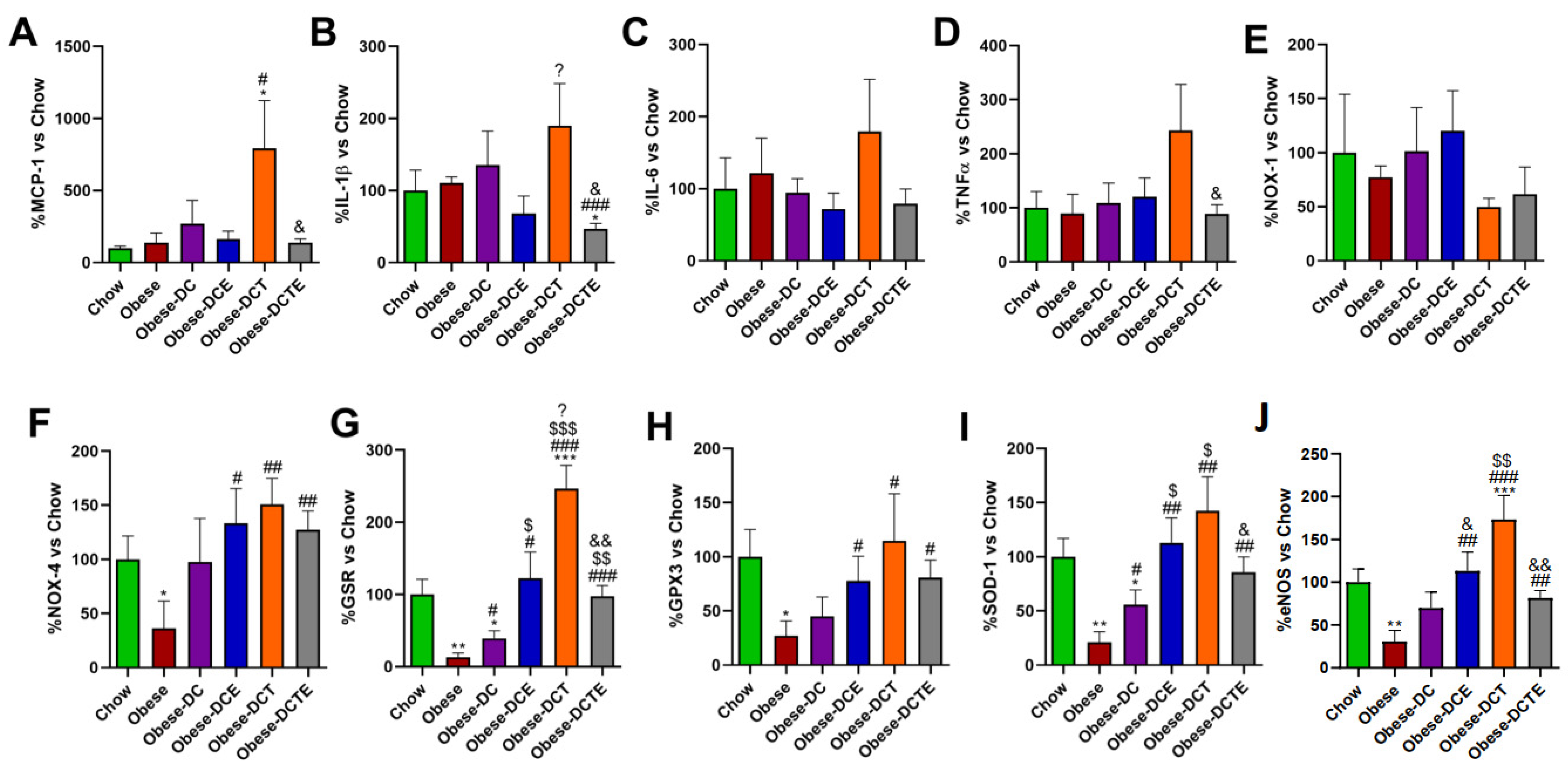
3.6. Lipid Content and Gene Expression of Pro-Inflammatory and Oxidative Stress Related Markers in Hepatic Tissue
3.7. Gene Expression of Pro-Inflammatory and Oxidative Stress Related Markers in Gastrocnemius Muscle
3.8. Adipocyte Area and Gene Expression of Pro-Inflammatory and Oxidative Stress Related Markers in Retroperitoneal Adipose Tissue
3.9. Vascular Function
3.10. Gene Expression of Pro-Inflammatory and Oxidative Stress Related Markers in Aortic Tissue
3.11. Vascular Content of Superoxide Anion in Arterial Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kendall, D.M.; Harmel, A.P. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: Understanding the role of insulin resistance. Am. J. Manag. Care 2002, 8, S635–S653. [Google Scholar] [PubMed]
- Turtle, J.R. The economic burden of insulin resistance. Int. J. Clin. Pract. Suppl. 2000, 113, 23–28. [Google Scholar]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Arner, P.; Langin, D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol. Metab. 2014, 25, 255–262. [Google Scholar] [CrossRef]
- Qatanani, M.; Lazar, M.A. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Mason, S.A.; Della Gatta, P.A.; Snow, R.J.; Russell, A.P.; Wadley, G.D. Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with type 2 diabetes: Findings of a randomized controlled study. Free Radic. Biol. Med. 2016, 93, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Goulas, V.; Georgiou, E. Utilization of Carob Fruit as Sources of Phenolic Compounds with Antioxidant Potential: Extraction Optimization and Application in Food Models. Foods 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.S.; Nakayama, T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Mekhoukhe, A.; Kicher, H.; Ladjouzi, A.; Medouni-Haroune, L.; Brahmi, F.; Medouni-Adrar, S.; Madani, K. Antioxidant activity of carob seeds and chemical composition of their bean gum by-products. J. Complement. Integr. Med. 2018, 16, 20170158. [Google Scholar] [CrossRef]
- Santonocito, D.; Granata, G.; Geraci, C.; Panico, A.; Siciliano, E.A.; Raciti, G.; Puglia, C. Carob Seeds: Food Waste or Source of Bioactive Compounds? Pharmaceutics 2020, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Fernandez, M.; Gonzalez-Hedstrom, D.; Amor, S.; Tejera-Munoz, A.; Fernandez, N.; Monge, L.; Almodovar, P.; Andres-Delgado, L.; Santamaria, L.; Prodanov, M.; et al. Supplementation with a Carob (Ceratonia siliqua L.) Fruit Extract Attenuates the Cardiometabolic Alterations Associated with Metabolic Syndrome in Mice. Antioxidants 2020, 9, 339. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Cao, H.; Lv, Q.; Tong, N. Modified high-sucrose diet-induced abdominally obese and normal-weight rats developed high plasma free fatty acid and insulin resistance. Oxid. Med. Cell Longev. 2012, 2012, 374346. [Google Scholar] [CrossRef]
- Granado, M.; Chowen, J.A.; Garcia-Caceres, C.; Delgado-Rubin, A.; Barrios, V.; Castillero, E.; Argente, J.; Frago, L.M. Ghrelin treatment protects lactotrophs from apoptosis in the pituitary of diabetic rats. Mol. Cell Endocrinol. 2009, 309, 67–75. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Granado, M.; Garcia-Caceres, C.; Frago, L.M.; Argente, J.; Chowen, J.A. The positive effects of growth hormone-releasing peptide-6 on weight gain and fat mass accrual depend on the insulin/glucose status. Endocrinology 2010, 151, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- García-Villalón, A.L.; Amor, S.; Monge, L.; Fernández, N.; Prodanov, M.; Muñoz, M.; Inarejos-García, A.M.; Granado, M. In vitro studies of an aged black garlic extract enriched in S-allyl cysteine and polyphenols with cardio protective effects. J. Funct. Foods 2016, 27, 189–200. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gonzalez-Hedstrom, D.; Moreno-Ruperez, A.; de la Fuente-Fernandez, M.; de la Fuente-Munoz, M.; Roman-Carmena, M.; Amor, S.; Garcia-Villalon, A.L.; Lopez-Calderon, A.; Isabel Martin, A.; Priego, T.; et al. A Nutraceutical Product Based on a Mixture of Algae and Extra Virgin Olive Oils and Olive Leaf Extract Attenuates Sepsis-Induced Cardiovascular and Muscle Alterations in Rats. Front. Nutr. 2022, 9, 918841. [Google Scholar] [CrossRef]
- De la Fuente-Munoz, M.; de la Fuente-Fernandez, M.; Roman-Carmena, M.; Iglesias de la Cruz, M.D.C.; Amor, S.; Martorell, P.; Enrique-Lopez, M.; Garcia-Villalon, A.L.; Inarejos-Garcia, A.M.; Granado, M. Supplementation with Two New Standardized Tea Extracts Prevents the Development of Hypertension in Mice with Metabolic Syndrome. Antioxidants 2022, 11, 1573. [Google Scholar] [CrossRef]
- Gaamouri, N.; Zouhal, H.; Hammami, M.; Hackney, A.C.; Abderrahman, A.B.; Saeidi, A.; El Hage, R.; Ounis, O.B. Effects of polyphenol (carob) supplementation on body composition and aerobic capacity in taekwondo athletes. Physiol. Behav. 2019, 205, 22–28. [Google Scholar] [CrossRef]
- Izaola, O.; Primo, D.; Rico Bargues, D.; Martin-Diana, A.B.; Martinez Villaluenga, C.; Miranda, J.; de Luis Roman, D.A. Effects of a snack enriched with carob and Undaria pinnatifida (wakame) on metabolic parameters in a double blind, randomized clinical trial in obese patients. Nutr. Hosp. 2020, 34, 465–473. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Orfanakos, N.; Farajian, P.; Kapetanakou, A.E.; Makariti, I.P.; Grivokostopoulos, N.; Ha, M.A.; Skandamis, P.N. Short-term effects of a low glycemic index carob-containing snack on energy intake, satiety, and glycemic response in normal-weight, healthy adults: Results from two randomized trials. Nutrition 2017, 42, 12–19. [Google Scholar] [CrossRef]
- Gruendel, S.; Garcia, A.L.; Otto, B.; Wagner, K.; Bidlingmaier, M.; Burget, L.; Weickert, M.O.; Dongowski, G.; Speth, M.; Katz, N.; et al. Increased acylated plasma ghrelin, but improved lipid profiles 24-h after consumption of carob pulp preparation rich in dietary fibre and polyphenols. Br. J. Nutr. 2007, 98, 1170–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruendel, S.; Otto, B.; Garcia, A.L.; Wagner, K.; Mueller, C.; Weickert, M.O.; Heldwein, W.; Koebnick, C. Carob pulp preparation rich in insoluble dietary fibre and polyphenols increases plasma glucose and serum insulin responses in combination with a glucose load in humans. Br. J. Nutr. 2007, 98, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Nemet, M.; Vasilic, M.; Tomas, A. Lipid-Lowering Effects of Carob Extracts (Ceratonia siliqua): Proposed Mechanisms and Clinical Importance. Front. Pharmacol. 2022, 13, 921123. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Norikura, T.; Matsui-Yuasa, I.; Kumazawa, S.; Honda, S.; Sonoda, T.; Kojima-Yuasa, A. Carob pod polyphenols suppress the differentiation of adipocytes through posttranscriptional regulation of C/EBPbeta. PLoS ONE 2021, 16, e0248073. [Google Scholar] [CrossRef]
- El Rabey, H.A.; Al-Seeni, M.N.; Al-Ghamdi, H.B. Comparison between the Hypolipidemic Activity of Parsley and Carob in Hypercholesterolemic Male Rats. Biomed. Res. Int. 2017, 2017, 3098745. [Google Scholar] [CrossRef]
- Macho-Gonzalez, A.; Garcimartin, A.; Lopez-Oliva, M.E.; Ruiz-Roso, B.; Martin de la Torre, I.; Bastida, S.; Benedi, J.; Sanchez-Muniz, F.J. Can Carob-Fruit-Extract-Enriched Meat Improve the Lipoprotein Profile, VLDL-Oxidation, and LDL Receptor Levels Induced by an Atherogenic Diet in STZ-NAD-Diabetic Rats? Nutrients 2019, 11, 332. [Google Scholar] [CrossRef]
- Valero-Munoz, M.; Ballesteros, S.; Ruiz-Roso, B.; Perez-Olleros, L.; Martin-Fernandez, B.; Lahera, V.; de Las Heras, N. Supplementation with an insoluble fiber obtained from carob pod (Ceratonia siliqua L.) rich in polyphenols prevents dyslipidemia in rabbits through SIRT1/PGC-1alpha pathway. Eur. J. Nutr. 2019, 58, 357–366. [Google Scholar] [CrossRef]
- Ruiz-Roso, B.; Quintela, J.C.; de la Fuente, E.; Haya, J.; Perez-Olleros, L. Insoluble carob fiber rich in polyphenols lowers total and LDL cholesterol in hypercholesterolemic sujects. Plant. Foods Hum. Nutr. 2010, 65, 50–56. [Google Scholar] [CrossRef]
- Zunft, H.J.; Luder, W.; Harde, A.; Haber, B.; Graubaum, H.J.; Koebnick, C.; Grunwald, J. Carob pulp preparation rich in insoluble fibre lowers total and LDL cholesterol in hypercholesterolemic patients. Eur. J. Nutr. 2003, 42, 235–242. [Google Scholar] [CrossRef]
- Macho-Gonzalez, A.; Garcimartin, A.; Naes, F.; Lopez-Oliva, M.E.; Amores-Arrojo, A.; Gonzalez-Munoz, M.J.; Bastida, S.; Benedi, J.; Sanchez-Muniz, F.J. Effects of Fiber Purified Extract of Carob Fruit on Fat Digestion and Postprandial Lipemia in Healthy Rats. J. Agric Food Chem. 2018, 66, 6734–6741. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, R.; Navarro-Alarcon, M.; Rodriguez-Martinez, C.; Fonolla-Joya, J. Effects on the lipid profile in humans of a polyphenol-rich carob (Ceratonia siliqua L.) extract in a dairy matrix like a functional food; a pilot study. Nutr. Hosp. 2013, 28, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Zunft, H.J.; Luder, W.; Harde, A.; Haber, B.; Graubaum, H.J.; Gruenwald, J. Carob pulp preparation for treatment of hypercholesterolemia. Adv. Ther. 2001, 18, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Van Rijs, P.; Fogliano, V. Roasting carob flour decreases the capacity to bind glycoconjugates of bile acids. Food Funct. 2020, 11, 5924–5932. [Google Scholar] [CrossRef] [PubMed]
- Wursch, P. Influence of tannin-rich carob pod fiber on the cholesterol metabolism in the rat. J. Nutr. 1979, 109, 685–692. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kurbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Bak, E.J.; Kim, J.; Jang, S.; Woo, G.H.; Yoon, H.G.; Yoo, Y.J.; Cha, J.H. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scand. J. Clin. Lab. Invest. 2013, 73, 607–614. [Google Scholar] [CrossRef]
- Mariee, A.D.; Abd-Allah, G.M.; El-Beshbishy, H.A. Protective effect of dietary flavonoid quercetin against lipemic-oxidative hepatic injury in hypercholesterolemic rats. Pharm. Biol. 2012, 50, 1019–1025. [Google Scholar] [CrossRef]
- Banuls, C.; Rovira-Llopis, S.; Lopez-Domenech, S.; Veses, S.; Victor, V.M.; Rocha, M.; Hernandez-Mijares, A. Effect of consumption of a carob pod inositol-enriched beverage on insulin sensitivity and inflammation in middle-aged prediabetic subjects. Food Funct. 2016, 7, 4379–4387. [Google Scholar] [CrossRef]
- Aboura, I.; Nani, A.; Belarbi, M.; Murtaza, B.; Fluckiger, A.; Dumont, A.; Benammar, C.; Tounsi, M.S.; Ghiringhelli, F.; Rialland, M.; et al. Protective effects of polyphenol-rich infusions from carob (Ceratonia siliqua) leaves and cladodes of Opuntia ficus-indica against inflammation associated with diet-induced obesity and DSS-induced colitis in Swiss mice. Biomed. Pharmacother. 2017, 96, 1022–1035. [Google Scholar] [CrossRef]
- Askari, G.; Ghiasvand, R.; Feizi, A.; Ghanadian, S.M.; Karimian, J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J. Res. Med. Sci. 2012, 17, 637–641. [Google Scholar]
- Xu, E.; Pereira, M.M.A.; Karakasilioti, I.; Theurich, S.; Al-Maarri, M.; Rappl, G.; Waisman, A.; Wunderlich, F.T.; Bruning, J.C. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat. Commun. 2017, 8, 14803. [Google Scholar] [CrossRef]
- Tishinsky, J.M.; Robinson, L.E.; Dyck, D.J. Insulin-sensitizing properties of adiponectin. Biochimie 2012, 94, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- El-Haskoury, R.; Al-Waili, N.; El-Hilaly, J.; Al-Waili, W.; Lyoussi, B. Antioxidant, hypoglycemic, and hepatoprotective effect of aqueous and ethyl acetate extract of carob honey in streptozotocin-induced diabetic rats. Vet. World 2019, 12, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Macho-Gonzalez, A.; Lopez-Oliva, M.E.; Merino, J.J.; Garcia-Fernandez, R.A.; Garcimartin, A.; Redondo-Castillejo, R.; Bastida, S.; Sanchez-Muniz, F.J.; Benedi, J. Carob fruit extract-enriched meat improves pancreatic beta-cell dysfunction, hepatic insulin signaling and lipogenesis in late-stage type 2 diabetes mellitus model. J. Nutr. Biochem. 2020, 84, 108461. [Google Scholar] [CrossRef] [PubMed]
- Qasem, M.A.; Noordin, M.I.; Arya, A.; Alsalahi, A.; Jayash, S.N. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ 2018, 6, e4788. [Google Scholar] [CrossRef]
- Banuls, C.; Rovira-Llopis, S.; Falcon, R.; Veses, S.; Monzo, N.; Victor, V.M.; Rocha, M.; Hernandez-Mijares, A. Chronic consumption of an inositol-enriched carob extract improves postprandial glycaemia and insulin sensitivity in healthy subjects: A randomized controlled trial. Clin. Nutr. 2016, 35, 600–607. [Google Scholar] [CrossRef]
- Lambert, C.; Cubedo, J.; Padro, T.; Vilahur, G.; Lopez-Bernal, S.; Rocha, M.; Hernandez-Mijares, A.; Badimon, L. Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism. Nutrients 2018, 10, 271. [Google Scholar] [CrossRef]
- Macho-Gonzalez, A.; Garcimartin, A.; Lopez-Oliva, M.E.; Bertocco, G.; Naes, F.; Bastida, S.; Sanchez-Muniz, F.J.; Benedi, J. Fiber purified extracts of carob fruit decrease carbohydrate absorption. Food Funct. 2017, 8, 2258–2265. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Saidani, K.; Sebai, H.; Amri, M.; Eto, B.; Marzouki, L. Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan-induced diabetes in rat. J. Sci. Food Agric. 2017, 97, 2664–2670. [Google Scholar] [CrossRef]
- Azab, A. D-Pinitol-Active Natural Product from Carob with Notable Insulin Regulation. Nutrients 2022, 14, 1453. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of quercetin supplementation on glycemic control among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tam, C.C.; Rolston, M.; Alves, P.; Chen, L.; Meng, S.; Hong, H.; Chang, S.K.C.; Yokoyama, W. Quercetin Ameliorates Insulin Resistance and Restores Gut Microbiome in Mice on High-Fat Diets. Antioxidants 2021, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Rtibi, K.; Selmi, S.; Jabri, M.A.; El-Benna, J.; Amri, M.; Marzouki, L.; Sebai, H. Protective Effect of Ceratonia siliqua L. Against a Dextran Sulfate Sodium-Induced Alterations in Liver and Kidney in Rat. J. Med. Food 2016, 19, 882–889. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Penas, E.; Rico, D.; Martin-Diana, A.B.; Portillo, M.P.; Macarulla, M.T.; de Luis, D.A.; Miranda, J. Potential Usefulness of a Wakame/Carob Functional Snack for the Treatment of Several Aspects of Metabolic Syndrome: From In Vitro to In Vivo Studies. Mar. Drugs 2018, 16, 512. [Google Scholar] [CrossRef]
- Chen, C.K.; Pace-Asciak, C.R. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen. Pharmacol. 1996, 27, 363–366. [Google Scholar] [CrossRef]
- Duarte, J.; Perez-Palencia, R.; Vargas, F.; Ocete, M.A.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef]
- Araujo, J.E.S.; Santos, R.M.D.; Oliveira, D.P.M.; Macedo, F.N.; Quintans, J.S.S.; Barreto, R.S.S.; Santos, S.L.; Santos, M.R.V.; Junior, L.J.Q.; Barreto, A.S. Resistance training increases insulin-induced vasodilation in the mesenteric artery of healthy rats. An. Acad. Bras. Cienc. 2021, 93, e20210222. [Google Scholar] [CrossRef]









| Weight (mg/cm) | Chow | Obese | Obese-DC | Obese-DCE | Obese-DCT | Obese-DCTE |
|---|---|---|---|---|---|---|
| Heart | 100.5 ± 3.4 | 110.9 ± 5.3 | 125.3 ± 9.3 | 115.0 ± 5.8 | 98.4 ± 12.8 | 106.3 ± 5.5 |
| Epididymal adipose tissue | 396.2 ± 31.1 | 1186.0 ± 86.6 *** | 897.9 ± 46.3 *** | 733.5 ± 65.3 * ### | 913.2 ± 106.1 *** | 907.9 ± 63.9 *** |
| Lumbar subcutaneous adipose tissue | 159.1 ± 28.1 | 1343.4 ± 45.32 *** | 1020.0 ± 71.6 *** # | 661.0 ± 99.7 *** ### $ | 799.8 ± 97.5 *** ### $ | 826.2 ± 81.8 *** ### $ |
| Interescapular brown adipose tissue | 55.4 ± 4.3 | 211.0 ± 21.8 *** | 156.8 ± 19.1 *** # | 124.2 ± 14.3 * ## | 126.9 ± 12.8 * ## $ | 120.6 ± 9.2 * ### $ |
| Retroperitoneal visceral adipose tissue | 244.2 ± 19.7 | 1089.0 ± 71.7 *** | 717.8 ± 43.2 *** ### | 556.9 ± 71.6 ** ### $ | 551.9 ± 50.9 ** ### $ | 594.6 ± 35.4 *** ### $ |
| Perivascular adipose tissue (PVAT) | 7.44 ± 0.48 | 23.90 ± 2.31 *** | 16.32 ± 2.38 * | 12.46 ± 1.74 ## | 13.6 ± 2.22 ## | 13.8 ± 1.15 ## |
| Kidneys | 157.4 ± 1.6 | 157.4 ± 1.6 | 191.3 ± 6.6 ** | 172.8 ± 7.2 | 175.1 ± 5.7 | 166.9 ± 7.6 # |
| Adrenal glands | 1.17 ± 0.12 | 2.36 ± 0.21 *** | 1.70 ± 0.11 ## | 1.55 ± 0.12 ### | 1.64 ± 0.11 ## | 1.95 ± 0.09 *** |
| Spleen | 33.8 ± 1.64 | 51.5 ± 3.18 *** | 50.7 ± 2.09 *** | 41.8 ± 1.21 # $ | 41.1 ± 2.4 # $ | 37.8 ± 1.32 ### $$$ |
| Liver | 595.7 ± 11.4 | 1418.1 ± 127.3 *** | 948.7 ± 54.2 ** ### | 748.0 ± 41.6 ### $ | 816.6 ± 72.2 ### | 691.2 ± 25.7 ### $ |
| Soleus | 5.30 ± 0.21 | 6.77 ± 0.51 ** | 6.67 ± 0.40 ** | 6.10 ± 0.49 | 6.54 ± 0.26 *** | 5.65 ± 0.31 # $ |
| Gastrocnemius | 68.6 ± 1.82 | 79.6 ± 4.24 ** | 75.7 ± 1.48 * | 79.5 ± 3.84 * | 73.6 ± 1.26 * | 69.5 ± 1.49 # $$ |
| Chow | Obese | Obese-DC | Obese-DCE | Obese-DCT | Obese-DCTE | |
|---|---|---|---|---|---|---|
| Total lipids (mg/dL) | 26.6 ± 9.5 | 253.1 ± 23.1 *** | 91.2 ± 28.3 * ### | 48.5 ± 15.2 ### | 79.8 ± 15.2 ** ## | 113.0 ± 33.5 * ## |
| Triglycerides (mg/dL) | 42.1 ± 2.8 | 98.4 ± 8.7 *** | 81.0 ± 6.9 *** | 60.1 ± 4.5 ** ## | 68.4 ± 5.5 *** # | 77.7 ± 6.6 *** # |
| Total cholesterol (mg/dL) | 152.4 ± 10.2 | 390.4 ± 21.8 *** | 254.4 ± 21.0 ** ### | 181.3 ± 22.1 ### $ | 225.6 ± 29.7 * ### | 167.4 ± 5.9 * ### $$ |
| LDL-c (mg/dL) | 55.7 ± 5.2 | 140.5 ± 9.4 *** | 86.0 ± 7.5 ** ### | 55.1 ± 6.9 ### $$ | 72.4 ± 10.9 ### | 45.6 ± 3.2 ### $$ |
| HDL-c (mg/dL) | 31.7 ± 2.1 | 68.0 ± 3.6 *** | 36.4 ± 2.7 ### | 37.6 ± 3.7 ### | 42.3 ± 4.0 * ### | 32.4 ± 2.6 ### |
| Glucose (mg/dL) | 86.3 ± 10.8 | 125.6 ± 7.2 * | 105.3 ± 5.5 | 79.7 ± 9.3 ## | 101.8 ± 8.2 | 93.4 ± 7.2 # |
| Leptin (ng/dL) | 4.1 ± 0.8 | 49.8 ± 1.8 *** | 30.3 ± 2.4 *** ### | 16.5 ± 3.6 * ### $$ | 23.8 ± 3.7 *** ### | 20.8 ± 2.4 *** ### |
| Insulin | 1.47 ± 0.32 | 14.39 ± 2.32 *** | 7.06 ± 0.94 * ## | 3.31 ± 0.64 ### | 4.49 ± 0.70 ### | 5.38 ± 0.97 ### |
| Adiponectin (ng/mL) | 12.017 ± 684 | 62.856 ± 316 *** | 6993 ± 327 *** | 7590 ± 275 *** # | 7452 ± 452 *** | 8079 ± 420 *** # |
| IL-6 (pg/mL) | 19.71 ± 2.34 | 41.6 ± 4.95 ** | 34.4 ± 6.96 | 26.4 ± 3.91 # | 36.8 ± 10.2 | 24.5 ± 1.14 ### |
| HOMA-IR | 0.49 ± 0.15 | 4.35 ± 0.75 *** | 1.65 ± 0.88 ### | 0.65 ± 0.15 ### | 1.13 ± 0.20 ### | 1.40 ± 0.32 ### |
| Adiposity Index | 1590 ± 101 | 3970 ± 206 *** | 3034 ± 149 *** ## | 2581 ± 244 ** ### | 2527 ± 172 ** ### | 2885 ± 144 *** ### |
| Mean blood pressure (mmHg) | 103.6 ± 2.1 | 125.7 ± 2.1 *** | 118.9 ± 1.5 *** | 111.6 ± 2.4 ### $ | 116.0 ± 3.2 ** | 119.1 ± 1.9 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Fuente-Fernández, M.; de la Fuente-Muñoz, M.; Román-Carmena, M.; Amor, S.; García-Redondo, A.B.; Blanco-Rivero, J.; González-Hedström, D.; Espinel, A.E.; García-Villalón, Á.L.; Granado, M. Carob Extract Supplementation Together with Caloric Restriction and Aerobic Training Accelerates the Recovery of Cardiometabolic Health in Mice with Metabolic Syndrome. Antioxidants 2022, 11, 1803. https://doi.org/10.3390/antiox11091803
de la Fuente-Fernández M, de la Fuente-Muñoz M, Román-Carmena M, Amor S, García-Redondo AB, Blanco-Rivero J, González-Hedström D, Espinel AE, García-Villalón ÁL, Granado M. Carob Extract Supplementation Together with Caloric Restriction and Aerobic Training Accelerates the Recovery of Cardiometabolic Health in Mice with Metabolic Syndrome. Antioxidants. 2022; 11(9):1803. https://doi.org/10.3390/antiox11091803
Chicago/Turabian Stylede la Fuente-Fernández, Maria, Mario de la Fuente-Muñoz, Marta Román-Carmena, Sara Amor, Ana Belén García-Redondo, Javier Blanco-Rivero, Daniel González-Hedström, Alberto E. Espinel, Ángel Luís García-Villalón, and Miriam Granado. 2022. "Carob Extract Supplementation Together with Caloric Restriction and Aerobic Training Accelerates the Recovery of Cardiometabolic Health in Mice with Metabolic Syndrome" Antioxidants 11, no. 9: 1803. https://doi.org/10.3390/antiox11091803






