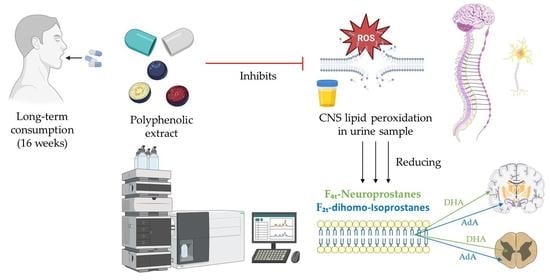
Ability of a Polyphenol-Rich Nutraceutical to Reduce Central Nervous System Lipid Peroxidation by Analysis of Oxylipins in Urine: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagent
2.2. Clinical Trial Design
2.3. Participants
2.4. Test Supplement
2.5. Extraction of Human Oxylipins in Urine Samples
2.6. UHPLC-QqQ-MS/MS Analysis of Oxylipins
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Oxylipins
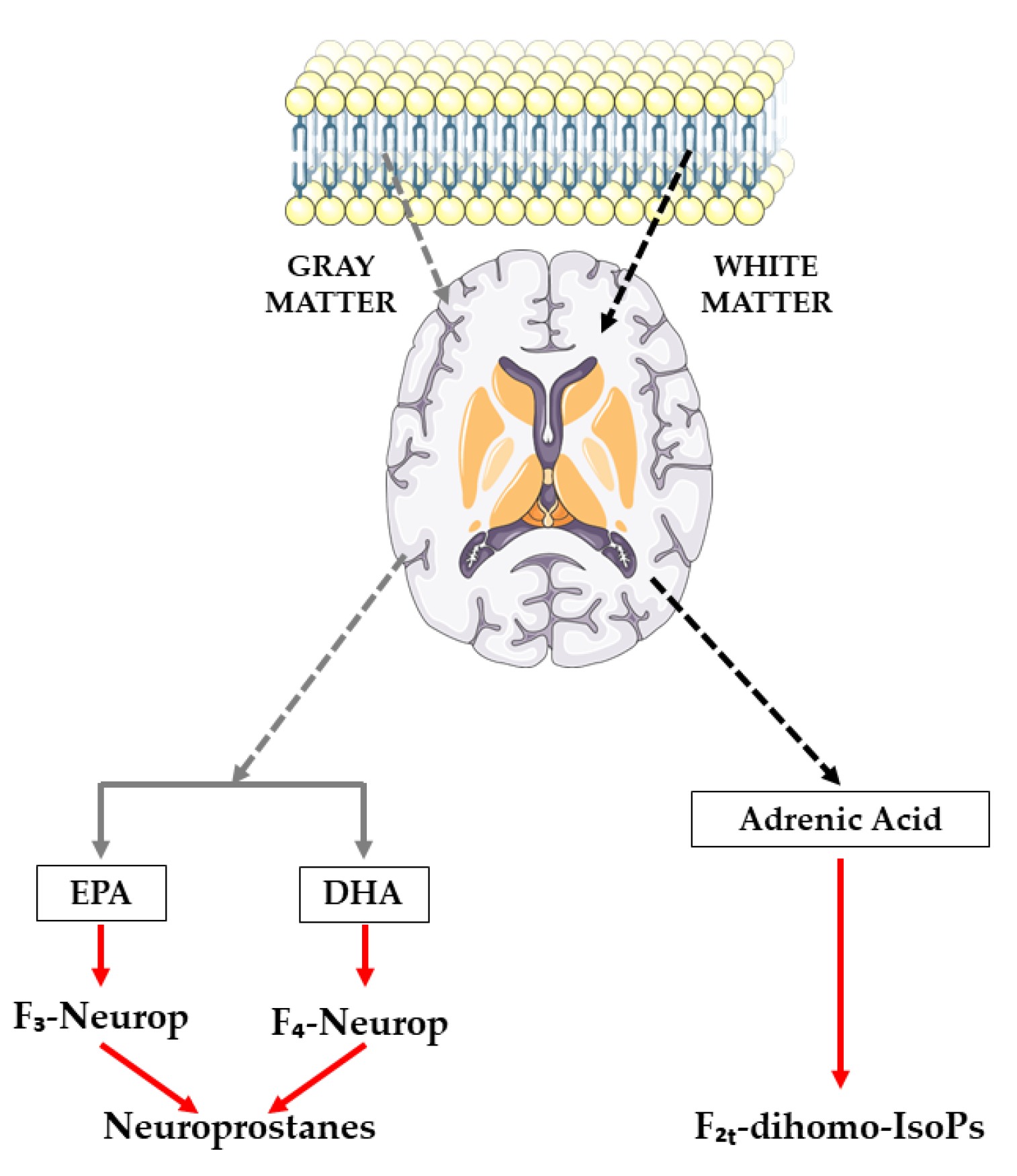
3.2.1. Oxylipins Derived from Adrenic Acid
- -
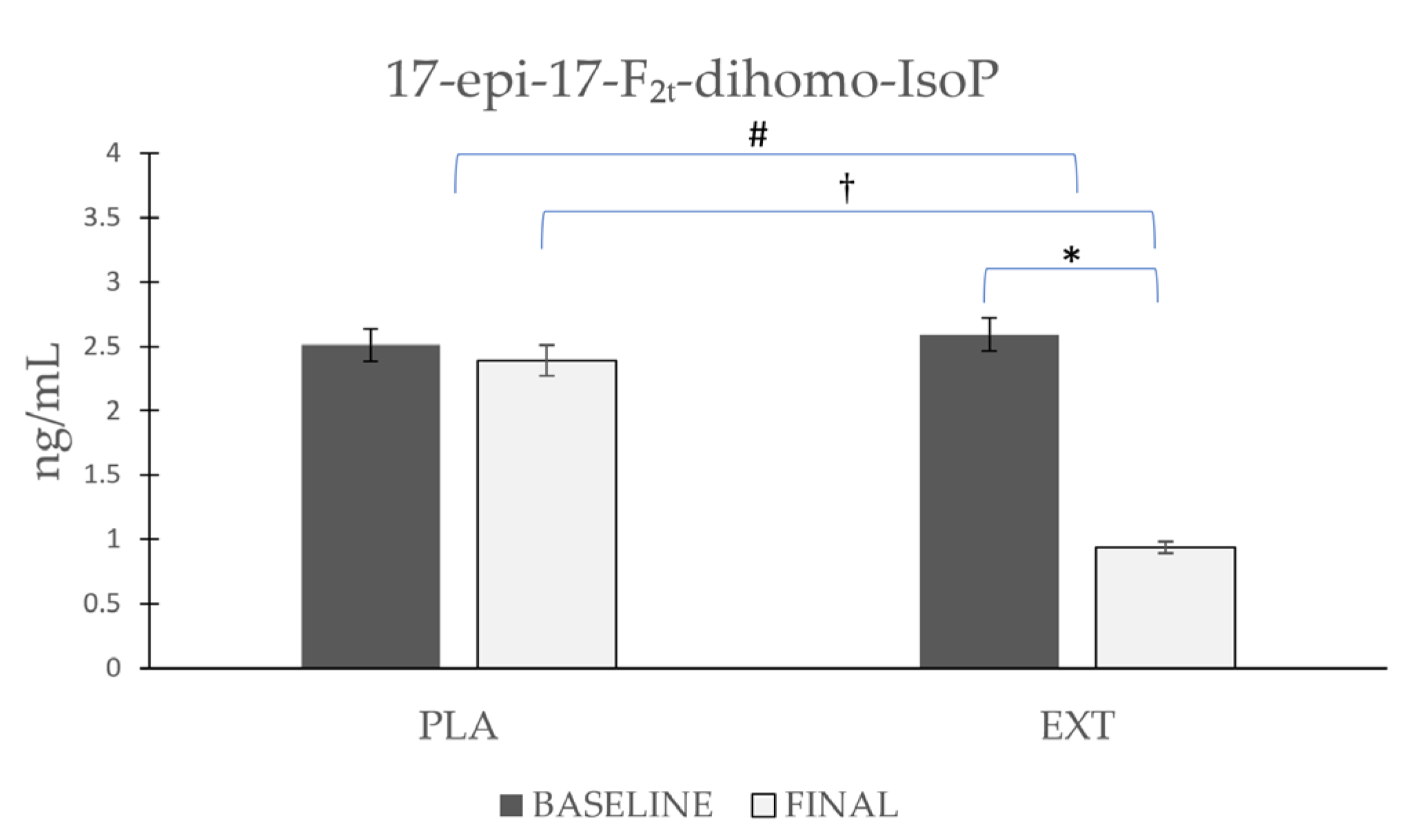
- 17-epi-17-F2t-dihomo-IsoP
- -
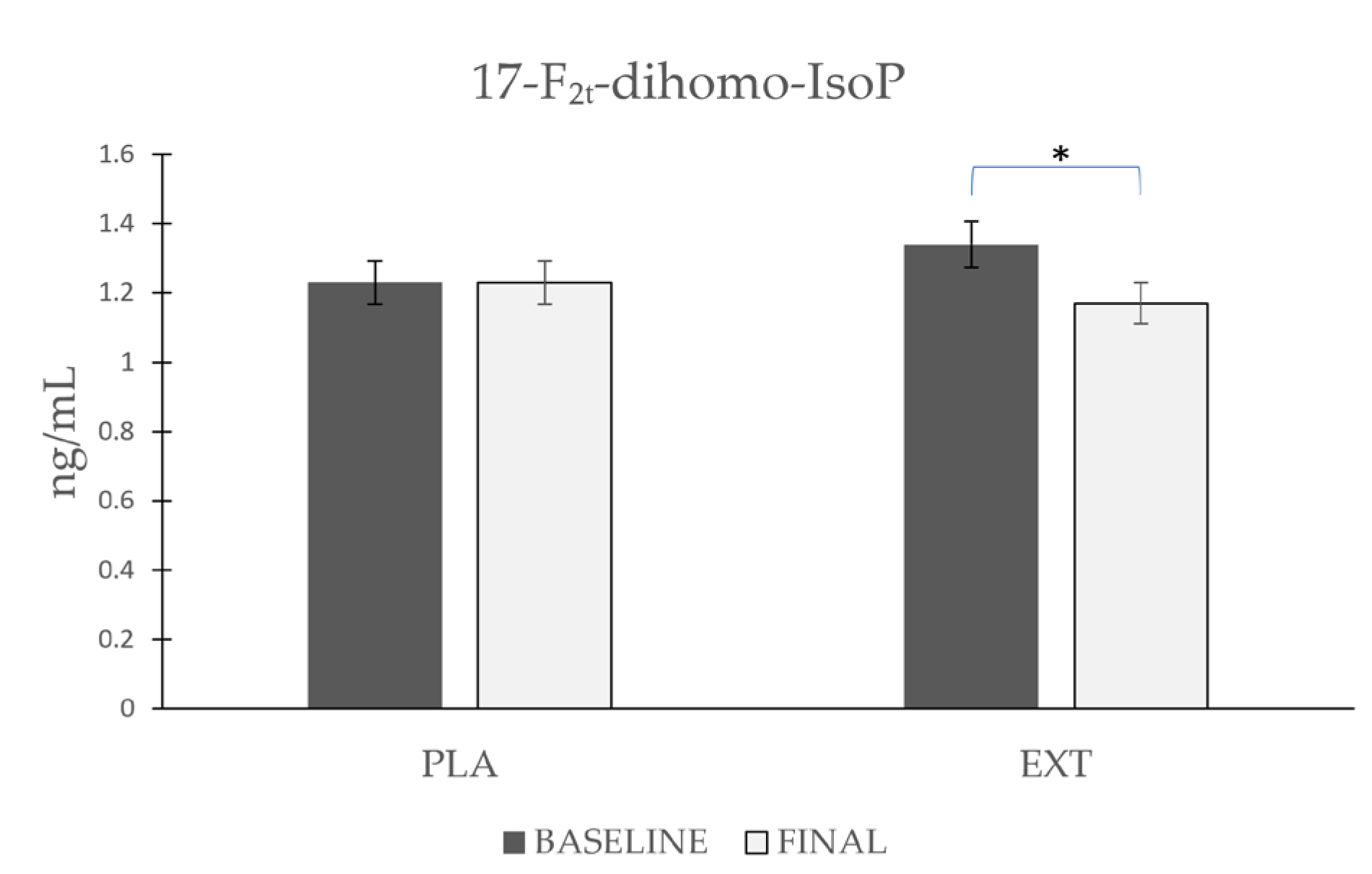
- 17-F2t-dihomo-IsoP
- -
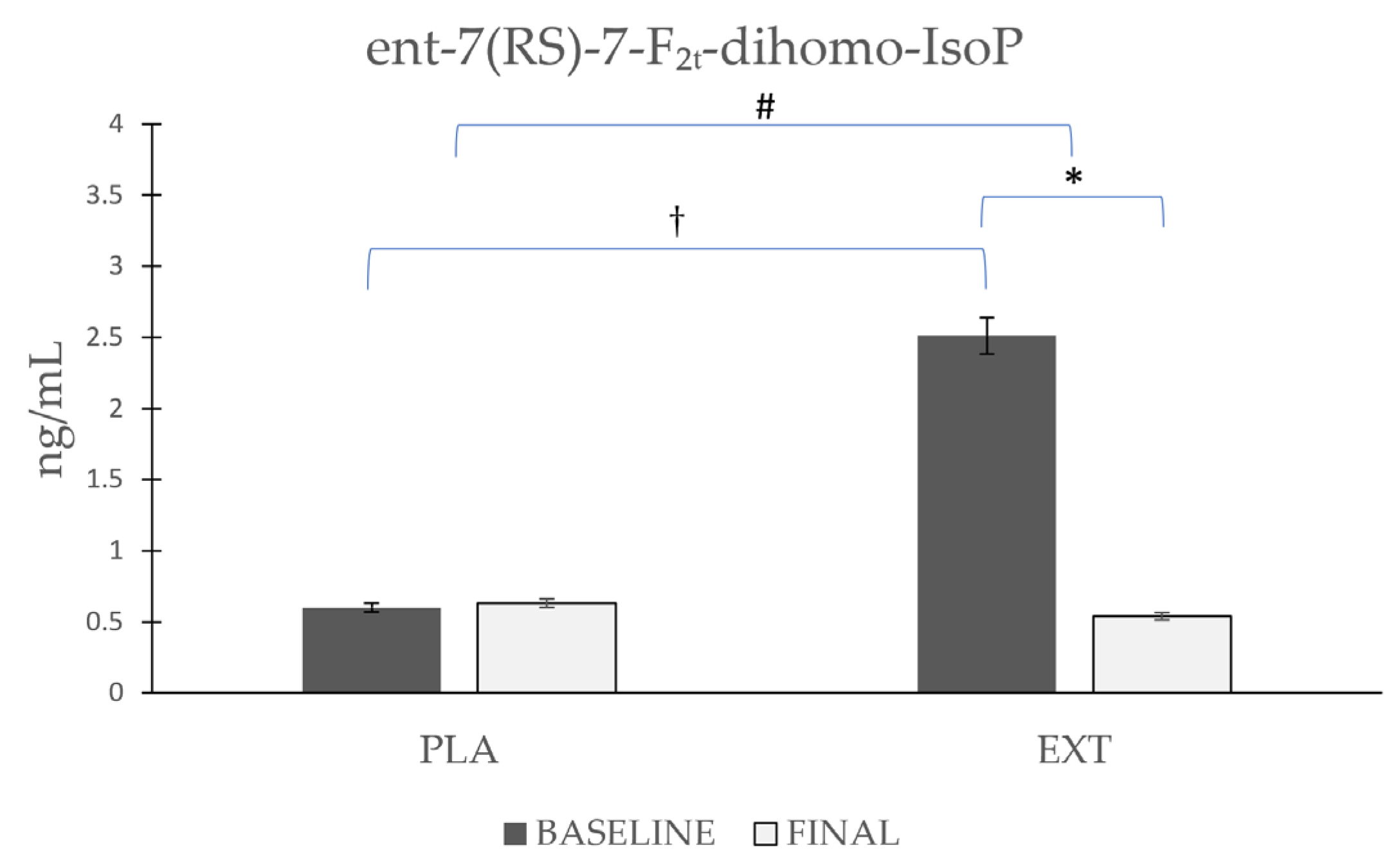
- ent-7(RS)-7-F2t-dihomo-IsoP
3.2.2. Oxylipins Derived from Docosahexaenoic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Kadiiska, M.B.; Gladen, B.C.; Baird, D.D.; Germolec, D.; Graham, L.B.; Parker, C.E.; Nyska, A.; Wachsman, J.T.; Ames, B.N.; Basu, S. Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005, 38, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Pompella, A.; Sies, H.; Wacker, R.; Brouns, F.; Grune, T.; Biesalski, H.K.; Frank, J. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition 2014, 30, 791–793. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Res. Int. 2013, 2013, 915436. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Nayeem, M.A. Role of oxylipins in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Gleim, S.; Stitham, J.; Tang, W.H.; Martin, K.A.; Hwa, J. An eicosanoid-centric view of atherothrombotic risk factors. Cell. Mol. Life Sci. 2012, 69, 3361–3380. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Massey, K.A.; Nicolaou, A. Lipidomics of oxidized polyunsaturated fatty acids. Free Radic. Biol. Med. 2013, 59, 45–55. [Google Scholar] [CrossRef]
- Tourdot, B.E.; Ahmed, I.; Holinstat, M. The emerging role of oxylipins in thrombosis and diabetes. Front. Pharmacol. 2014, 4, 176. [Google Scholar] [CrossRef]
- Morrow, J.D.; Harris, T.M.; Roberts, L.J., II. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: Analytical ramifications for measurement of eicosanoids. Anal. Biochem. 1990, 184, 1–10. [Google Scholar] [CrossRef]
- Rokach, J.; Kim, S.; Bellone, S.; Lawson, J.A.; Praticò, D.; Powell, W.S.; FitzGerald, G.A. Total synthesis of isoprostanes: Discovery and quantitation in biological systems. Chem. Phys. Lipids 2004, 128, 35–56. [Google Scholar] [CrossRef]
- Milne, G.L.; Dai, Q.; Roberts, L.J., 2nd. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Medina, S.; Domínguez-Perles, R.; Cejuela-Anta, R.; Villaño, D.; Martínez-Sanz, J.M.; Gil, P.; García-Viguera, C.; Ferreres, F.; Gil, J.I.; Gil-Izquierdo, A. Assessment of oxidative stress markers and prostaglandins after chronic training of triathletes. Prostaglandins Other Lipid Mediat. 2012, 99, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Awad, J.A.; Kato, T.; Takahashi, K.; Badr, K.F.; Roberts, L.J., 2nd; Burk, R.F. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J. Clin. Investig. 1992, 90, 2502–2507. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J.; Morrow, J.D. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000, 28, 505–513. [Google Scholar] [CrossRef] [PubMed]
- García-Flores, L.A.; Medina, S.; Martínez-Hernández, P.; Oger, C.; Galano, J.-M.; Durand, T.; Casas-Pina, T.; Ferreres, F.; Gil-Izquierdo, Á. Snapshot situation of oxidative degradation of the nervous system, kidney, and adrenal glands biomarkers-neuroprostane and dihomo-isoprostanes-urinary biomarkers from infancy to elderly adults. Redox Biol. 2017, 11, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Morel, A.; Saso, L.; Saluk, J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2014, 2014, 572491. [Google Scholar] [CrossRef]
- Galano, J.-M.; Mas, E.; Barden, A.; Mori, T.A.; Signorini, C.; De Felice, C.; Barrett, A.; Opere, C.; Pinot, E.; Schwedhelm, E.; et al. Isoprostanes and neuroprostanes: Total synthesis, biological activity and biomarkers of oxidative stress in humans. Prostaglandins Other Lipid Mediat. 2013, 107, 95–102. [Google Scholar] [CrossRef]
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; Le Faouder, P.; Galano, J.-M.; Lee, J.C.-Y.; Durand, T. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B Anal. Technol. Biomed. life Sci. 2014, 964, 65–78. [Google Scholar] [CrossRef]
- Galano, J.-M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C.-Y. Isoprostanes, neuroprostanes and phytoprostanes: An overview of 25years of research in chemistry and biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef]
- Milne, G.L.; Yin, H.; Hardy, K.D.; Davies, S.S.; Roberts, L.J. Isoprostane generation and function. Chem. Rev. 2011, 111, 5973–5996. [Google Scholar] [CrossRef]
- Yen, H.-C.; Wei, H.-J.; Lin, C.-L. Unresolved issues in the analysis of F2-isoprostanes, F4-neuroprostanes, isofurans, neurofurans, and F2-dihomo-isoprostanes in body fluids and tissue using gas chromatography/negative-ion chemical-ionization mass spectrometry. Free Radic. Res. 2015, 49, 861–880. [Google Scholar] [CrossRef] [PubMed]
- García-Blanco, A.; Peña-Bautista, C.; Oger, C.; Vigor, C.; Galano, J.-M.; Durand, T.; Martín-Ibáñez, N.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Reliable determination of new lipid peroxidation compounds as potential early Alzheimer Disease biomarkers. Talanta 2018, 184, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Reich, E.E.; Markesbery, W.R.; Roberts, L.J., 2nd; Swift, L.L.; Morrow, J.D.; Montine, T.J. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Am. J. Pathol. 2001, 158, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Friedman, J. Why is the nervous system vulnerable to oxidative stress? In Oxidative Stress and Free Radical Damage in Neurology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–27. [Google Scholar]
- Arcusa, R.; Carrillo, J.Á.; Cerdá, B.; Durand, T.; Gil-Izquierdo, Á.; Medina, S.; Galano, J.-M.; Villaño Valencia, D.; Marhuenda, J.; Zafrilla, P. Anti-Inflammatory and Antioxidant Capacity of a Fruit and Vegetable-Based Nutraceutical Measured by Urinary Oxylipin Concentration in a Healthy Population: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Antioxidants 2022, 11, 1342. [Google Scholar] [CrossRef]
- Arcusa, R.; Carrillo, J.Á.; Xandri-Martínez, R.; Cerdá, B.; Villaño, D.; Marhuenda, J.; Zafrilla, M.P. Effects of a Fruit and Vegetable-Based Nutraceutical on Biomarkers of Inflammation and Oxidative Status in the Plasma of a Healthy Population: A Placebo-Controlled, Double-Blind, and Randomized Clinical Trial. Molecules 2021, 26, 3604. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Cossu, M.; Mena, P.; Sayegh, M.; Ray, S.; Del Rio, D. (Poly) phenolic characterization of three food supplements containing 36 different fruits, vegetables and berries. PharmaNutrition 2015, 3, 11–19. [Google Scholar] [CrossRef]
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.-E.; Lamprecht, M.; et al. Absorption Profile of (Poly)Phenolic Compounds after Consumption of Three Food Supplements Containing 36 Different Fruits, Vegetables, and Berries. Nutrients 2017, 9, 194. [Google Scholar] [CrossRef]
- Medina, S.; Domínguez-Perles, R.; Gil, J.I.; Ferreres, F.; García-Viguera, C.; Martínez-Sanz, J.M.; Gil-Izquierdo, A. A ultra-pressure liquid chromatography/triple quadrupole tandem mass spectrometry method for the analysis of 13 eicosanoids in human urine and quantitative 24 hour values in healthy volunteers in a controlled constant diet. Rapid Commun. Mass Spectrom. 2012, 26, 1249–1257. [Google Scholar] [CrossRef]
- Medina, S.; De Miguel-Elízaga, I.; Oger, C.; Galano, J.-M.; Durand, T.; Martínez-Villanueva, M.; Gil-Del Castillo, M.L.; Villegas-Martínez, I.; Ferreres, F.; Martínez-Hernández, P. Dihomo-isoprostanes—Nonenzymatic metabolites of AdA—Are higher in epileptic patients compared to healthy individuals by a new ultrahigh pressure liquid chromatography–triple quadrupole–tandem mass spectrometry method. Free Radic. Biol. Med. 2015, 79, 154–163. [Google Scholar] [CrossRef]
- Marhuenda, J.; Medina, S.; Martínez-Hernández, P.; Arina, S.; Zafrilla, P.; Mulero, J.; Oger, C.; Galano, J.-M.; Durand, T.; Solana, A.; et al. Effect of the dietary intake of melatonin- and hydroxytyrosol-rich wines by healthy female volunteers on the systemic lipidomic-related oxylipins. Food Funct. 2017, 8, 3745–3757. [Google Scholar] [CrossRef]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef]
- Di Maio, R. Neuronal oxidative injury in the development of the epileptic disease: A potential target for novel therapeutic approaches. Malta Med. J. 2011, 23, 15–18. [Google Scholar]
- Barden, A.E.; Corcoran, T.B.; Mas, E.; Durand, T.; Galano, J.-M.; Roberts, L.J.; Paech, M.; Muchatuta, N.A.; Phillips, M.; Mori, T.A. Is there a role for isofurans and neuroprostanes in pre-eclampsia and normal pregnancy? Antioxid. Redox Signal. 2012, 16, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Lipid peroxidation triggers neurodegeneration: A redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 2013, 62, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; De Felice, C.; Leoncini, S.; Giardini, A.; D’Esposito, M.; Filosa, S.; Della Ragione, F.; Rossi, M.; Pecorelli, A.; Valacchi, G. F4-neuroprostanes mediate neurological severity in Rett syndrome. Clin. Chim. Acta 2011, 412, 1399–1406. [Google Scholar] [CrossRef]
- Manna, C.; Officioso, A.; Trojsi, F.; Tedeschi, G.; Leoncini, S.; Signorini, C.; Ciccoli, L.; De Felice, C. Increased non-protein bound iron in Down syndrome: Contribution to lipid peroxidation and cognitive decline. Free Radic. Res. 2016, 50, 1422–1431. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Lee, C.-Y.J.; Loke, W.M.; Huang, S.H.; Huang, H.; Looi, W.F.; Chew, E.S.; Quek, A.M.L.; Lim, E.C.H.; Halliwell, B. Biomarkers of oxidative damage in cigarette smokers: Which biomarkers might reflect acute versus chronic oxidative stress? Free Radic. Biol. Med. 2011, 50, 1787–1793. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Lee, C.-Y.J.; Lim, E.C.H.; Quek, A.M.L.; Huang, H.; Huang, S.H.; Looi, W.F.; Long, L.H.; Halliwell, B. Oral zinc supplementation does not improve oxidative stress or vascular function in patients with type 2 diabetes with normal zinc levels. Atherosclerosis 2011, 219, 231–239. [Google Scholar] [CrossRef]
- Signorini, C.; De Felice, C.; Durand, T.; Galano, J.-M.; Oger, C.; Leoncini, S.; Ciccoli, L.; Carone, M.; Ulivelli, M.; Manna, C.; et al. Relevance of 4-F(4t)-neuroprostane and 10-F(4t)-neuroprostane to neurological diseases. Free Radic. Biol. Med. 2018, 115, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J., 2nd; Fessel, J.P. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem. Phys. Lipids 2004, 128, 173–186. [Google Scholar] [CrossRef]
- De Felice, C.; Signorini, C.; Durand, T.; Oger, C.; Guy, A.; Bultel-Poncé, V.; Galano, J.-M.; Ciccoli, L.; Leoncini, S.; D’Esposito, M.; et al. F2-dihomo-isoprostanes as potential early biomarkers of lipid oxidative damage in Rett syndrome. J. Lipid Res. 2011, 52, 2287–2297. [Google Scholar] [CrossRef]
- García-Flores, L.A.; Medina, S.; Cejuela, R.; Martínez-Sanz, J.M.; Oger, C.; Galano, J.-M.; Durand, T.; Casas-Pina, T.; Martínez-Hernández, P.; Ferreres, F.; et al. Assessment of oxidative stress biomarkers-neuroprostanes and dihomo-isoprostanes-in the urine of elite triathletes after two weeks of moderate-altitude training. Free Radic. Res. 2016, 50, 485–494. [Google Scholar] [CrossRef] [PubMed]
- García-Flores, L.A.; Medina, S.; Oger, C.; Galano, J.-M.; Durand, T.; Cejuela, R.; Martínez-Sanz, J.M.; Ferreres, F.; Gil-Izquierdo, Á. Lipidomic approach in young adult triathletes: Effect of supplementation with a polyphenols-rich juice on neuroprostane and F(2)-dihomo-isoprostane markers. Food Funct. 2016, 7, 4343–4355. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Medina, S.; Martínez-Hernández, P.; Arina, S.; Zafrilla, P.; Mulero, J.; Oger, C.; Galano, J.-M.; Durand, T.; Ferreres, F.; et al. Melatonin and hydroxytyrosol protect against oxidative stress related to the central nervous system after the ingestion of three types of wine by healthy volunteers. Food Funct. 2017, 8, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Scheepens, A. Vascular action of polyphenols. Mol. Nutr. Food Res. 2009, 53, 322–331. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Halliwell, B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F.; Andrés, A.-C.J.; Florencia, A.; Carolina, E.; Felicia, R.-M. Neuroprotective actions of flavones and flavonols: Mechanisms and relationship to flavonoid structural features. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 30–35. [Google Scholar] [CrossRef]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood–brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol Microbial Metabolites Exhibit Gut and Blood–Brain Barrier Permeability and Protect Murine Microglia against LPS-Induced Inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; De Freitas, V.; Mateus, N.; Calhau, C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell. Mol. Biol. Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Kritchevsky, J.; Hargett, K.; Feller, K.; Klobusnik, R.; Song, B.J.; Cooper, B.; Jouni, Z.; Ferruzzi, M.G.; Janle, E.M. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol. Nutr. Food Res. 2015, 59, 2432–2447. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Nguyen, T.T.J. Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Wollen, K.A. Alzheimer’s disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern. Med. Rev. 2010, 15, 223–244. [Google Scholar]

| NeuroPs/F2t-dihomo-IsoPs | Retention Time (min) | MRM Transition (m/z) | Molecular Weight (g/mol) |
|---|---|---|---|
| Neuroprostanes derivates from DHA | |||
| 4(RS)-4-F4t-NeuroP | 5.26 | 377.1 > 271.2 | 378.5 |
| 4-epi-4-F3t-NeuroP | 7.18 | 379.0 > 219.0 | 378.5 |
| 4-F4t-NeuroP * | 4.10 | 377.1 > 333.1 | 378.5 |
| F2t-dihomo-Isoprostanes derivates from AdA | |||
| 17-epi-17-F2t-dihomo-IsoP * | 5.90 | 381.0 > 337.1 | 382.5 |
| 17-F2t-dihomo-IsoP * | 6.54 | 381.0 > 337.1 | 382.5 |
| Ent-7(RS)-7F2t-dihomo-IsoP * | 5.89 | 381.1 > 363.2 | 382.5 |
| Variable | Total | N1 | N2 |
|---|---|---|---|
| N | 92 | 48 | 44 |
| Men | 45 | 20 | 25 |
| Women | 47 | 28 | 19 |
| Age (years) | 34 ± 11 | 33 ± 10 | 36 ± 12 |
| Weight (kg) | 73.10 ± 14.29 | 70.68 ± 13.88 | 75.68 ± 14.44 |
| Height (m) | 1.72 ± 9 | 1.71 ± 9 | 1.73 ± 9 |
| BMI (kg/m2) | 24.40 ± 3.43 | 23.87 ± 3.42 | 24.99 ± 3.38 |
| Oxylipins (ng/mL) | Product | Baseline | Final | p-1 | p-2 | p-3 |
|---|---|---|---|---|---|---|
| F2t-dihomo-IsoPs | ||||||
| 17-epi-17-F2t-dihomo-IsoP | Placebo | 2.51 ± 0.881 | 2.39 ± 0.828 | 0.621 | 0.001 # | 0.001 † |
| Extract | 2.59 ± 0.853 | 0.94 ± 0.261 | 0.001 * | |||
| 17-F2t-dihomo-IsoP | Placebo | 1.23 ± 0.358 | 1.23 ± 0.406 | 0.983 | 0.084 | 0.572 |
| Extract | 1.34 ± 0.425 | 1.17 ± 0.308 | 0.015 * | |||
| ent-7(RS)-7-F2t-dihomo-IsoP | Placebo | 0.60 ± 0.182 | 0.63 ± 0.181 | 0.971 | 0.152 | 0.001 † |
| Extract | 2.51 ± 0.643 | 0.54 ± 0.149 | 0.001 * | |||
| NeuroPs | ||||||
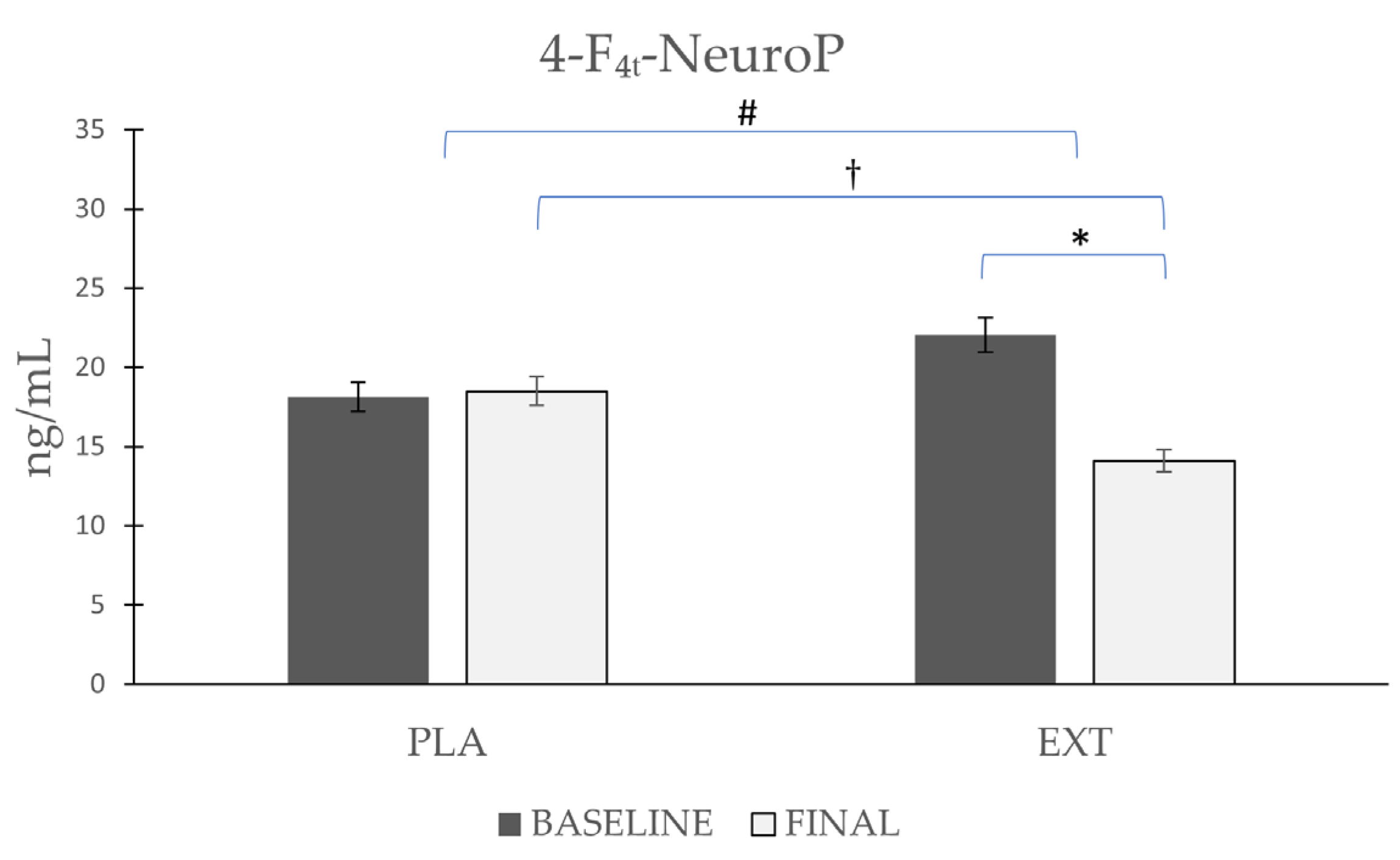
| 4-F4t-NeuroP | Placebo | 18.12 ± 6.41 | 18.50 ± 5.86 | 0.86 | 0.02 # | 0.025 † |
| Extract | 22.04 ± 7.47 | 14.10 ± 5.35 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcusa, R.; Carillo, J.Á.; Cerdá, B.; Durand, T.; Gil-Izquierdo, Á.; Medina, S.; Galano, J.-M.; Zafrilla, M.P.; Marhuenda, J. Ability of a Polyphenol-Rich Nutraceutical to Reduce Central Nervous System Lipid Peroxidation by Analysis of Oxylipins in Urine: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Antioxidants 2023, 12, 721. https://doi.org/10.3390/antiox12030721
Arcusa R, Carillo JÁ, Cerdá B, Durand T, Gil-Izquierdo Á, Medina S, Galano J-M, Zafrilla MP, Marhuenda J. Ability of a Polyphenol-Rich Nutraceutical to Reduce Central Nervous System Lipid Peroxidation by Analysis of Oxylipins in Urine: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Antioxidants. 2023; 12(3):721. https://doi.org/10.3390/antiox12030721
Chicago/Turabian StyleArcusa, Raúl, Juan Ángel Carillo, Begoña Cerdá, Thierry Durand, Ángel Gil-Izquierdo, Sonia Medina, Jean-Marie Galano, María Pilar Zafrilla, and Javier Marhuenda. 2023. "Ability of a Polyphenol-Rich Nutraceutical to Reduce Central Nervous System Lipid Peroxidation by Analysis of Oxylipins in Urine: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Antioxidants 12, no. 3: 721. https://doi.org/10.3390/antiox12030721
APA StyleArcusa, R., Carillo, J. Á., Cerdá, B., Durand, T., Gil-Izquierdo, Á., Medina, S., Galano, J.-M., Zafrilla, M. P., & Marhuenda, J. (2023). Ability of a Polyphenol-Rich Nutraceutical to Reduce Central Nervous System Lipid Peroxidation by Analysis of Oxylipins in Urine: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Antioxidants, 12(3), 721. https://doi.org/10.3390/antiox12030721