Dietary Freeze-Dried Flaxseed and Alfalfa Sprouts as Additional Ingredients to Improve the Bioactive Compounds and Reduce the Cholesterol Content of Hen Eggs
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Alfalfa and Flax Sprouts
Drying Treatments
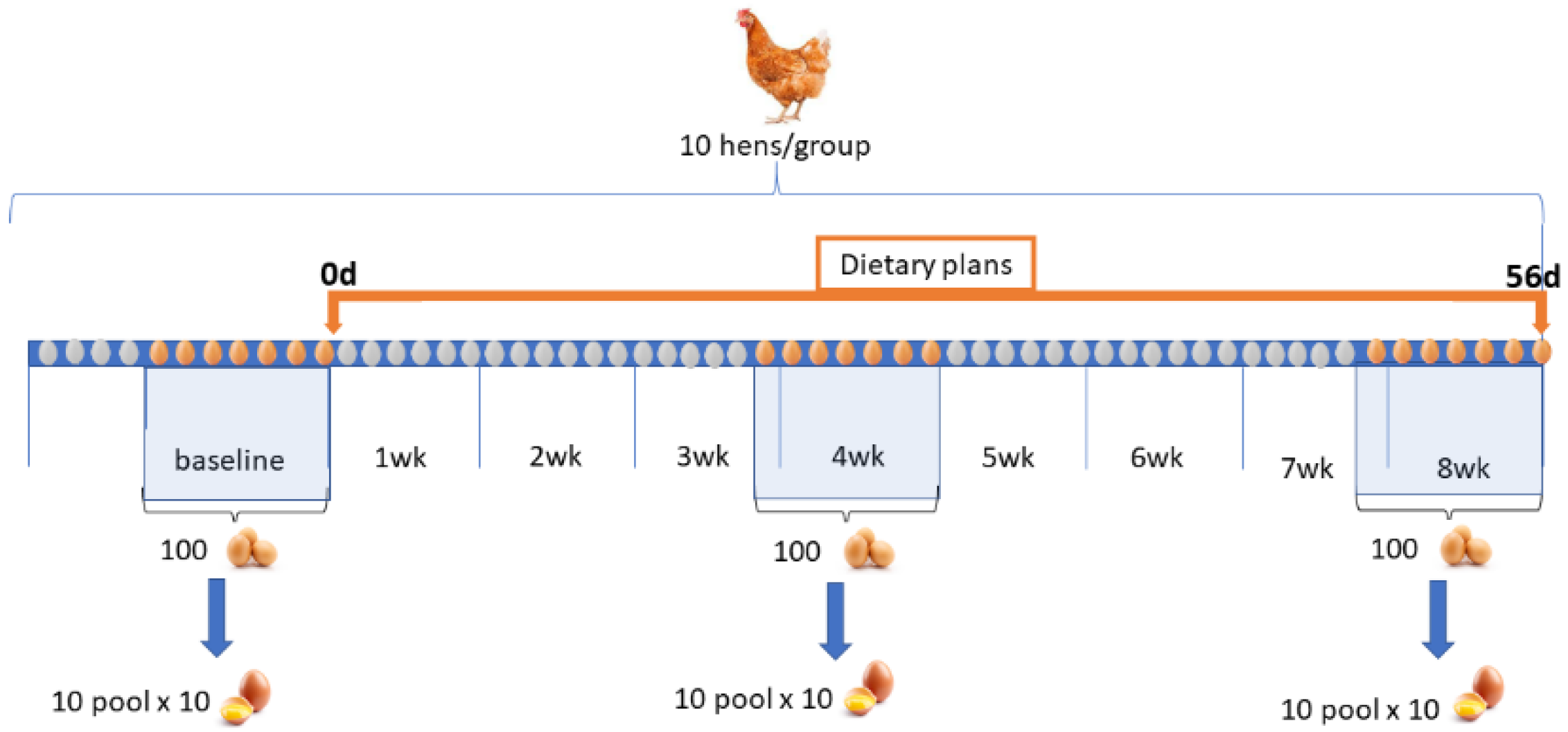
2.2. Animals and Diets
- Standard diet (C);
- Standard diet + 3% of freeze-dried alfalfa sprouts (A);
- Standard diet + 3% of freeze-dried flaxseed sprouts (F).
2.3. Eggs Sampling
2.4. Analytical Determination
2.4.1. Fatty Acids
2.4.2. Antioxidants
2.4.3. Phytoestrogens
2.4.4. Sterols and Vitamin D
2.4.5. Oxidation Assays: Cholesterol-Oxidized Products and Thiobarbituric Acid Reactive Substance
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knowles, N.R. Egg quality. Int. J. Food Sci. Nutr. 1961, 15, 77–82. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.J. The fifty year rehabilitation of the egg. Nutrients 2015, 7, 8716–8722. [Google Scholar] [CrossRef]
- Buttriss, J.L.; Lanham-New, S.A. Is a vitamin D fortification strategy needed? Nutr. Bull. 2020, 45, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Nda, A.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; Moseley, B.; Neuhäuser-berthold, M.; Van Loveren, H.; Correspondence, H.V. Scientific Opinion on the substantiation of health claims related to vitamin E and protection of DNA, proteins and lipids from oxidative damage (ID 160, 162, 1947), maintenance of the normal function of the immune system (ID 161, 163), maintenance of norm. EFSA J. 2010, 8, 1–30. [Google Scholar]
- Mattioli, S.; Ruggeri, S.; Sebastiani, B.; Brecchia, G.; Dal Bosco, A.; Mancinelli, A.C.; Castellini, C. Performance and egg quality of laying hens fed flaxseed: Highlights on n-3 fatty acids, cholesterol, lignans and isoflavones. Animal 2017, 11, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Sepehr, A.; Kashani, R.B.; Esmaeili, M.; Safari, O.; Rombenso, A. Effects of extruded, milled, and whole flaxseed (Linum usitatissimum) on egg performance, lipid components, and fatty acids concentrations in yolk and blood, and antioxidant system of commercial laying hens. Anim. Feed Sci. Technol. 2021, 276, 114877. [Google Scholar] [CrossRef]
- Yang, J.; Ding, X.M.; Bai, S.P.; Wang, J.P.; Zeng, Q.F.; Peng, H.W.; Xuan, Y.; Su, Z.W.; Zhang, K.Y. Effects of dietary vitamin E supplementation on laying performance, hatchability, and antioxidant status in molted broiler breeder hens. J. Appl. Poult. Res. 2021, 30, 1–8. [Google Scholar] [CrossRef]
- Clark, A.; Kuznesof, S.; Davies, S.; Waller, A.; Ritchie, A.; Wilson, S.; Harbord, L.; Hill, T. Egg enrichment with vitamin D: The Sunshine Eggs projects. Nutr. Bull. 2021, 46, 332–338. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Mohamed, D.A.; Chwen, L.T.; Akit, H.; Samsudin, A.A. Effect of selenium sources on laying performance, egg quality characteristics, intestinal morphology, microbial population and digesta volatile fatty acids in laying hens. Animals 2021, 11, 1681. [Google Scholar] [CrossRef] [PubMed]
- Kralik, G.; Kralik, Z.; Grčević, M.; Hanžek, D.; Margeta, P.; Galović, O. Enrichment of table eggs with lutein. Poljoprivreda 2020, 26, 56–63. [Google Scholar] [CrossRef]
- Leeson, S.; Caston, L. Enrichment of eggs with lutein. Poult. Sci. 2004, 83, 1709–1712. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Martino, M.; Ruggeri, S.; Marconi, O.; Sileoni, V.; Falcinelli, B.; Castellini, C.; Benincasa, P. Alfalfa and flax sprouts supplementation enriches the content of bioactive compounds and lowers the cholesterol in hen egg. J. Funct. Foods 2016, 22, 454–462. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Mir, S.A.; Farooq, S.; Shah, M.A.; Sofi, S.A.; Dar, B.N.; Hamdani, A.M.; Mousavi Khaneghah, A. An overview of sprouts nutritional properties, pathogens and decontamination technologies. Lwt 2021, 141, 110900. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Castellini, C.; Falcinelli, B.; Sileoni, V.; Marconi, O.; Mancinelli, A.C.; Cotozzolo, E.; Benincasa, P. Effect of heat- and freeze-drying treatments on phytochemical content and fatty acid profile of alfalfa and flax sprouts. J. Sci. Food Agric. 2019, 99, 4029–4035. [Google Scholar] [CrossRef]
- The European Parliament and the Council. Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes; Official Journal of the European Union: Luxembourg, 2010. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Mugnai, C.; Sossidou, E.N.; Dal Bosco, A.; Ruggeri, S.; Mattioli, S.; Castellini, C. The effects of husbandry system on the grass intake and egg nutritive characteristics of laying hens. J. Sci. Food Agric. 2014, 94, 459–467. [Google Scholar] [CrossRef]
- Cherian, G.; Traber, M.G.; Goeger, M.P.; Leonard, S.W. Conjugated linoleic acid and fish oil in laying hen diets: Effects on egg fatty acids, thiobarbituric acid reactive substances, and tocopherols during storage. Poult. Sci. 2007, 86, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.O.; Ofosu, F.K.; Kilonzi, S.M.; Shabbir, U.; Oh, D.H. Edible plant sprouts: Health benefits, trends, and opportunities for novel exploration. Nutrients 2021, 13, 2882. [Google Scholar] [CrossRef]
- Ostlund, R.E. Phytosterols and cholesterol metabolism. Curr. Opin. Lipidol. 2004, 15, 37–41. [Google Scholar] [CrossRef]
- Wang, L. M ammalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B 2002, 777, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of beverages, nuts, seeds, and oils. J. Agric. Food Chem. 2008, 56, 7311–7315. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Desai, P.B.; Zimmer-Nechimias, L.; Wolfe, B.; Jakate, A.S.; Creutzinger, V.; Heubi, J.E. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J. Nutr. 2003, 133, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.S.; Wilcox, G.; Wahlqvist, M.L.; Griffiths, K. Determination of lignans and isoflavonoids in human 553 female plasma following dietary supplementation. J. Endoc. 1994, 142, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Milinsk, M.C.; Murakami, A.E.; Gomes, S.T.M.; Matsushita, M.; De Souza, N.E. Fatty acid profile of egg yolk lipids from hens fed diets rich in n-3 fatty acids. Food Chem. 2003, 83, 287–292. [Google Scholar] [CrossRef]
- Mugnai, C.; dal Bosco, A.; Castellini, C. Effect of rearing system and season on the performance and egg characteristics of Ancona laying hens. Ital. J. Anim. Sci. 2009, 8, 175–188. [Google Scholar] [CrossRef]
- El-Bahr, S.M.; Shousha, S.; Alfattah, M.A.; Al-Sultan, S.; Khattab, W.; Sabeq, I.I.; Ahmed-Farid, O.; El-Garhy, O.; Albusadah, K.A.; Alhojaily, S.; et al. Enrichment of broiler chickens’ meat with dietary linseed oil and lysine mixtures: Influence on nutritional value, carcass characteristics and oxidative stress biomarkers. Foods 2021, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Kralik, G.; Škrtić, Z.; Suchý, P.; Straková, E.; Gajčević, Z. Feeding fish oil and linseed oil to laying hens to increase the n-3 PUFA of egg yolk. Acta Vet. Brno 2008, 77, 561–568. [Google Scholar] [CrossRef]
- Li, J.; Zhai, J.; Gu, L.; Su, Y.; Gong, L.; Yang, Y.; Chang, C. Hen egg yolk in food industry-A review of emerging functional modifications and applications. Trends Food Sci. Technol. 2021, 115, 12–21. [Google Scholar] [CrossRef]
- Bell, M.V.; Tocher, D.R. Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: General pathways and new directions. In Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009; ISBN 9780387893662. [Google Scholar]
- Lee, S.H.; Kim, Y.B.; Kim, D.H.; Lee, D.W.; Lee, H.G.; Jha, R.; Lee, K.W. Dietary soluble flaxseed oils as a source of omega-3 polyunsaturated fatty acids for laying hens. Poult. Sci. 2021, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lemahieu, C.; Bruneel, C.; Termote-Verhalle, R.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of feed supplementation with different omega-3 rich microalgae species on enrichment of eggs of laying hens. Food Chem. 2013, 141, 4051–4059. [Google Scholar] [CrossRef] [PubMed]
- Christensen, V.L.; Donaldson, W.E.; McMurtry, J.P. Physiological differences in late embryos from turkey breeders at different ages. Poult. Sci. 1996, 75, 172–178. [Google Scholar] [CrossRef]
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Dixit, A.K.; Bhatnagar, D.; Kumar, V.; Chawla, D.; Fakhruddin, K.; Bhatnagar, D. Antioxidant potential and radioprotective effect of soy isoflavone against gamma irradiation induced oxidative stress. J. Funct. Foods 2012, 4, 197–206. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant defense mechanisms: From the beginning to the end (of the beginning). Free Rad. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Pérez-gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Thurnham, D.I. Bioequivalence of β-carotene and retinol. J. Sci. Food Agric. 2007, 87, 13–39. [Google Scholar] [CrossRef]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O.; Kohen, R.; Katzhendler, J.; Alon, A.; Barenholz, Y. Oxidative stress effect on the integrity of lipid bilayers is modulated by cholesterol level of bilayers. Chem. Phys. Lipids 1997, 87, 17–22. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural vitamin D content in animal products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Barnkob, L.L.; Argyraki, A.; Jakobsen, J. Naturally enhanced eggs as a source of vitamin D: A review. Trends Food Sci. Technol. 2020, 102, 62–70. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
| Ingredients | C | A | F |
|---|---|---|---|
| Corn | 450 | 450 | 450 |
| Extruded soybean flakes | 200 | 200 | 200 |
| Maize gluten feed | 160 | 160 | 160 |
| Sunflower meal | 88 | 88 | 85 |
| Alfalfa meal | 30 | 27 | 30 |
| Freeze-dried alfalfa sprouts | - | 3 | - |
| Freeze-dried linseed sprouts | - | - | 3 |
| Vitamin mineral premix † | 10 | 10 | 10 |
| Calcium carbonate | 50 | 50 | 50 |
| Dicalcium phosphate | 5 | 5 | 5 |
| Sodium bicarbonate | 5 | 5 | 5 |
| Salt | 2 | 2 | 2 |
| Nutrient composition | |||
| Water | 110 | 122 | 118 |
| Crude protein | 178 | 179 | 178 |
| Ether extract | 53 | 54 | 56 |
| Crude fiber | 56 | 56 | 55 |
| Ash | 111 | 111 | 112 |
| Experimental Diets | |||
|---|---|---|---|
| C | A | F | |
| Fatty acids | |||
| C16:0 | 17.25 ± 1.12 | 16.43 ± 0.88 | 16.20 ± 1.08 |
| C18:0 | 21.12 ± 2.41 | 20.41 ± 1.09 | 20.45 ± 2.47 |
| C18:1n-9 | 17.50 ± 1.02 | 17.48 ± 0.87 | 17.30 ± 1.04 |
| C18:2n-6 | 22.25 ± 3.04 | 22.41 ± 2.74 | 21.78 ± 2.49 |
| C18:3n-3 | 16.02 ± 0.98 | 18.10 ± 1.17 | 19.25 ± 1.97 |
| Antioxidants | |||
| α-tocopherol | 120.45 ± 13.47 | 124.55 ± 12.39 | 123.85 ± 11.98 |
| α-tocopheryl acetate | 50.85 ± 6.12 | 52.30 ± 4.97 | 52.30 ± 5.01 |
| γ-tocopherol | 0.45 ± 0.04 | 1.55 ± 0.47 | 1.18 ± 0.68 |
| δ-tocopherol | 3.25 ± 0.12 | 3.11 ± 0.09 | 3.05 ± 2.98 |
| α-tocotrienol | 3.99 ± 0.09 | 4.85 ± 0.74 | 4.82 ± 0.45 |
| γ-tocotrienol | 5.65 ± 1.74 | 6.78 ± 1.47 | 6.75 ± 5.98 |
| Σ Tocols | 184.64 ± 22.64 | 193.14 ± 20.74 | 191.95 ± 19.47 |
| Retinol | 58.12 ± 6.12 | 57.44 ± 5.71 | 59.85 ± 6.47 |
| Lutein | 6.12 ± 1.04 | 9.34 ± 1.14 | 6.25 ± 0.98 |
| Zeaxanthin | 17.25 ± 1.70 | 39.86 ± 6.47 | 20.48 ± 1.98 |
| β-carotene | 11.78 ± 0.61 | 15.06 ± 1.14 | 10.85 ± 1.15 |
| Phytoestrogens | |||
| SDG | 415.80 ± 23.51 | 415.15 ± 23.36 | 1761.21 ± 361.20 |
| ISO | 1.22 ± 0.13 | 2.90 ± 0.11 | 31.34 ± 2.74 |
| SECO | n.d. | 1.84 ± 0.23 | 36.84 ± 1.54 |
| MATA | n.d. | 0.41 ± 0.10 | 21.36 ± 3.22 |
| Σ Lignans | 417.12 ± 2.55 | 420.30 ± 28.71 | 1850.75 ± 194.70 |
| Daidzein | 70.61 ± 7.11 | 75.10 ± 3.14 | 74.71 ± 14.79 |
| Genistein | 39.82 ± 2.74 | 40.22 ± 4.79 | 40.22 ± 0.86 |
| Glycitein | 26.55 ± 0.61 | 25.12 ± 2.74 | 25.11 ± 0.91 |
| Σ Isoflavones | 153.67 ± 11.54 | 142.47 ± 13.61 | 140.04 ± 13.61 |
| Coumestrol | n.d. | 2.03 ± 0.11 | 42.67 ± 3.22 |
| Phytosterols | |||
| β-sitosterol | 78.41 ± 7.11 | 82.71 ± 10.43 | 91.41 ± 10.54 |
| Stigmasterol | n.d. | 25.46 ± 8.55 | 13.21 ± 3.64 |
| Campesterol | 43.64 ± 1.42 | 42.54 ± 9.74 | 48.35 ± 8.71 |
| Avenasterol | 40.3 ± 3.22 | 47.11 ± 36.90 | 35.54 ± 6.33 |
| Brassicasterol | n.d. | n.d. | 9.37 ± 0.26 |
| Σ Sterols | 162.35 ± 20.90 | 197.82 ± 27.71 | 197.88 ± 37.44 |
| Baseline | 4 wk | 8 wk | RMSE | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | A | F | C | A | F | Diet | Time | Diet × Time | |||
| Antioxidants | |||||||||||
| Retinol | 215.99 | 277.31 | 598.30 | 317.79 | 252.08 | 550.38 | 407.15 | 3.84 | <0.001 | 0.002 | 0.001 |
| Lutein | 8.74 | 8.67 | 14.03 | 9.88 | 8.75 | 19.05 | 12.92 | 0.63 | <0.001 | <0.001 | <0.001 |
| Zeaxanthin | 8.51 | 8.55 | 16.42 | 23.97 | 8.35 | 28.24 | 28.81 | 0.93 | <0.001 | <0.001 | <0.001 |
| β-carotene | 0.95 | 1.00 | 1.73 | 1.07 | 0.96 | 1.64 | 1.10 | 0.18 | 0.002 | 0.021 | 0.037 |
| Σ Carotenes | 18.21 | 18.23 | 32.19 | 34.94 | 18.06 | 48.94 | 42.84 | 1.08 | <0.001 | <0.001 | <0.001 |
| δ-Tocopherol | 0.58 | 0.34 | 1.04 | 1.22 | 0.77 | 1.17 | 1.06 | 0.13 | 0.013 | 0.349 | 0.375 |
| γ-Tocopherol | 1.82 | 2.33 | 3.32 | 2.77 | 2.61 | 2.30 | 2.42 | 0.20 | 0.235 | 0.305 | 0.454 |
| α-Tocopherol | 22.97 | 28.06 | 94.04 | 71.40 | 28.49 | 90.74 | 78.51 | 1.62 | <0.001 | 0.177 | 0.720 |
| Sterols | |||||||||||
| Cholesterol, mg/egg | 186.07 | 181.85 | 169.30 | 151.31 | 219.02 | 131.21 | 138.16 | 5.93 | 0.038 | 0.025 | 0.063 |
| Cholesterol, mg/100 g egg | 310.11 | 303.08 | 282.16 | 252.18 | 365.03 | 218.68 | 230.26 | 12.35 | 0.018 | 0.01 | 0.050 |
| Campesterol | 2.86 | 4.88 | 5.77 | 6.89 | 2.64 | 5.95 | 10.18 | 1.64 | <0.001 | <0.000 | 0.001 |
| β-sitosterol | 0.11 | 0.17 | 1.18 | 1.42 | 0.59 | 1.95 | 1.29 | 0.70 | <0.001 | <0.001 | 0.041 |
| Vitamin D | |||||||||||
| Vitamin D3 | 1.40 | 1.41 | 1.59 | 1.54 | 1.41 | 1.64 | 1.69 | 0.14 | 0.025 | 0.012 | 0.667 |
| 25-OH Vitamin D3 | 0.46 | 0.50 | 0.62 | 0.59 | 0.50 | 0.67 | 0.62 | 0.11 | 0.019 | 0.081 | 0.438 |
| Σ Vitamin D | 1.85 | 1.90 | 2.20 | 2.13 | 1.90 | 2.31 | 2.30 | 0.16 | 0.008 | 0.011 | 0.434 |
| Lignans | |||||||||||
| Daidzeina | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | - | - |
| Genistein | n.d. | n.d. | n.d. | 32.98 | n.d. | 41.82 | 58.32 | 5.09 | <0.001 | <0.001 | <0.001 |
| Isoflavones | |||||||||||
| PINO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | - | - |
| MATA | n.d. | n.d. | 76.92 | 116.76 | n.d. | 170.93 | 192.11 | 7.23 | <0.001 | <0.001 | <0.001 |
| Coumestrol | 38.85 | 37.23 | 42.01 | 51.07 | 37.23 | 65.74 | 97.74 | 4.93 | <0.001 | <0.001 | <0.001 |
| Metabolites | |||||||||||
| ENL | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | - | - |
| END | n.d. | n.d. | n.d. | n.d. | n.d. | 43.88 | 35.90 | 2.38 | <0.001 | <0.001 | <0.001 |
| Equol | 87.31 | 89.69 | 119.39 | 164.49 | 89.69 | 224.32 | 262.30 | 8.46 | <0.001 | <0.001 | <0.001 |
| Baseline | 4 wk | 8 wk | RMSE | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | A | F | C | A | F | Diet | Time | Diet × Time | |||
| Lipids | 30.3 | 31.24 | 30.56 | 30.12 | 30.97 | 30.27 | 30.22 | 1.81 | 0.525 | 0.765 | 0.562 |
| Oxidation | |||||||||||
| 7-OH | 123.87 | 131.05 | 59.38 | 53.24 | 128.09 | 42.34 | 45.06 | 16.05 | <0.001 | <0.001 | 0.002 |
| 7-Keto | 6.25 | 4.92 | 4.37 | 5.47 | 7.38 | 3.22 | 4.74 | 1.24 | 0.559 | 0.977 | 0.126 |
| Σ COPs | 130.13 | 135.97 | 63.75 | 58.70 | 135.47 | 45.56 | 49.80 | 6.18 | <0.001 | <0.001 | 0.002 |
| TBARS | 114.37 | 93.18 | 112.50 | 96.09 | 105.79 | 110.76 | 124.23 | 11.58 | 0.057 | 0.014 | 0.005 |
| Fatty acids | |||||||||||
| 18:2n-6, LA | 801.49 | 827.09 | 208.83 | 217.57 | 877.87 | 203.73 | 212.27 | 12.20 | <0.001 | 0.270 | 0.700 |
| 18:3n-3, ALA | 44.80 | 46.93 | 24.96 | 31.10 | 40.96 | 25.60 | 31.89 | 1.30 | <0.001 | 0.365 | 0.827 |
| 20:4n-6, AA | 135.47 | 133.33 | 20.77 | 16.40 | 136.85 | 20.27 | 16.00 | 1.21 | <0.001 | 0.276 | 0.785 |
| 20:5n-3, EPA | 3.09 | 3.09 | 12.17 | 11.96 | 3.09 | 12.48 | 12.27 | 0.60 | <0.001 | 0.144 | 0.702 |
| 22:5n-3, DPA | 2.97 | 2.76 | 11.44 | 13.00 | 2.97 | 11.73 | 13.33 | 1.12 | <0.001 | 0.349 | 0.915 |
| 22:6n-3, DHA | 31.25 | 30.72 | 31.62 | 54.08 | 31.25 | 32.43 | 55.47 | 1.44 | <0.001 | 0.332 | 0.646 |
| INQ | 0.29 | 0.29 | 0.43 | 0.62 | 0.29 | 0.44 | 0.63 | 0.14 | <0.001 | 0.150 | 0.649 |
| Σ Antioxidants | Cholesterol | Σ Phytosterols | Σ COPs | Σ Phytoestrogens | Σ vitamin D | Σ n−3 | |
|---|---|---|---|---|---|---|---|
| Σ Antioxidants | 1 | −0.547 ** | 0.628 ** | −0.777 ** | 0.598 ** | 0.580 ** | 0.059 |
| 0.002 | <0.001 | <0.001 | <0.001 | 0.001 | 0.758 | ||
| Cholesterol | 1 | −0.595 ** | 0.613 ** | −0.437 * | −0.265 + | −0.294 | |
| 0.001 | <0.001 | 0.016 | 0.051 | 0.115 | |||
| Σ Phytosterols | 1 | −0.759 ** | 0.843 ** | 0.691 ** | 0.510 ** | ||
| <0.001 | <0.001 | <0.001 | 0.004 | ||||
| Σ COPs | 1 | −0.797 ** | −0.687 ** | −0.404 * | |||
| <0.001 | <0.001 | 0.027 | |||||
| Σ Phytoestrogens | 1 | 0.695 ** | 0.503 ** | ||||
| <0.001 | 0.005 | ||||||
| Σ Vitamin D | 1 | 0.306 | |||||
| 0.100 | |||||||
| Σ n−3 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattioli, S.; Cartoni Mancinelli, A.; Bravi, E.; Angelucci, E.; Falcinelli, B.; Benincasa, P.; Castellini, C.; Sileoni, V.; Marconi, O.; Dal Bosco, A. Dietary Freeze-Dried Flaxseed and Alfalfa Sprouts as Additional Ingredients to Improve the Bioactive Compounds and Reduce the Cholesterol Content of Hen Eggs. Antioxidants 2023, 12, 103. https://doi.org/10.3390/antiox12010103
Mattioli S, Cartoni Mancinelli A, Bravi E, Angelucci E, Falcinelli B, Benincasa P, Castellini C, Sileoni V, Marconi O, Dal Bosco A. Dietary Freeze-Dried Flaxseed and Alfalfa Sprouts as Additional Ingredients to Improve the Bioactive Compounds and Reduce the Cholesterol Content of Hen Eggs. Antioxidants. 2023; 12(1):103. https://doi.org/10.3390/antiox12010103
Chicago/Turabian StyleMattioli, Simona, Alice Cartoni Mancinelli, Elisabetta Bravi, Elisa Angelucci, Beatrice Falcinelli, Paolo Benincasa, Cesare Castellini, Valeria Sileoni, Ombretta Marconi, and Alessandro Dal Bosco. 2023. "Dietary Freeze-Dried Flaxseed and Alfalfa Sprouts as Additional Ingredients to Improve the Bioactive Compounds and Reduce the Cholesterol Content of Hen Eggs" Antioxidants 12, no. 1: 103. https://doi.org/10.3390/antiox12010103
APA StyleMattioli, S., Cartoni Mancinelli, A., Bravi, E., Angelucci, E., Falcinelli, B., Benincasa, P., Castellini, C., Sileoni, V., Marconi, O., & Dal Bosco, A. (2023). Dietary Freeze-Dried Flaxseed and Alfalfa Sprouts as Additional Ingredients to Improve the Bioactive Compounds and Reduce the Cholesterol Content of Hen Eggs. Antioxidants, 12(1), 103. https://doi.org/10.3390/antiox12010103












