Relationships between the Reversible Oxidation of the Single Cysteine Residue and the Physiological Function of the Mitochondrial Glutaredoxin S15 from Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Heterologous Expression in E. coli and Protein Purification
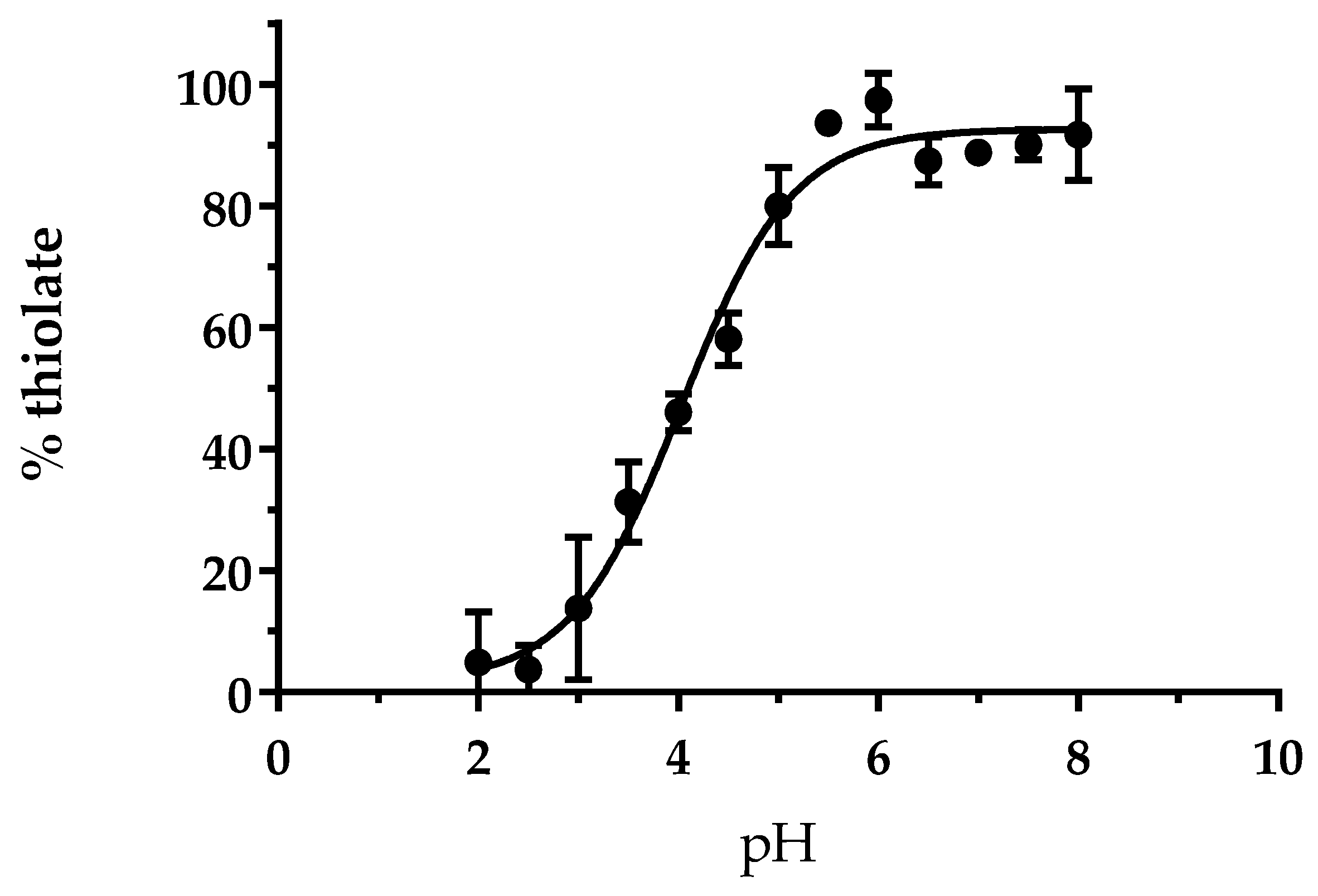
2.2. pKa Determination
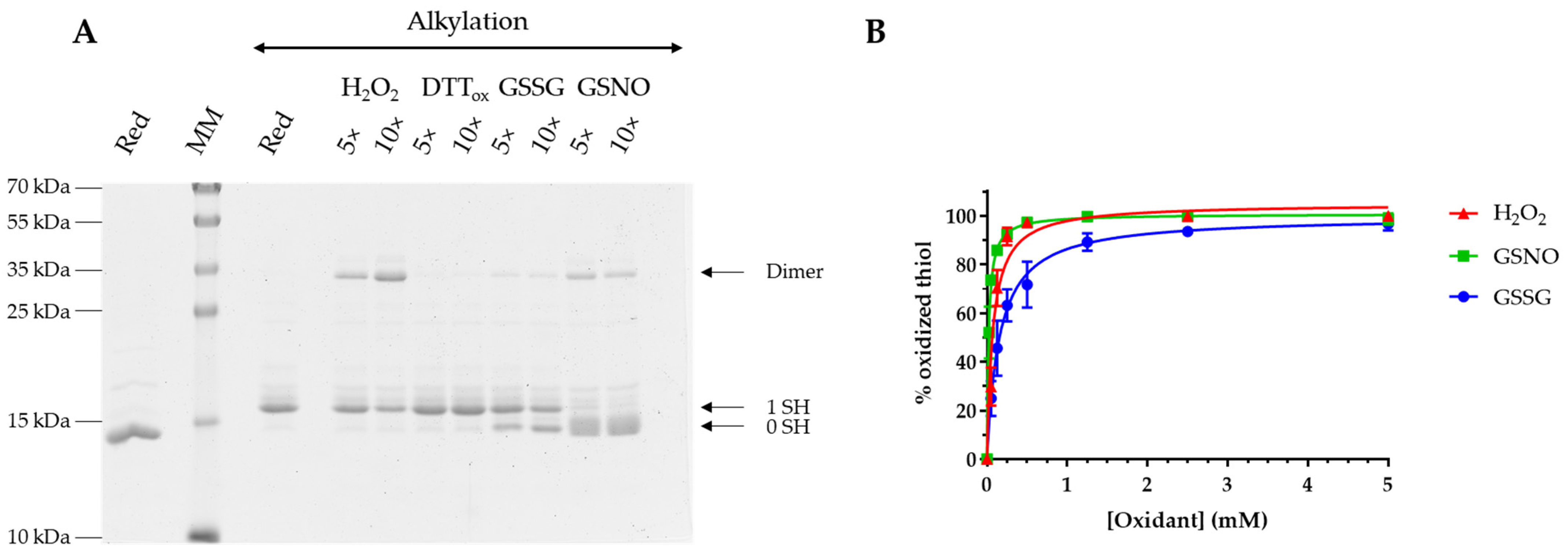
2.3. Sensibility to Oxidants
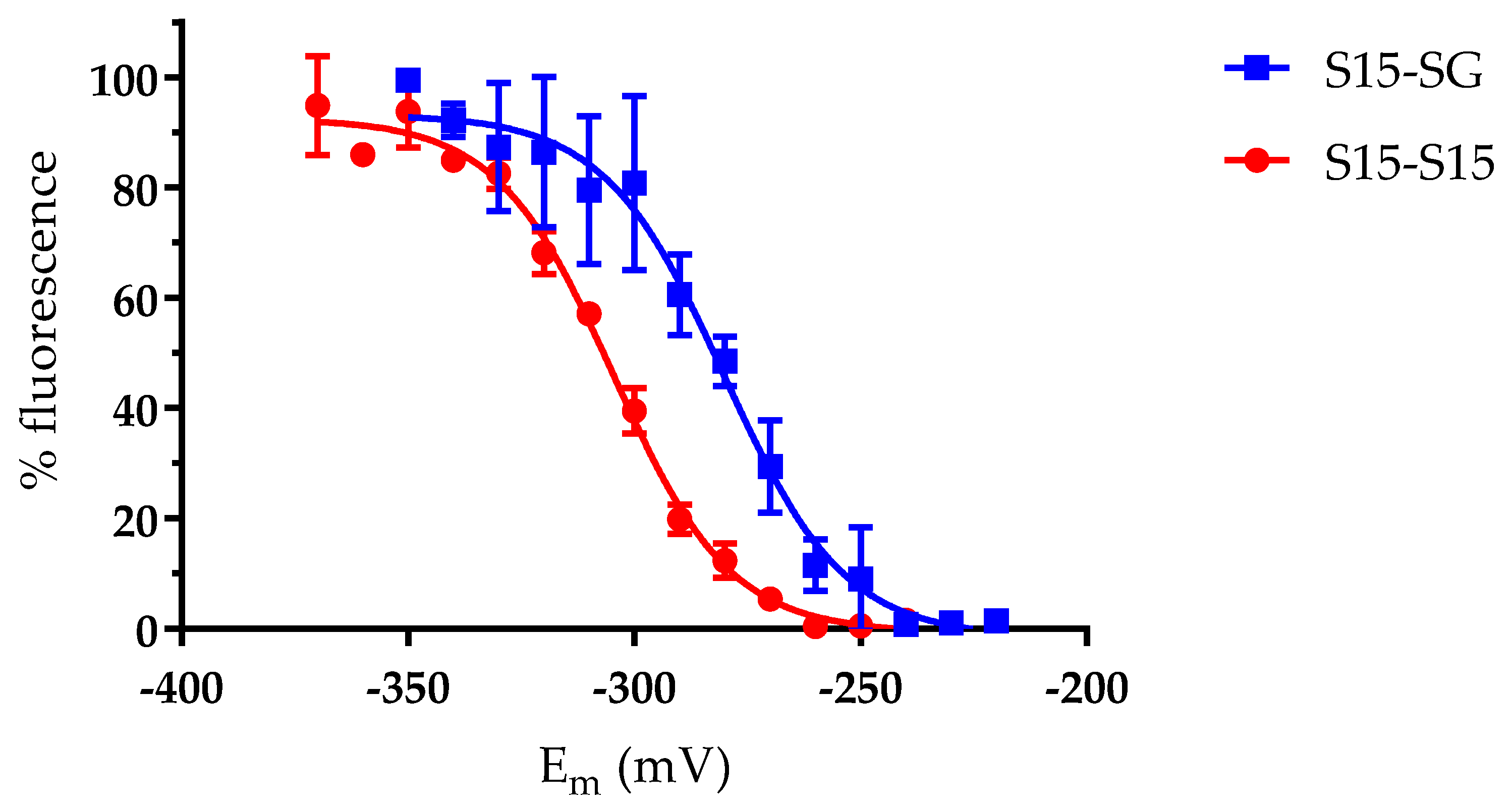
2.4. Redox Potential Determination of Oxidized Forms
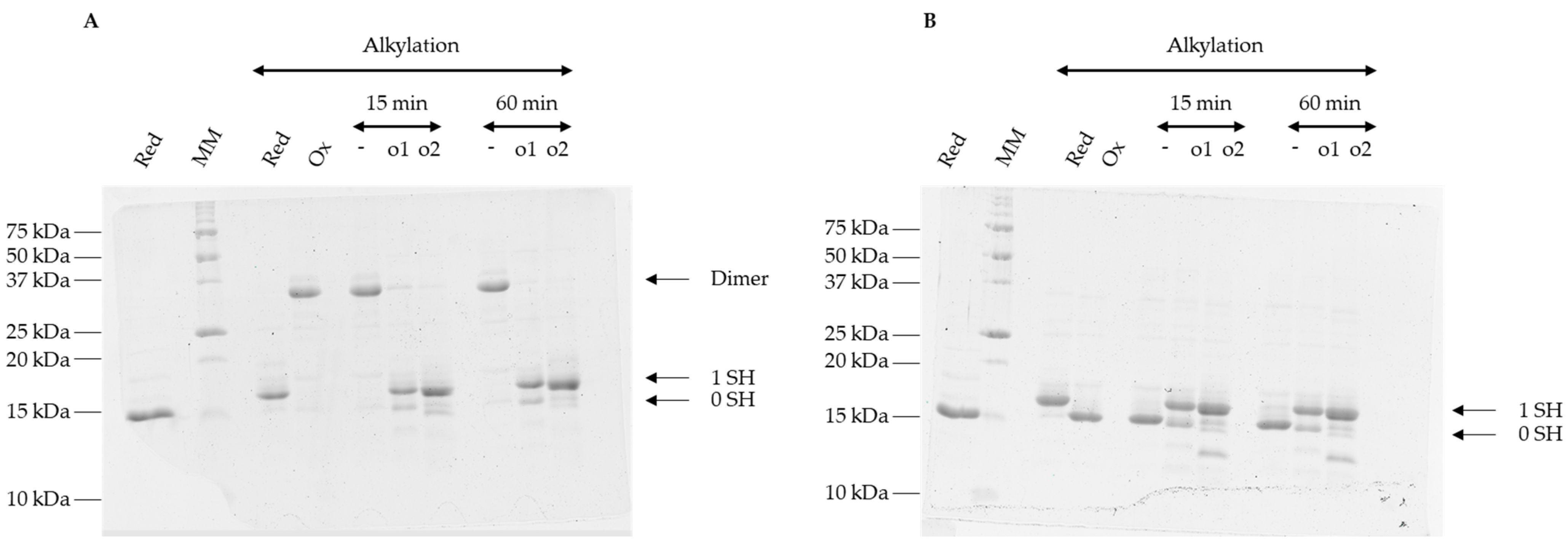
2.5. Reduction Tests
2.6. Replicates
3. Results and Discussion
3.1. The Cysteine of AtGRXS15 Has an Acidic pKa
3.2. AtGRXS15 Is Prone to Oxidation by Physiological Oxidants
3.3. The AtGRXS15 Intermolecular Disulfide Bonds Have Low Redox Potentials
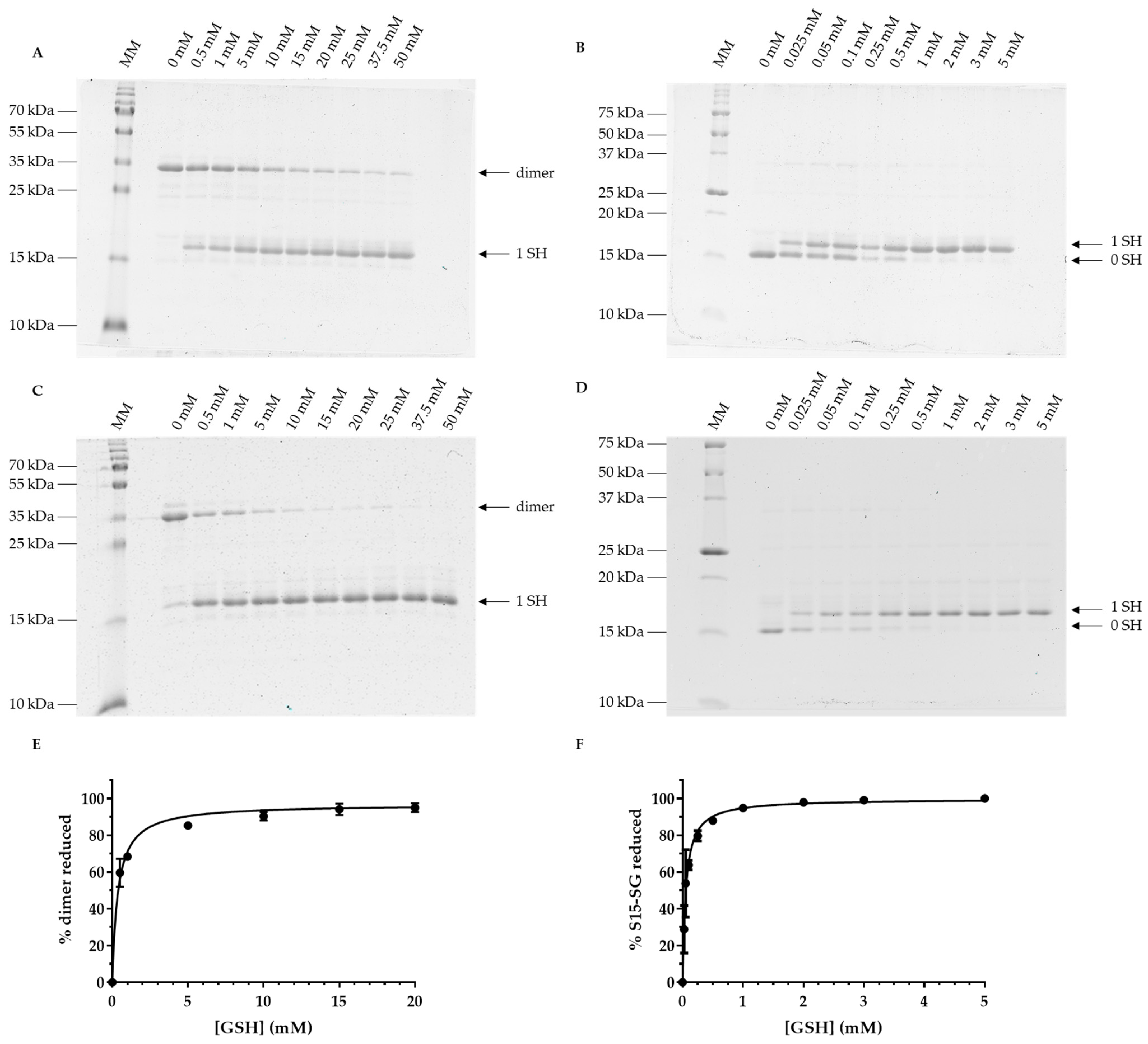
3.4. Oxidized Forms of GRXS15 Are Reduced by Mitochondrial Reduction Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves, R.; Vilaprinyo, E.; Sorribas, A.; Herrero, E. Evolution based on domain combinations: The case of glutaredoxins. BMC Evol. Biol. 2009, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L. Thioredoxin—A fold for all reasons. Structure 1995, 3, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Jacquot, J.-P.; Rouhier, N. Evolution and diversity of glutaredoxins in photosynthetic organisms. Cell. Mol. Life Sci. 2009, 66, 2539–2557. [Google Scholar] [CrossRef]
- Lillig, C.H.; Berndt, C. Glutaredoxins in Thiol/Disulfide Exchange. Antioxid. Redox Signal. 2013, 18, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Jacquot, J.-P.; Rouhier, N. Toward a refined classification of class I dithiol glutaredoxins from poplar: Biochemical basis for the definition of two subclasses. Front. Plant Sci. 2013, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Rouhier, N.; Lemaire, S.D.; Jacquot, J.-P. The Role of Glutathione in Photosynthetic Organisms: Emerging Functions for Glutaredoxins and Glutathionylation. Annu. Rev. Plant Biol. 2008, 59, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Bedhomme, M.; Lemaire, S.D.; Trost, P. The emerging roles of protein glutathionylation in chloroplasts. Plant Sci. 2012, 185–186, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Fermani, S.; Marchand, C.H.; Costa, A.; Sparla, F.; Rouhier, N.; Geigenberger, P.; Lemaire, S.D.; Trost, P. Redox Homeostasis in Photosynthetic Organisms: Novel and Established Thiol-Based Molecular Mechanisms. Antioxid. Redox Signal. 2019, 31, 155–210. [Google Scholar] [CrossRef]
- Couturier, J.; Ströher, E.; Albetel, A.-N.; Roret, T.; Muthuramalingam, M.; Tarrago, L.; Seidel, T.; Tsan, P.; Jacquot, J.-P.; Johnson, M.K.; et al. Arabidopsis Chloroplastic Glutaredoxin C5 as a Model to Explore Molecular Determinants for Iron-Sulfur Cluster Binding into Glutaredoxins. J. Biol. Chem. 2011, 286, 27515–27527. [Google Scholar] [CrossRef]
- Rouhier, N.; Unno, H.; Bandyopadhyay, S.; Masip, L.; Kim, S.-K.; Hirasawa, M.; Gualberto, J.M.; Lattard, V.; Kusunoki, M.; Knaff, D.B.; et al. Functional, structural, and spectroscopic characterization of a glutathione-ligated [2Fe–2S] cluster in poplar glutaredoxin C1. Proc. Natl. Acad. Sci. USA 2007, 104, 7379–7384. [Google Scholar] [CrossRef]
- Johansson, C.; Kavanagh, K.L.; Gileadi, O.; Oppermann, U. Reversible Sequestration of Active Site Cysteines in a 2Fe-2S-bridged Dimer Provides a Mechanism for Glutaredoxin 2 Regulation in Human Mitochondria. J. Biol. Chem. 2007, 282, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Berndt, C.; Christ, L.; Rouhier, N.; Mühlenhoff, U. Glutaredoxins with iron-sulphur clusters in eukaryotes—Structure, function and impact on disease. Biochim. Biophys. Acta BBA-Bioenerg. 2021, 1862, 148317. [Google Scholar] [CrossRef]
- Zimmermann, J.; Oestreicher, J.; Hess, S.; Herrmann, J.M.; Deponte, M.; Morgan, B. One cysteine is enough: A monothiol Grx can functionally replace all cytosolic Trx and dithiol Grx. Redox Biol. 2020, 36, 101598. [Google Scholar] [CrossRef] [PubMed]
- Begas, P.; Liedgens, L.; Moseler, A.; Meyer, A.J.; Deponte, M. Glutaredoxin catalysis requires two distinct glutathione interaction sites. Nat. Commun. 2017, 8, 14835. [Google Scholar] [CrossRef] [PubMed]
- Zannini, F.; Moseler, A.; Bchini, R.; Dhalleine, T.; Meyer, A.J.; Rouhier, N.; Couturier, J. The thioredoxin-mediated recycling of Arabidopsis thaliana GRXS16 relies on a conserved C-terminal cysteine. Biochim. Biophys. Acta BBA-Gen. Subj. 2019, 1863, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Fladvad, M.; Berndt, C.; Andrésen, C.; Lillig, C.H.; Neubauer, P.; Sunnerhagen, M.; Holmgren, A.; Vlamis-Gardikas, A. A Novel Monothiol Glutaredoxin (Grx4) from Escherichia coli Can Serve as a Substrate for Thioredoxin Reductase. J. Biol. Chem. 2005, 280, 24544–24552. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Michelet, L.; Massot, V.; Trost, P.; Lemaire, S.D. Biochemical Characterization of Glutaredoxins from Chlamydomonas reinhardtii Reveals the Unique Properties of a Chloroplastic CGFS-type Glutaredoxin. J. Biol. Chem. 2008, 283, 8868–8876. [Google Scholar] [CrossRef]
- Johansson, C.; Lillig, C.H.; Holmgren, A. Human Mitochondrial Glutaredoxin Reduces S-Glutathionylated Proteins with High Affinity Accepting Electrons from Either Glutathione or Thioredoxin Reductase. J. Biol. Chem. 2004, 279, 7537–7543. [Google Scholar] [CrossRef]
- Moseler, A.; Aller, I.; Wagner, S.; Nietzel, T.; Przybyla-Toscano, J.; Mühlenhoff, U.; Lill, R.; Berndt, C.; Rouhier, N.; Schwarzländer, M.; et al. The mitochondrial monothiol glutaredoxin S15 is essential for iron-sulfur protein maturation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 13735–13740. [Google Scholar] [CrossRef]
- Tamarit, J.; Bellí, G.; Cabiscol, E.; Herrero, E.; Ros, J. Biochemical Characterization of Yeast Mitochondrial Grx5 Monothiol Glutaredoxin. J. Biol. Chem. 2003, 278, 25745–25751. [Google Scholar] [CrossRef]
- Liedgens, L.; Zimmermann, J.; Wäschenbach, L.; Geissel, F.; Laporte, H.; Gohlke, H.; Morgan, B.; Deponte, M. Quantitative assessment of the determinant structural differences between redox-active and inactive glutaredoxins. Nat. Commun. 2020, 11, 1725. [Google Scholar] [CrossRef] [PubMed]
- Trnka, D.; Engelke, A.D.; Gellert, M.; Moseler, A.; Hossain, M.F.; Lindenberg, T.T.; Pedroletti, L.; Odermatt, B.; de Souza, J.V.; Bronowska, A.K.; et al. Molecular basis for the distinct functions of redox-active and FeS-transfering glutaredoxins. Nat. Commun. 2020, 11, 3445. [Google Scholar] [CrossRef] [PubMed]
- Przybyla-Toscano, J.; Christ, L.; Keech, O.; Rouhier, N. Iron–sulfur proteins in plant mitochondria: Roles and maturation. J. Exp. Bot. 2021, 72, 2014–2044. [Google Scholar] [CrossRef] [PubMed]
- Azam, T.; Przybyla-Toscano, J.; Vignols, F.; Couturier, J.; Rouhier, N.; Johnson, M.K. The Arabidopsis Mitochondrial Glutaredoxin GRXS15 Provides [2Fe-2S] Clusters for ISCA-Mediated [4Fe-4S] Cluster Maturation. Int. J. Mol. Sci. 2020, 21, 9237. [Google Scholar] [CrossRef]
- Ströher, E.; Grassl, J.; Carrie, C.; Fenske, R.; Whelan, J.; Millar, A.H. Glutaredoxin S15 Is Involved in Fe-S Cluster Transfer in Mitochondria Influencing Lipoic Acid-Dependent Enzymes, Plant Growth, and Arsenic Tolerance in Arabidopsis. Plant Physiol. 2016, 170, 1284–1299. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Fricker, M.D.; Sweetlove, L.J. Monitoring the in vivo redox state of plant mitochondria: Effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim. Biophys. Acta 2009, 1787, 468–475. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Fricker, M.D.; Müller, C.; Marty, L.; Brach, T.; Novak, J.; Sweetlove, L.J.; Hell, R.; Meyer, A.J. Confocal imaging of glutathione redox potential in living plant cells. J. Microsc. 2008, 231, 299–316. [Google Scholar] [CrossRef]
- Zhang, B.; Bandyopadhyay, S.; Shakamuri, P.; Naik, S.G.; Huynh, B.H.; Couturier, J.; Rouhier, N.; Johnson, M.K. Monothiol glutaredoxins can bind linear [Fe3S4]+ and [Fe4S4]2+ clusters in addition to [Fe2S2]2+ clusters: Spectroscopic characterization and functional implications. J. Am. Chem. Soc. 2013, 135, 15153–15164. [Google Scholar] [CrossRef]
- Nietzel, T.; Mostertz, J.; Ruberti, C.; Née, G.; Fuchs, P.; Wagner, S.; Moseler, A.; Müller-Schüssele, S.J.; Benamar, A.; Poschet, G.; et al. Redox-mediated kick-start of mitochondrial energy metabolism drives resource-efficient seed germination. Proc. Natl. Acad. Sci. USA 2020, 117, 741–751. [Google Scholar] [CrossRef]
- Schenk, P.M.; Baumann, S.; Mattes, R.; Steinbiss, H.H. Improved high-level expression system for eukaryotic genes in Escherichia coli using T7 RNA polymerase and rare ArgtRNAs. BioTechniques 1995, 19, 196–198, 200. [Google Scholar]
- Zannini, F.; Roret, T.; Przybyla-Toscano, J.; Dhalleine, T.; Rouhier, N.; Couturier, J. Mitochondrial Arabidopsis thaliana TRXo Isoforms Bind an Iron–Sulfur Cluster and Reduce NFU Proteins In Vitro. Antioxidants 2018, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, J.-P.; Rivera-Madrid, R.; Marinho, P.; Kollarova, M.; Le Maréchal, P.; Miginiac-Maslow, M.; Meyer, Y. Arabidopsis thaliana NADPH Thioredoxin Reductase. J. Mol. Biol. 1994, 235, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Zannini, F.; Couturier, J.; Keech, O.; Rouhier, N. In Vitro Alkylation Methods for Assessing the Protein Redox State. Methods Mol. Biol. Clifton NJ 2017, 1653, 51–64. [Google Scholar] [CrossRef]
- Couturier, J.; Koh, C.S.; Zaffagnini, M.; Winger, A.M.; Gualberto, J.M.; Corbier, C.; Decottignies, P.; Jacquot, J.-P.; Lemaire, S.D.; Didierjean, C.; et al. Structure-Function Relationship of the Chloroplastic Glutaredoxin S12 with an Atypical WCSYS Active Site. J. Biol. Chem. 2009, 284, 9299–9310. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Bedhomme, M.; Marchand, C.H.; Couturier, J.; Gao, X.-H.; Rouhier, N.; Trost, P.; Lemaire, S.D. Glutaredoxin S12: Unique Properties for Redox Signaling. Antioxid. Redox Signal. 2012, 16, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-H.; Zaffagnini, M.; Bedhomme, M.; Michelet, L.; Cassier-Chauvat, C.; Decottignies, P.; Lemaire, S.D. Biochemical characterization of glutaredoxins from Chlamydomonas reinhardtii: Kinetics and specificity in deglutathionylation reactions. FEBS Lett. 2010, 584, 2242–2248. [Google Scholar] [CrossRef]
- Bedhomme, M.; Adamo, M.; Marchand, C.H.; Couturier, J.; Rouhier, N.; Lemaire, S.D.; Zaffagnini, M.; Trost, P. Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem. J. 2012, 445, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Greetham, D.; Vickerstaff, J.; Shenton, D.; Perrone, G.G.; Dawes, I.W.; Grant, C.M. Thioredoxins function as deglutathionylase enzymes in the yeast Saccharomyces cerevisiae. BMC Biochem. 2010, 11, 3. [Google Scholar] [CrossRef]
- Kehr, S.; Jortzik, E.; Delahunty, C.; Yates, J.R.; Rahlfs, S.; Becker, K. Protein S-glutathionylation in malaria parasites. Antioxid. Redox Signal. 2011, 15, 2855–2865. [Google Scholar] [CrossRef]
- Bonilla, M.; Denicola, A.; Marino, S.M.; Gladyshev, V.N.; Salinas, G. Linked thioredoxin-glutathione systems in platyhelminth parasites: Alternative pathways for glutathione reduction and deglutathionylation. J. Biol. Chem. 2011, 286, 4959–4967. [Google Scholar] [CrossRef]
- Subramani, J.; Kundumani-Sridharan, V.; Hilgers, R.H.P.; Owens, C.; Das, K.C. Thioredoxin Uses a GSH-independent Route to Deglutathionylate Endothelial Nitric-oxide Synthase and Protect against Myocardial Infarction. J. Biol. Chem. 2016, 291, 23374–23389. [Google Scholar] [CrossRef] [PubMed]
- Moseler, A.; Kruse, I.; Maclean, A.E.; Pedroletti, L.; Franceschetti, M.; Wagner, S.; Wehler, R.; Fischer-Schrader, K.; Poschet, G.; Wirtz, M.; et al. The function of glutaredoxin GRXS15 is required for lipoyl-dependent dehydrogenases in mitochondria. Plant Physiol. 2021, 186, 1507–1525. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christ, L.; Couturier, J.; Rouhier, N. Relationships between the Reversible Oxidation of the Single Cysteine Residue and the Physiological Function of the Mitochondrial Glutaredoxin S15 from Arabidopsis thaliana. Antioxidants 2023, 12, 102. https://doi.org/10.3390/antiox12010102
Christ L, Couturier J, Rouhier N. Relationships between the Reversible Oxidation of the Single Cysteine Residue and the Physiological Function of the Mitochondrial Glutaredoxin S15 from Arabidopsis thaliana. Antioxidants. 2023; 12(1):102. https://doi.org/10.3390/antiox12010102
Chicago/Turabian StyleChrist, Loïck, Jérémy Couturier, and Nicolas Rouhier. 2023. "Relationships between the Reversible Oxidation of the Single Cysteine Residue and the Physiological Function of the Mitochondrial Glutaredoxin S15 from Arabidopsis thaliana" Antioxidants 12, no. 1: 102. https://doi.org/10.3390/antiox12010102
APA StyleChrist, L., Couturier, J., & Rouhier, N. (2023). Relationships between the Reversible Oxidation of the Single Cysteine Residue and the Physiological Function of the Mitochondrial Glutaredoxin S15 from Arabidopsis thaliana. Antioxidants, 12(1), 102. https://doi.org/10.3390/antiox12010102







