Tracking the Biostimulatory Effect of Fractions from a Commercial Plant Protein Hydrolysate in Greenhouse-Grown Lettuce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions, Experimental Design and Plant Material
2.2. Biostimulant Characteristics
2.3. Biostimulant Treatments
2.4. Yield, Growth Assessment, Leaf Colorimetric Measurement and Sampling
2.5. Carotenoids and Total Ascorbic Acid Determination
2.6. Leaf Mineral Analysis
2.7. Leaf Polyphenolic Content
2.8. Statistical Analysis, Cluster Analysis and Heatmap
3. Results and Discussion
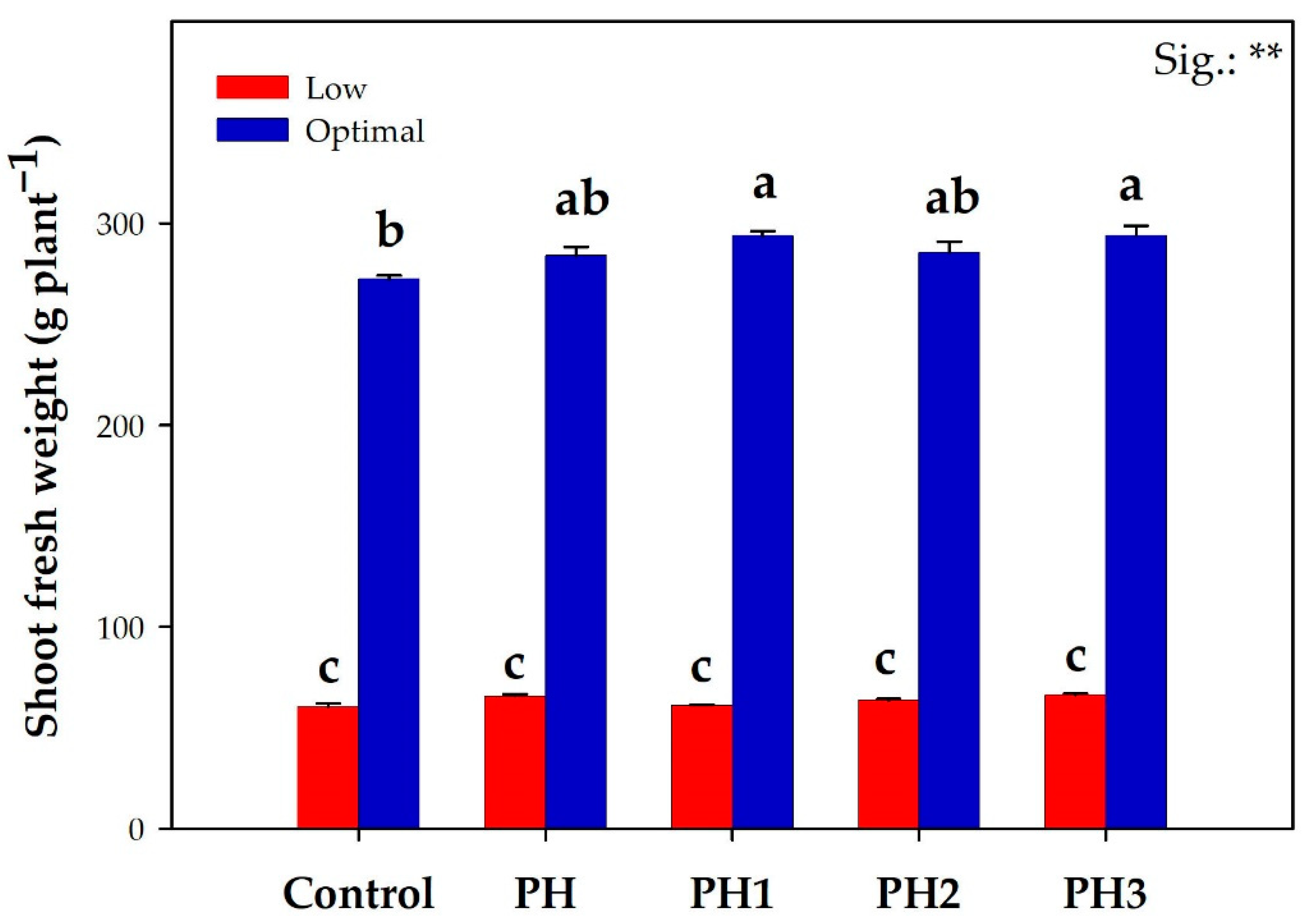
3.1. Lettuce Fresh Yield and Leaf Colorimetry Indices
3.2. Leaf Mineral and Organic Acid Contents
3.3. Leaf Pigments and Total Ascorbic Acid Content
3.4. Leaf Polyphenolics
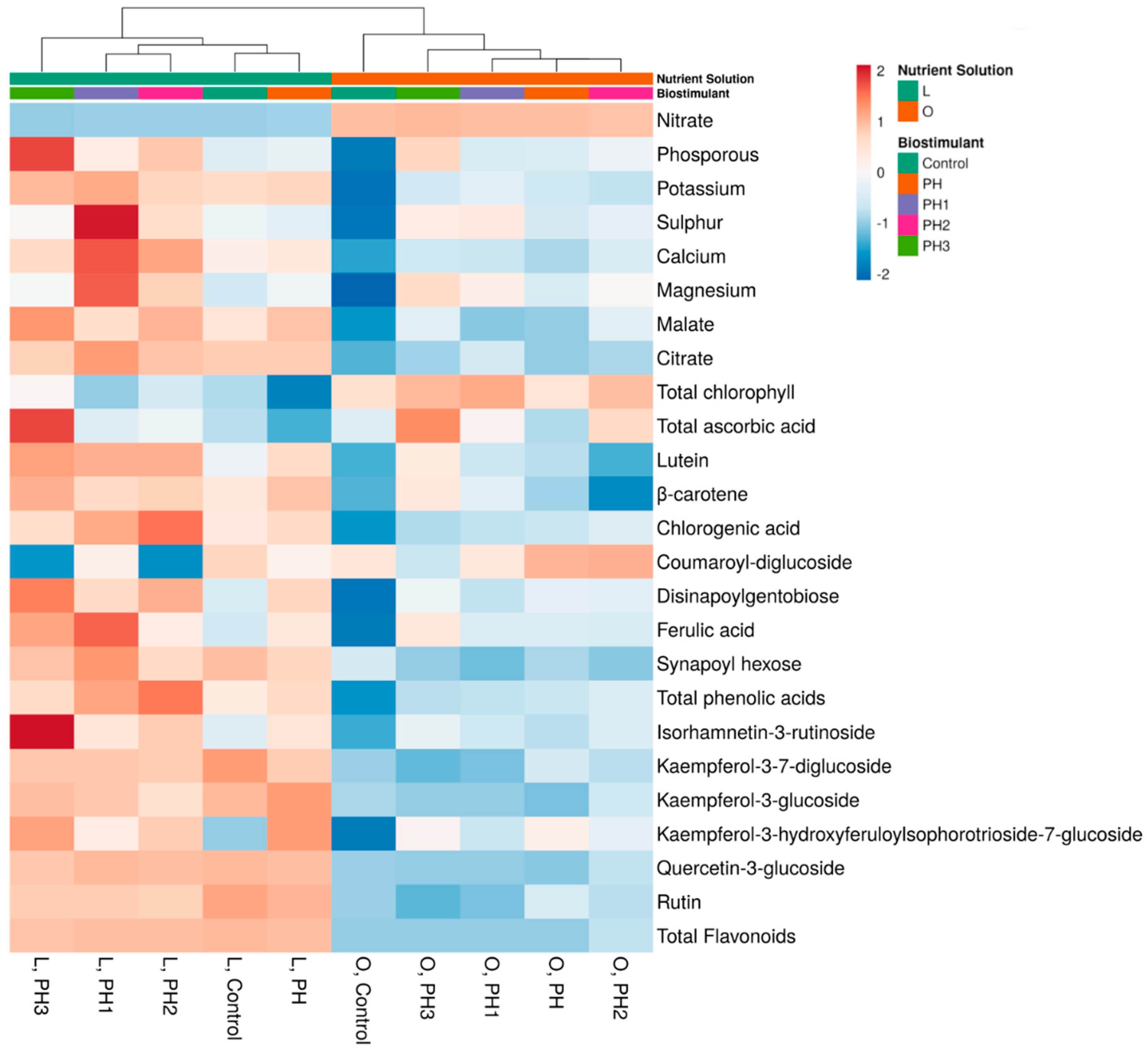
3.5. Cluster Analysis and Heatmap of the Accumulation of Phytochemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Agency. Nitrate in Vegetables-Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 689. [Google Scholar] [CrossRef]
- Mancuso, G.; Bencresciuto, G.F.; Lavrnić, S.; Toscano, A. Diffuse Water Pollution from Agriculture: A Review of Nature-Based Solutions for Nitrogen Removal and Recovery. Water 2021, 13, 1893. [Google Scholar] [CrossRef]
- EU (European Union). Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance); European Union: Brussels, Belgium, 2019; Volume 170. [Google Scholar]
- Cristofano, F.; El-Nakhel, C.; Pannico, A.; Giordano, M.; Colla, G.; Rouphael, Y. Foliar and Root Applications of Vegetal-Derived Protein Hydrolysates Differentially Enhance the Yield and Qualitative Attributes of Two Lettuce Cultivars Grown in Floating System. Agronomy 2021, 11, 1194. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Elia, A. Foliar Application of Protein Hydrolysates on Baby-Leaf Spinach Grown at Different N Levels. Agronomy 2022, 12, 36. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [Green Version]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Cardarelli, M.; Colla, G. Combining Molecular Weight Fractionation and Metabolomics to Elucidate the Bioactivity of Vegetal Protein Hydrolysates in Tomato Plants. Front. Plant Sci. 2020, 11, 976. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Kubo, M. Soybean Peptide: Novel Plant Growth Promoting Peptide from Soybean. In Soybean and Nutrition; IntechOpen: London, UK, 2011; pp. 215–230. [Google Scholar] [CrossRef] [Green Version]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar Application of Different Vegetal-Derived Protein Hydrolysates Distinctively Modulates Tomato Root Development and Metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Sorrentino, M.; De Diego, N.; Ugena, L.; Spíchal, L.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Rouphael, Y.; Colla, G.; Panzarová, K. Seed Priming With Protein Hydrolysates Improves Arabidopsis Growth and Stress Tolerance to Abiotic Stresses. Front. Plant Sci. 2021, 12, 837. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown Under Limited Water Availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 Color-Difference Formula: Implementation Notes, Supplementary Test Data, and Mathematical Observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional Quality in Novel Food Sources: Genotypic Variation in the Nutritive and Phytochemical Composition of Thirteen Microgreens Species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and Determination of Ascorbate and Dehydroascorbate from Plant Tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar Applications of a Legume-Derived Protein Hydrolysate Elicit Dose-Dependent Increases of Growth, Leaf Mineral Composition, Yield and Fruit Quality in Two Greenhouse Tomato Cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.B.; Rouphael, Y.; De Pasquale, C.; Iapichino, G.; D’anna, F.; La Bella, S. Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment. Agronomy 2021, 11, 1023. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-Alone and Combinatorial Effects of Plant-Based Biostimulants on the Production and Leaf Quality of Perennial Wall Rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Richard-Molard, C.; Krapp, A.; Brun, F.; Ney, B.; Daniel-Vedele, F.; Chaillou, S. Plant Response to Nitrate Starvation Is Determined by N Storage Capacity Matched by Nitrate Uptake Capacity in Two Arabidopsis Genotypes. J. Exp. Bot. 2008, 59, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Anjana, S.U.; Iqbal, M. Nitrate Accumulation in Plants, Factors Affecting the Process, and Human Health Implications. A Review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Pannico, A.; El-Nakhel, C.; Fascella, G.; Duri, L.G.; Cristofano, F.; Gentile, B.R.; Giordano, M.; Rouphael, Y.; et al. Nutrient Solution Deprivation as a Tool to Improve Hydroponics Sustainability: Yield, Physiological, and Qualitative Response of Lettuce. Agronomy 2021, 11, 1469. [Google Scholar] [CrossRef]
- Becker, C.; Urlić, B.; Špika, M.J.; Kläring, H.-P.; Krumbein, A.; Baldermann, S.; Ban, S.G.; Perica, S.; Schwarz, D. Nitrogen Limited Red and Green Leaf Lettuce Accumulate Flavonoid Glycosides, Caffeic Acid Derivatives, and Sucrose While Losing Chlorophylls, B-Carotene and Xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Fahnenstich, H.; Sonnewald, U. Maize Source Leaf Adaptation to Nitrogen Deficiency Affects Not Only Nitrogen and Carbon Metabolism But Also Control of Phosphate Homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef] [Green Version]
- Radin, J.W.; Eidenbock, M.P. Carbon Accumulation during Photosynthesis in Leaves of Nitrogen- and Phosphorus-Stressed Cotton. Plant Physiol. 1986, 82, 869–871. [Google Scholar] [CrossRef]
- Gao, S.; Wang, S.; Hu, Y.; Chen, S.; Guo, J.; Shi, L. Comparative Differences in Photosynthetic Characteristics, Ion Balance, and Nitrogen Metabolism between Young and Old Wild Soybean Leaves under Nitrogen Deficiency. Plant Stress 2022, 4, 100064. [Google Scholar] [CrossRef]
- Kotopoulou, S.; Zampelas, A.; Magriplis, E. Dietary Nitrate and Nitrite and Human Health: A Narrative Review by Intake Source. Nutr. Rev. 2022, 80, 762–773. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality—A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Micronutrient Inadequacies in the US Population: An Overview. Available online: https://lpi.oregonstate.edu/mic/micronutrient-inadequacies/overview (accessed on 10 November 2022).
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; El-Nakhel, C.; Carillo, P.; Colla, G.; Graziani, G.; Di Mola, I.; Mori, M.; Kyriacou, M.C.; Rouphael, Y.; Soteriou, G.A.; et al. Plant-Derived Biostimulants Differentially Modulate Primary and Secondary Metabolites and Improve the Yield Potential of Red and Green Lettuce Cultivars. Agronomy 2022, 12, 1361. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an Alfalfa Protein Hydrolysate on the Gene Expression and Activity of Enzymes of the Tricarboxylic Acid (TCA) Cycle and Nitrogen Metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-Wide Identification of Differentially Expressed Genes in Solanum lycopersicon L. in Response to an Alfalfa-Protein Hydrolysate Using Microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [Green Version]
- El-Nakhel, C.; Geelen, D.; De Paepe, J.; Clauwaert, P.; De Pascale, S.; Rouphael, Y. An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System. Sustainability 2021, 13, 4218. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The Role of the Xanthophyll Cycle and of Lutein in Photoprotection of Photosystem II. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Torres-Montilla, S.; Rodriguez-Concepcion, M. Making Extra Room for Carotenoids in Plant Cells: New Opportunities for Biofortification. Prog. Lipid Res. 2021, 84, 101128. [Google Scholar] [CrossRef]
- Gest, N.; Gautier, H.; Stevens, R. Ascorbate as Seen through Plant Evolution: The Rise of a Successful Molecule? J. Exp. Bot. 2013, 64, 33–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Vitamin a Deficiency. Available online: https://www.who.int/data/nutrition/nlis/info/vitamin-a-deficiency (accessed on 7 November 2022).
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; Cimini, S.; De Gara, L. Strategies to Increase Vitamin C in Plants: From Plant Defense Perspective to Food Biofortification. Front. Plant Sci. 2013, 4, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive Compounds in Lettuce: Highlighting the Benefits to Human Health and Impacts of Preharvest and Postharvest Practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering Plants with Increased Levels of the Antioxidant Chlorogenic Acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic Acid From Plant Biomass: A Phytochemical With Promising Antiviral Properties. Front. Nutr. 2022, 8, 777576. [Google Scholar] [CrossRef]
| Source of Variance | NO3 | P | K | S | Ca | Mg | Malate | Citrate |
|---|---|---|---|---|---|---|---|---|
| (mg kg−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | |
| Nutrient Solution (NS) | ||||||||
| Optimal N (O) | 1060 ± 46 | 20.5 ± 0.5 | 264 ± 11 | 3.51 ± 0.12 | 33.6 ± 1.4 | 18.7 ± 0.6 | 192 ± 6 | 19.6 ± 1.1 |
| Low N (L) | 11.3 ± 0.6 | 22.1 ± 0.6 | 407 ± 9 | 3.87 ± 0.15 | 57.7 ± 3.1 | 20.1 ± 0.5 | 283 ± 7 | 60.0 ± 2.4 |
| t-test | *** | * | *** | * | *** | * | *** | *** |
| Biostimulant (B) | ||||||||
| Control | 534 ± 242 | 19.3 ± 0.9 b | 291 ± 44 b | 3.28 ± 0.23 b | 37.0 ± 4.7 c | 16.8 ± 0.7 c | 211 ± 25 c | 35.9 ± 9.3 b |
| PH | 543 ± 243 | 20.6 ± 0.8 b | 336 ± 30 ab | 3.5 ± 0.19 ab | 41.0 ± 4.1 bc | 18.7 ± 0.4 bc | 234 ± 22 abc | 37.3 ± 8.6 b |
| PH1 | 526 ± 233 | 21.0 ± 0.3 ab | 368 ± 32 a | 4.2 ± 0.21 a | 54.9 ± 9.1 a | 21.5 ± 0.9 a | 225 ± 21 bc | 48.6 ± 10.8 a |
| PH2 | 505 ± 229 | 21.9 ± 0.8 ab | 332 ± 30 ab | 3.74 ± 0.17 ab | 50.0 ± 6.5 ab | 20.2 ± 0.8 ab | 255 ± 17 ab | 40.0 ± 9.7 ab |
| PH3 | 568 ± 254 | 23.5 ± 0.5 a | 352 ± 32 a | 3.73 ± 0.16 ab | 45.3 ± 5.0 abc | 20.0 ± 0.5 ab | 263 ± 23 a | 37.2 ± 8.1 b |
| n.s. | ** | ** | * | ** | *** | ** | * | |
| NS × B | ||||||||
| O × Control | 1058 ± 139 | 18.1 ± 1.0 | 194 ± 15 | 2.96 ± 0.28 | 27.2 ± 3.1 e | 15.4 ± 0.5 | 161 ± 9 | 15.4 ± 1.1 |
| O × PH | 1074 ± 121 | 20.4 ± 1.2 | 275 ± 11 | 3.44 ± 0.05 | 32.7 ± 1.0 de | 18.3 ± 0.4 | 184 ± 1 | 18.4 ± 1.1 |
| O × PH1 | 1041 ± 77 | 20.4 ± 0.4 | 301 ± 17 | 3.82 ± 0.26 | 35.1 ± 3.4 de | 19.9 ± 1.2 | 183 ± 17 | 24.8 ± 3.4 |
| O × PH2 | 999 ± 139 | 20.9 ± 0.6 | 269 ± 15 | 3.56 ± 0.18 | 37.0 ± 3.0 cde | 19.4 ± 1. | 216 ± 6 | 20.1 ± 1.1 |
| O × PH3 | 1127 ± 106 | 22.5 ± 0.6 | 280 ± 10 | 3.77 ± 0.21 | 35.8 ± 1.9 de | 20.7 ± 0.6 | 213 ± 1 | 19.3 ± 0.5 |
| L × Control | 10.7 ± 2.0 | 20.5 ± 1.3 | 387 ± 15 | 3.61 ± 0.27 | 46.8 ± 1.5 bcd | 18.1 ± 0.3 | 260 ± 25 | 56.4 ± 3.2 |
| L × PH | 12.9 ± 1.8 | 20.8 ± 1.2 | 397 ± 27 | 3.56 ± 0.43 | 49.4 ± 3.7 bcd | 19.1 ± 0.8 | 284 ± 7 | 56.3 ± 2.5 |
| L × PH1 | 11.4 ± 0.6 | 21.6 ± 0.1 | 435 ± 19 | 4.57 ± 0.14 | 74.6 ± 3.6 a | 23.1 ± 0.5 | 267 ± 15 | 72.4 ± 1.5 |
| L × PH2 | 11.4 ± 1.9 | 22.8 ± 1.4 | 394 ± 18 | 3.92 ± 0.27 | 62.9 ± 5.6 ab | 21.0 ± 1.1 | 293 ± 5 | 59.9 ± 8.8 |
| L × PH3 | 10.0 ± 1.0 | 24.6 ± 0.2 | 423 ± 9 | 3.69 ± 0.29 | 54.7 ± 5.5 bc | 19.3 ± 0.6 | 313 ± 11 | 55.2 ± 1.4 |
| n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. | n.s. |
| Source of Variance | Total Chlorophylls | TAA | β-Carotene | Lutein |
|---|---|---|---|---|
| (mg 100 g−1 fw) | (mg AA 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | |
| Nutrient Solution (NS) | ||||
| Optimal N (O) | 95.0 ± 3.4 | 139 ± 6.00 | 1.54 ± 0.11 | 1.54 ± 0.1 |
| Low N (L) | 58.5 ± 3.4 | 132 ± 7.43 | 2.47 ± 0.12 | 2.30 ± 0.06 |
| t-test | *** | n.s. | *** | *** |
| Biostimulant (B) | ||||
| Control | 71.9 ± 7.0 ab | 121 ± 6 bc | 1.53 ± 0.16 c | 1.69 ± 0.18 b |
| PH | 64.8 ± 11.0 b | 112 ± 3 c | 1.95 ± 0.24 bc | 1.91 ± 0.23 ab |
| PH1 | 79.0 ± 11.7 ab | 131 ± 6 bc | 2.12 ± 0.25 ab | 1.99 ± 0.13 ab |
| PH2 | 80.7 ± 8.8 ab | 141 ± 10 b | 1.96 ± 0.35 b | 1.72 ± 0.28 b |
| PH3 | 87.5 ± 6.8 a | 172 ± 5 a | 2.48 ± 0.18 a | 2.29 ± 0.15 a |
| * | *** | *** | ** | |
| NS × B | ||||
| H × Control | 86.9 ± 2.6 | 126 ± 13 | 1.23 ± 0.08 e | 1.30 ± 0.01 |
| H × PH | 86.6 ± 9.8 | 117 ± 7 | 1.51 ± 0.08 de | 1.45 ± 0.05 |
| H × PH1 | 103 ± 8.2 | 136 ± 11 | 1.57 ± 0.09 cde | 1.72 ± 0.12 |
| H × PH2 | 98.8 ± 7.0 | 149 ± 8 | 1.23 ± 0.17 e | 1.14 ± 0.08 |
| H × PH3 | 99.4 ± 7.2 | 166 ± 11 | 2.17 ± 0.24 abcd | 2.10 ± 0.25 |
| L × Control | 56.9 ± 3.4 | 117 ± 0 | 1.83 ± 0.17 bcde | 2.09 ± 0.08 |
| L × PH | 43.1 ± 6.5 | 107 ± 2 | 2.39 ± 0.28 abc | 2.38 ± 0.20 |
| L × PH1 | 54.7 ± 5.4 | 125 ± 7 | 2.67 ± 0.07 ab | 2.26 ± 0.05 |
| L × PH2 | 62.5 ± 2.8 | 133 ± 20 | 2.7 ± 0.24 ab | 2.30 ± 0.22 |
| L × PH3 | 75.6 ± 6.0 | 177 ± 2 | 2.78 ± 0.15 a | 2.48 ± 0.09 |
| n.s. | n.s. | * | n.s. |
| Source of Variance | Chlorogenic Acid | Coumaroyl-Diglucoside | Disinapoylgentobiose | Ferulic Acid | Synapoyl-Hexose | Total Phenolic Acids |
|---|---|---|---|---|---|---|
| (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | |
| Nutrient Solution (NS) | ||||||
| Optimal N (O) | 9736 ± 245 | 13.1 ± 0.8 | 3.17 ± 0.12 | 753 ± 32 | 32.1 ± 1.3 | 10537 ± 255 |
| Low N (L) | 13431 ± 416 | 11.4 ± 1.0 | 4.26 ± 0.15 | 899 ± 39 | 80.3 ± 4.8 | 14426 ± 436 |
| t-test | ** | ** | *** | n.s. | *** | ** |
| Biostimulant (B) | ||||||
| Control | 10308 ± 907 b | 13.8 ± 2.0 | 2.83 ± 0.21 d | 685 ± 57 b | 60.0 ± 11.1 | 11069 ± 955 b |
| PH | 11545 ± 936 ab | 13.4 ± 1.3 | 3.85 ± 0.19 bc | 813 ± 37 ab | 53.7 ± 11.6 | 12429 ± 980 ab |
| PH1 | 11990 ± 1046 ab | 12.9 ± 1.6 | 3.68 ± 0.26 c | 903 ± 81 a | 61.1 ± 14.6 | 12971 ± 1123 ab |
| PH2 | 12847 ± 1043 a | 11.6 ± 1.4 | 3.98 ± 0.27 b | 809 ± 55 ab | 50.8 ± 10.4 | 13722 ± 1070 a |
| PH3 | 11227 ± 680 ab | 9.59 ± 0.6 | 4.25 ± 0.33 a | 919 ± 46 a | 55.5 ± 12.5 | 12216 ± 718 ab |
| ** | n.s. | *** | * | n.s. | ** | |
| NS × B | ||||||
| O × Control | 8450 ± 403 | 13.7 ± 3.9 | 2.38 ± 0.09 d | 610 ± 23 | 38.5 ± 2.5 | 9114 ± 418 |
| O × PH | 10017 ± 428 | 14.4 ± 1.1 | 3.43 ± 0.05 c | 759 ± 15 | 32.9 ± 2.6 | 10827 ± 446 |
| O × PH1 | 9959 ± 736 | 12.8 ± 1.2 | 3.12 ± 0.12 c | 761 ± 44 | 28.5 ± 1.9 | 10765 ± 690 |
| O × PH2 | 10521 ± 130 | 14.4 ± 0.4 | 3.40 ± 0.13 c | 765 ± 100 | 30.2 ± 3.3 | 11335 ± 71 |
| O × PH3 | 9732 ± 32 | 10.4 ± 0.8 | 3.54 ± 0.12 c | 869 ± 75. | 30.7 ± 1.8 | 10645 ± 106 |
| L × Control | 12166 ± 709 | 13.8 ± 1.9 | 3.28 ± 0.02 c | 760 ± 100 | 81.5 ± 12.2 | 13024 ± 754 |
| L × PH | 13073 ± 1364 | 12.5 ± 2.5 | 4.27 ± 0.06 b | 867 ± 61 | 74.6 ± 15.3 | 14031 ± 142 |
| L × PH1 | 14022 ± 893 | 13.0 ± 3.3 | 4.23 ± 0.06 b | 1045 ± 104 | 93.8 ± 0.9 | 15178 ± 980 |
| L × PH2 | 15173 ± 118 | 8.74 ± 1.1 | 4.56 ± 0.05 ab | 852 ± 56 | 71.4 ± 10.5 | 16109 ± 143 |
| L × PH3 | 12722 ± 278 | 8.87 ± 0.5 | 4.96 ± 0.15 a | 970 ± 46 | 80.4 ± 12.7 | 13786 ± 322 |
| n.s. | n.s. | * | n.s. | n.s. | n.s. |
| Source of Variance | Isorhamnetin 3-Rutinoside | Kaempferol 3,7-Diglucoside | Kaempferol 3-Glucoside | Kaempferol 3-Hydroxyferuloyl-Sophorotrioside-7-Glucoside | Quercetin 3-Glucoside | Rutin | Total Flavonoids |
|---|---|---|---|---|---|---|---|
| (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | (µg 100 g−1 fw) | |
| Nutrient Solution (NS) | |||||||
| Optimal N (O) | 3.33 ± 0.12 | 0.89 ± 0.11 | 3.82 ± 0.30 | 1.55 ± 0.10 | 67.8 ± 4.5 | 1.78 ± 0.22 | 80.0 ± 4.8 |
| Low N (L) | 5.50 ± 0.42 | 6.59 ± 0.78 | 12.6 ± 0.70 | 1.81 ± 0.14 | 495 ± 15 | 13.8 ± 1.52 | 535 ± 16 |
| t-test | *** | *** | *** | *** | *** | *** | *** |
| Biostimulant (B) | |||||||
| Control | 3.12 ± 0.26 d | 5.11 ± 2.58 | 8.60 ± 2.16 | 0.69 ± 0.09 d | 295 ± 98 | 10.2 ± 5.16 | 323 ± 107 |
| PH | 4.08 ± 0.41 c | 3.60 ± 1.08 | 9.25 ± 2.72 | 1.92 ± 0.17 a | 279 ± 97 | 8.69 ± 2.70 | 307 ± 103 |
| PH1 | 4.17 ± 0.36 bc | 3.23 ± 1.25 | 7.75 ± 2.02 | 1.32 ± 0.13 c | 294 ± 102 | 6.46 ± 2.50 | 316 ± 109 |
| PH2 | 4.58 ± 0.47 b | 3.41 ± 1.09 | 7.32 ± 1.32 | 1.63 ± 0.17 b | 291 ± 90 | 6.82 ± 2.18 | 315 ± 95 |
| PH3 | 6.11 ± 0.99 a | 3.85 ± 1.28 | 8.25 ± 2.29 | 1.89 ± 0.18 a | 268 ± 89 | 7.69 ± 2.55 | 294 ± 96 |
| *** | n.s. | n.s. | *** | n.s. | n.s. | n.s. | |
| NS × B | |||||||
| O × Control | 2.56 ± 0.09 d | 0.73 ± 0.10 | 4.06 ± 1.07 | 0.51 ± 0.03 g | 77.1 ± 18.6 | 1.46 ± 0.20 | 86.5 ± 20.0 |
| O × PH | 3.18 ± 0.09 cd | 1.40 ± 0.07 | 3.32 ± 0.78 | 1.55 ± 0.07 c | 67.8 ± 7.6 | 2.79 ± 0.15 | 80.0 ± 8.2 |
| O × PH1 | 3.41 ± 0.09 c | 0.52 ± 0.12 | 3.50 ± 0.02 | 1.03 ± 0.06 ef | 71.9 ± 5.0 | 1.05 ± 0.23 | 81.4 ± 4.9 |
| O × PH2 | 3.59 ± 0.07 c | 1.02 ± 0.31 | 4.62 ± 0.63 | 1.26 ± 0.02 de | 91.5 ± 8.2 | 2.04 ± 0.63 | 104 ± 9.7 |
| O × PH3 | 3.90 ± 0.04 c | 0.74 ± 0.28 | 3.59 ± 0.60 | 1.49 ± 0.01 cd | 72.0 ± 5.7 | 1.49 ± 0.57 | 82.5 ± 6.2 |
| L × Control | 3.68 ± 0.06 cd | 9.48 ± 3.76 | 13.1 ± 1.22 | 0.88 ± 0.06 f | 513 ± 19.8 | 19.0 ± 7.52 | 559 ± 30 |
| L × PH | 4.97 ± 0.11 b | 5.80 ± 0.98 | 15.2 ± 1.10 | 2.30 ± 0.03 a | 491 ± 42.7 | 14.6 ± 1.28 | 534 ± 45 |
| L × PH1 | 4.93 ± 0.22 b | 5.94 ± 0.70 | 12.0 ± 1.52 | 1.60 ± 0.07 c | 515 ± 58.2 | 11.9 ± 1.41 | 552 ± 62 |
| L × PH2 | 5.56 ± 0.34 b | 5.79 ± 0.36 | 10.0 ± 1.02 | 1.99 ± 0.06 b | 491 ± 20.5 | 11.6 ± 0.72 | 526 ± 22 |
| L × PH3 | 8.33 ± 0.09 a | 5.91 ± 0.22 | 12.9 ± 2.06 | 2.28 ± 0.03 a | 464 ± 30.6 | 11.8 ± 0.45 | 506 ± 32 |
| *** | n.s. | n.s. | ** | n.s. | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristofano, F.; El-Nakhel, C.; Colla, G.; Cardarelli, M.; Pii, Y.; Lucini, L.; Rouphael, Y. Tracking the Biostimulatory Effect of Fractions from a Commercial Plant Protein Hydrolysate in Greenhouse-Grown Lettuce. Antioxidants 2023, 12, 107. https://doi.org/10.3390/antiox12010107
Cristofano F, El-Nakhel C, Colla G, Cardarelli M, Pii Y, Lucini L, Rouphael Y. Tracking the Biostimulatory Effect of Fractions from a Commercial Plant Protein Hydrolysate in Greenhouse-Grown Lettuce. Antioxidants. 2023; 12(1):107. https://doi.org/10.3390/antiox12010107
Chicago/Turabian StyleCristofano, Francesco, Christophe El-Nakhel, Giuseppe Colla, Mariateresa Cardarelli, Youry Pii, Luigi Lucini, and Youssef Rouphael. 2023. "Tracking the Biostimulatory Effect of Fractions from a Commercial Plant Protein Hydrolysate in Greenhouse-Grown Lettuce" Antioxidants 12, no. 1: 107. https://doi.org/10.3390/antiox12010107
APA StyleCristofano, F., El-Nakhel, C., Colla, G., Cardarelli, M., Pii, Y., Lucini, L., & Rouphael, Y. (2023). Tracking the Biostimulatory Effect of Fractions from a Commercial Plant Protein Hydrolysate in Greenhouse-Grown Lettuce. Antioxidants, 12(1), 107. https://doi.org/10.3390/antiox12010107











