Nutritional and Functional Properties of Novel Italian Spray-Dried Cricket Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
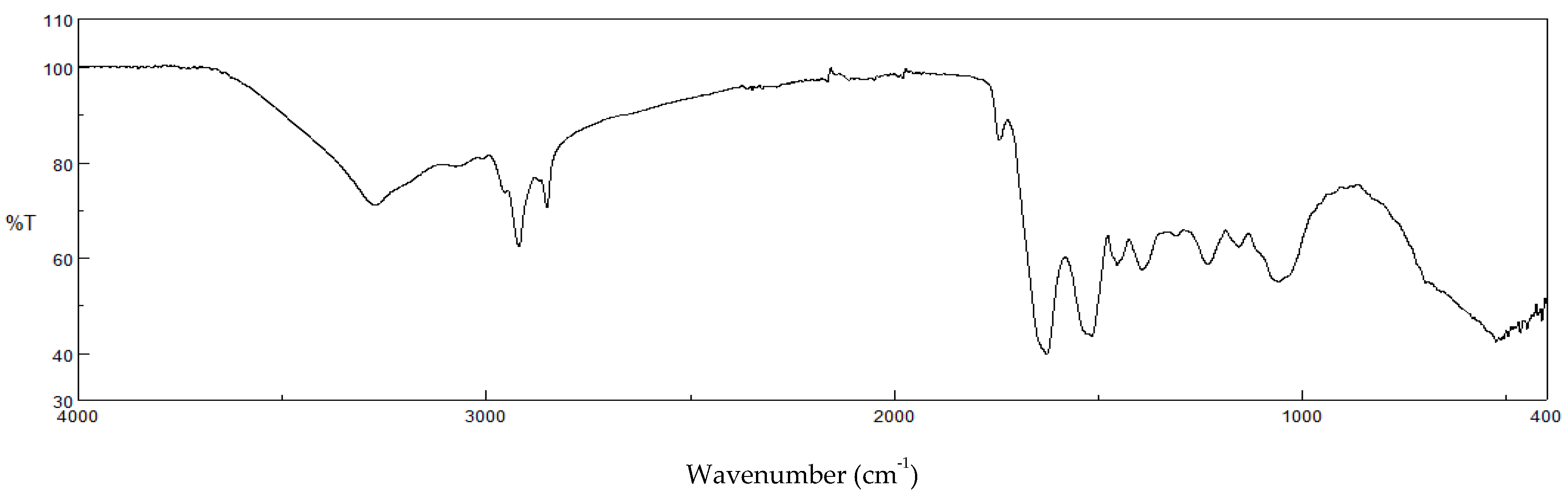
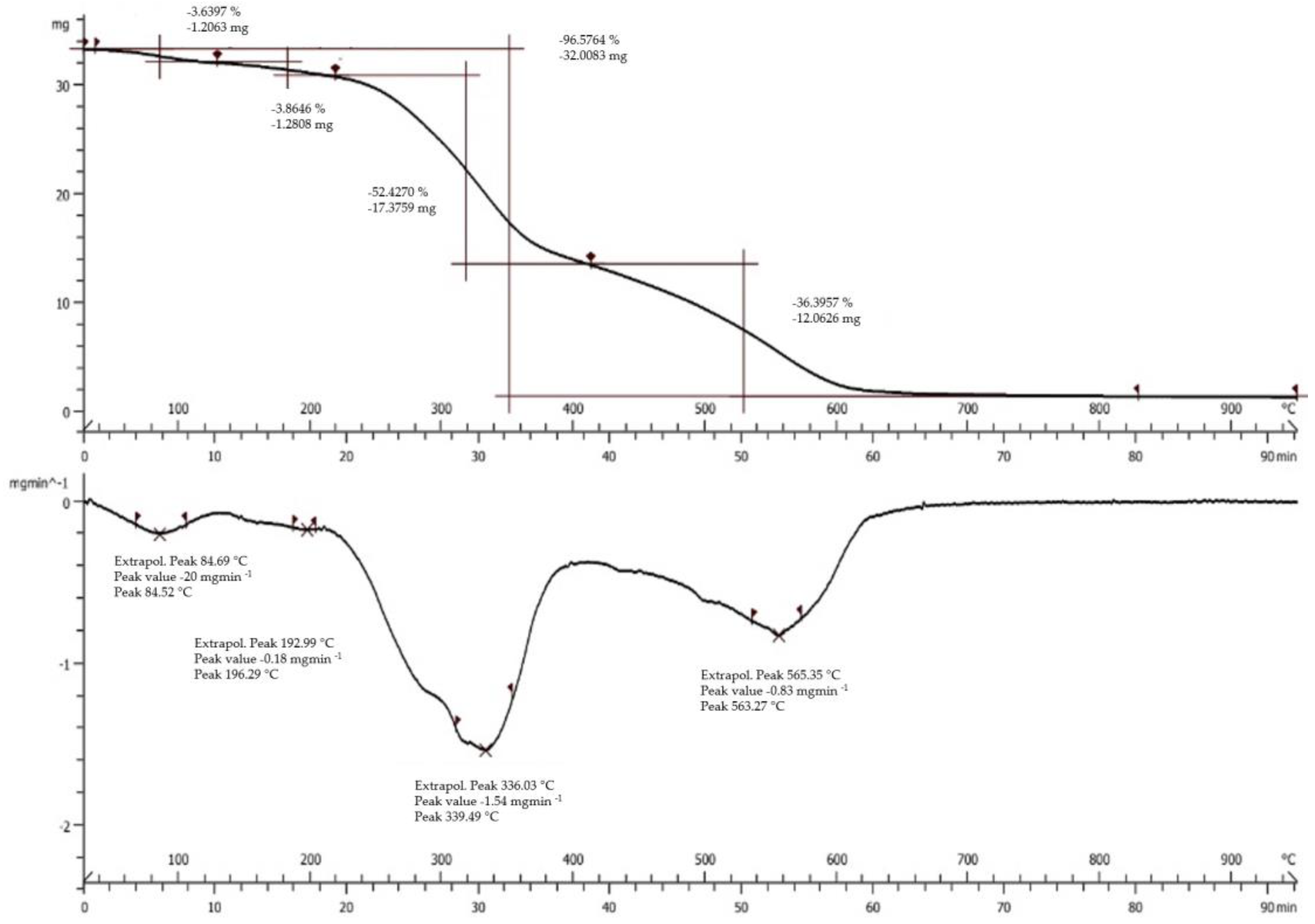
2.2. Physico-Chemical Characterization
2.3. Chitin Quantification
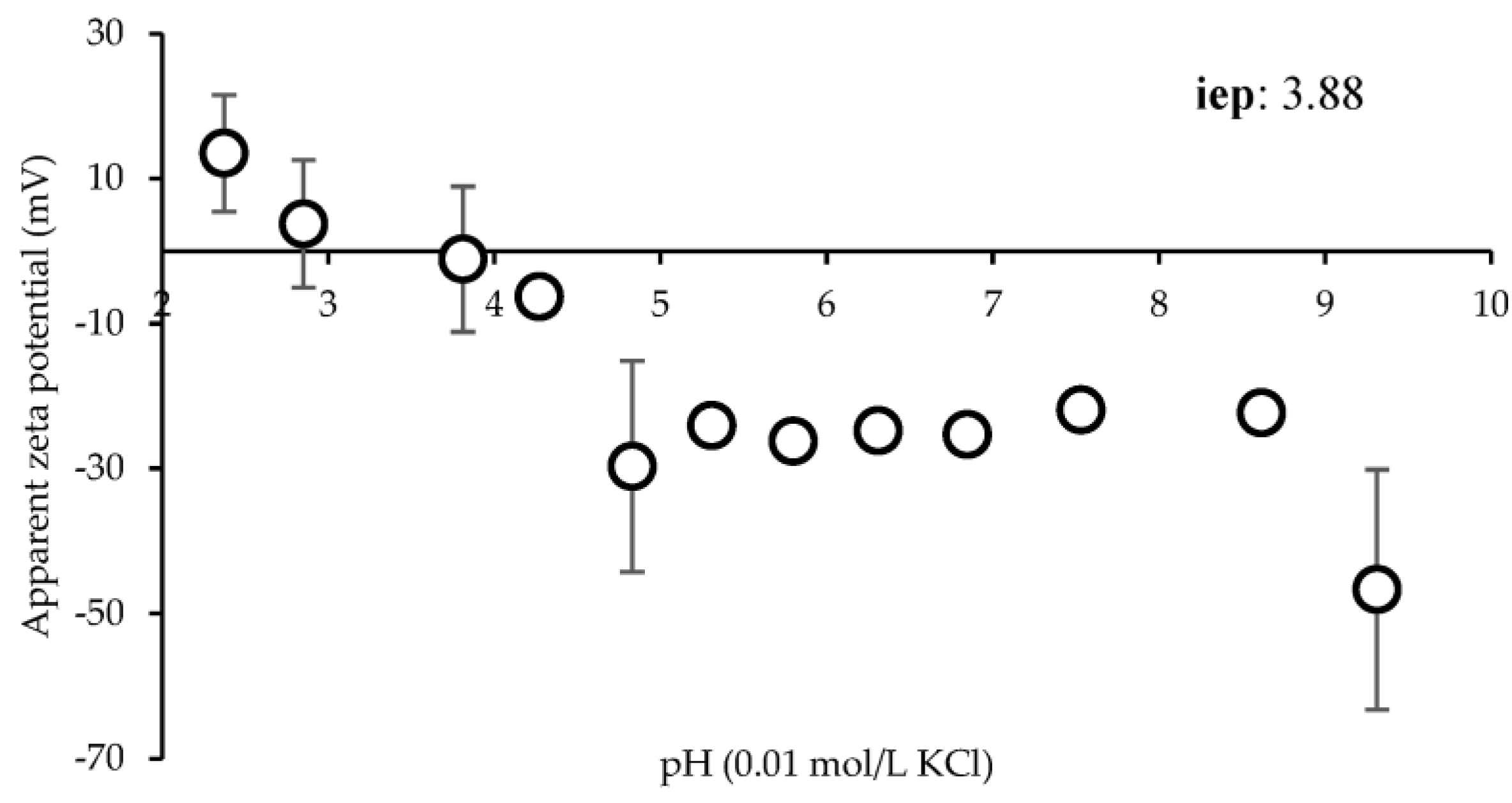
2.4. Surface Zeta Potential (ζ)
2.5. Protein Digestability Assay
2.6. Antioxidant Properties
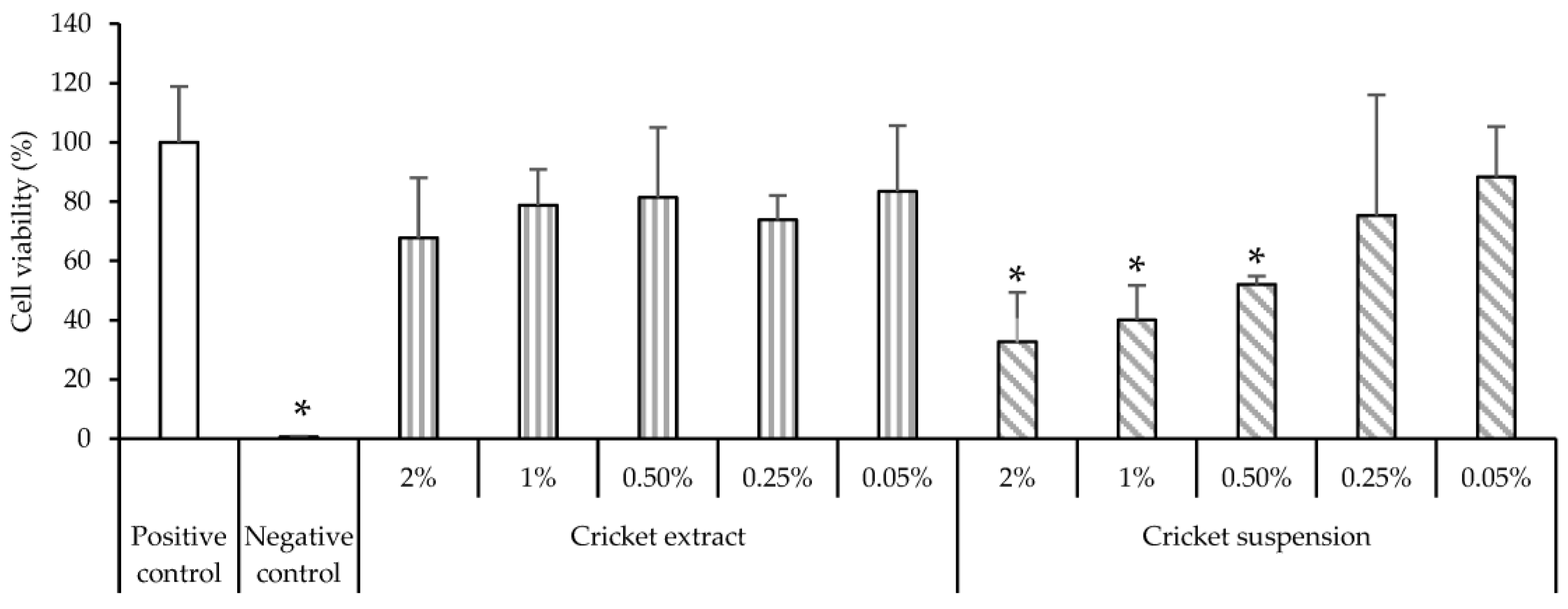
2.7. Cell Biocompatibility
2.8. Cytocompatibility of Macrophages and Pro-Inflammatory Immune Response
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characterization
3.2. Chitin Quantification
3.3. Surface Zeta Potential (ζ)
3.4. Protein Digestability Assay
3.5. Antioxidant Properties
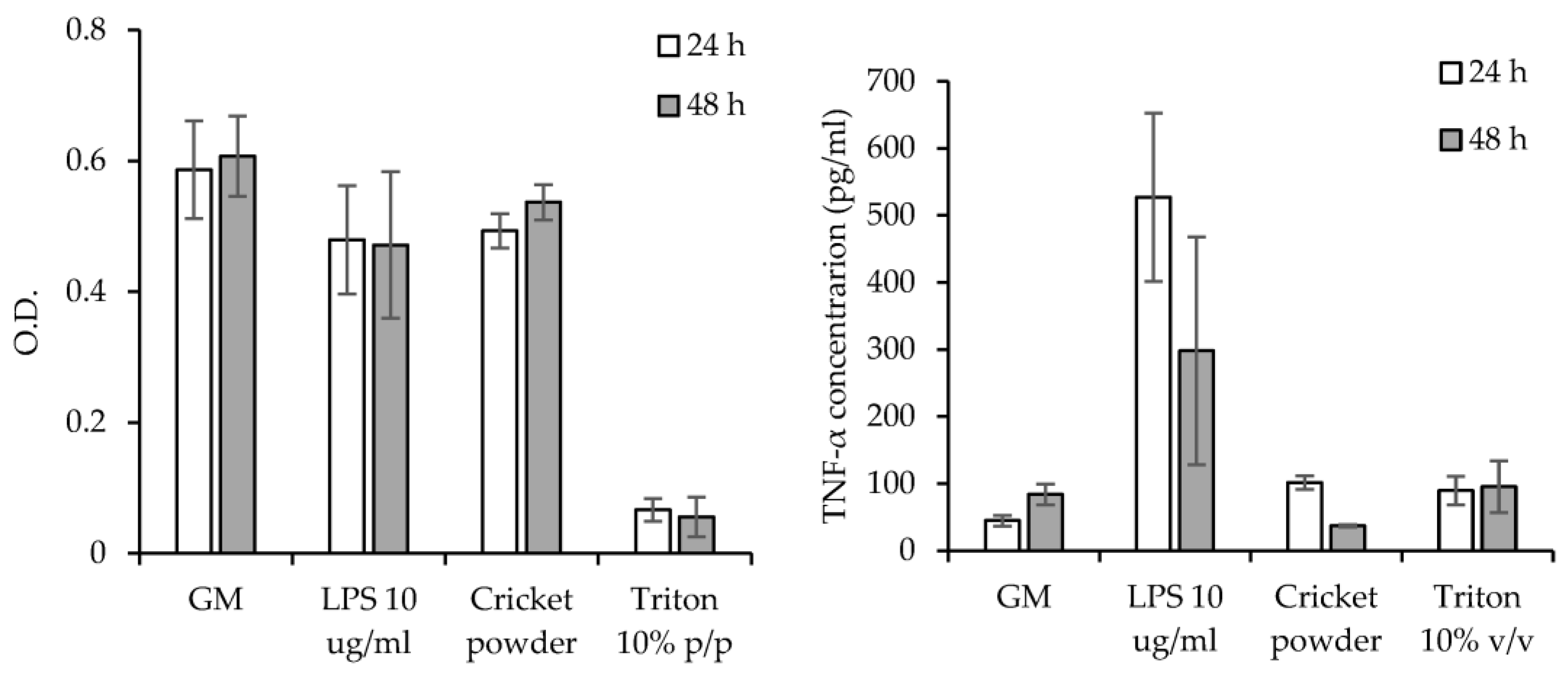
3.6. Cytocompatibility and Pro-Inflammatory Immune Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans—A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006. [Google Scholar]
- Rojas-Downing, M.M.; Pouyan Nejadhashemi, A.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nischalke, S.; Wagler, I.; Tanga, C.; Allan, D.; Phankaew, C.; Ratompoarison, C.; Razafindrakotomamonjy, A.; Kusia, E. How to turn collectors of edible insects into mini-livestock farmers: Multidimensional sustainability challenges to a thriving industry. Glob. Food Secur. 2020, 26, 100376. [Google Scholar] [CrossRef]
- Sorjonen, J.M.; Karhapää, M.; Holm, S.; Valtonen, A.; Roininen, H. Performance of the house cricket (Acheta domesticus) on by-product diets in small-scale production. J. Insects Food Feed 2021, 8, 289–294. [Google Scholar] [CrossRef]
- FAO. Looking at Edible Insects from a Food Safety Perspective. Challenges and Opportunities for the Sector; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M.M.; Frank, J.; Barker, J.H.; Becker, H. Effect of extracts fromthe Chinese and European mole cricket on wound epithelialization and neovascularization: In vivo studies in the hairless mouse ear wound model. Wound Rep. Reg. 2006, 14, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathia, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Tan, H.S.G.; Fischer, A.R.H.; Tinchan, P.; Stieger, M.; Steenbekkers, L.P.A.; van Trijp, H.C.M. Insects as food: Exploring cultural exposure and individual experience asdetermi-nants of acceptance. Food Qual. Prefer. 2015, 42, 78–89. [Google Scholar] [CrossRef]
- Bassett, F.S. Comparison of Functional, Nutritional, and Sensory Properties of Spray-Dried and Oven-Dried Cricket (Acheta domesticus) Powder. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2018. [Google Scholar]
- Deshmukh, R.; Wagh, P.; Naik, J. Solvent evaporation and spray drying technique for micro- and nanospheres/particles preparation: A review. Dry Technol. 2016, 34, 1758–1772. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwalla, T.; Zibek, S. New methods for high-accuracy insect chitin measurement. J. Sci. Food Agric. 2018, 98, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Vigani, B.; Boselli, C.; Icaro Cornaglia, A.; Colombo, D.; Sànchez-Espejo, R.; Del Favero, E.; Mandras, N.; Roana, J.; Cavallo, L.; et al. Smart nano-in-microparticles to tackle bacterial infections in skin tissue engineering. Mater. Today Bio 2022, 16, 100418. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Bianchi, E.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Malavasi, L.; Carzino, R.; Suarato, G.; Sánchez-Espejo, R.; Athanassiou, A.; et al. Maltodextrin-amino acids electrospun scaffolds cross-linked with Maillard-type reaction for skin tissue engineering. Biomater. Adv. 2022, 133, 112593. [Google Scholar] [CrossRef]
- Kaneko, F.; Katagiri, C.; Nagashima, K.; Sazaki, G.; Ikemoto, Y. Cuticular Lipid Topology on Insect Body Surfaces Studied by Synchrotron Radiation FTIR ATR Microspectroscopy. J. Phys. Chem. B 2021, 125, 9757–9767. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Plachý, V.; Božik, M.; Adámková, A.; Vrabec, V. Effect of sex on the nutritional value of house cricket, Acheta domestica L. Food Chem. 2019, 272, 267–272. [Google Scholar] [CrossRef]
- Psarianos, M.; Dimopoulos, G.; Ojha, S.; Cavini, A.C.M.; Bußler, S.; Taoukis, P.; Schlüter, O.K. Effect of pulsed electric fields on cricket (Acheta domesticus) flour: Extraction yield (protein, fat and chitin) and techno-functional properties. Innov. Food Sci. Emerg. Technol. 2022, 76, 102908. [Google Scholar] [CrossRef]
- Araújo, D.; Ferreira, I.C.; Torres, C.A.; Neves, L.; Freitas, F. Chitinous polymers: Extraction from fungal sources, characterization and processing towards value-added applications. J. Chem. Technol. Biotechnol. 2020, 95, 1277–1289. [Google Scholar] [CrossRef] [Green Version]
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional Potential of Selected Insect Species Reared on the Island of Sumatra. Int. J. Environ. Res. Public Health 2017, 14, 521. [Google Scholar] [CrossRef]
- Dong, L.; Ariëns, R.M.C.; Tomassen, M.M.; Wichers, H.J.; Govers, C. In Vitro Studies Toward the Use of Chitin as Nutraceutical: Impact on the Intestinal Epithelium, Macrophages, and Microbiota. Mol. Nutr. Food Res. 2020, 64, e2000324. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. In vitro Protein Digestibility of Selected Seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. The Protein Digestibility–Corrected Amino Acid Score. J. Nutr. 2000, 130, 1865S–1867S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez López, M.M.; Kizlansky, A.; López, L.B. Assessment of protein quality in foods by calculating the amino acids score corrected by digestibility. Nutr. Hosp. 2006, 21, 47–51. [Google Scholar] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wan, S.; Liu, J.; Zou, Y.; Liao, S. Antioxidant Activity and Stability Study of Peptides from Enzymatically Hydrolyzed Male Silkmoth. J. Food Process. Preserv. 2017, 41, e13081. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ICS: Waltham, MA, USA, 2009.
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Scientific Opinion on the safety of frozen and dried formulations from whole house crickets (Acheta domesticus) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 8, e06779. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggeri, M.; Bianchi, E.; Vigani, B.; Sánchez-Espejo, R.; Spano, M.; Totaro Fila, C.; Mannina, L.; Viseras, C.; Rossi, S.; Sandri, G. Nutritional and Functional Properties of Novel Italian Spray-Dried Cricket Powder. Antioxidants 2023, 12, 112. https://doi.org/10.3390/antiox12010112
Ruggeri M, Bianchi E, Vigani B, Sánchez-Espejo R, Spano M, Totaro Fila C, Mannina L, Viseras C, Rossi S, Sandri G. Nutritional and Functional Properties of Novel Italian Spray-Dried Cricket Powder. Antioxidants. 2023; 12(1):112. https://doi.org/10.3390/antiox12010112
Chicago/Turabian StyleRuggeri, Marco, Eleonora Bianchi, Barbara Vigani, Rita Sánchez-Espejo, Mattia Spano, Carlotta Totaro Fila, Luisa Mannina, César Viseras, Silvia Rossi, and Giuseppina Sandri. 2023. "Nutritional and Functional Properties of Novel Italian Spray-Dried Cricket Powder" Antioxidants 12, no. 1: 112. https://doi.org/10.3390/antiox12010112
APA StyleRuggeri, M., Bianchi, E., Vigani, B., Sánchez-Espejo, R., Spano, M., Totaro Fila, C., Mannina, L., Viseras, C., Rossi, S., & Sandri, G. (2023). Nutritional and Functional Properties of Novel Italian Spray-Dried Cricket Powder. Antioxidants, 12(1), 112. https://doi.org/10.3390/antiox12010112










