Molecular and Antioxidant Characterization of Opuntia robusta Fruit Extract and Its Protective Effect against Diclofenac-Induced Acute Liver Injury in an In Vivo Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Sample Preparation
2.3. Phenolic Compound Extraction
2.4. Determination of Total Soluble Phenols and Flavonoids
2.5. Betalain Content
2.6. Determination of Anti-Free Radical and Ferric Reducing Activities
2.7. UPLC-QTOF-MS/MS Analysis
2.7.1. Sample Preparation
2.7.2. Liquid Chromatography and Mass Spectrometry
2.8. Animals
2.9. Experimental Design
2.10. Biomarkers of Oxidative Stress
2.11. Molecular Biomarkers
2.11.1. Quantitative RT-PCR Analysis
2.11.2. Western Blot
2.11.3. Immunohistochemistry (IHC) for Active Caspase-3
2.12. Statistical Analysis
3. Results
3.1. Determination of Antioxidant Properties of O. robusta Fruit Extract
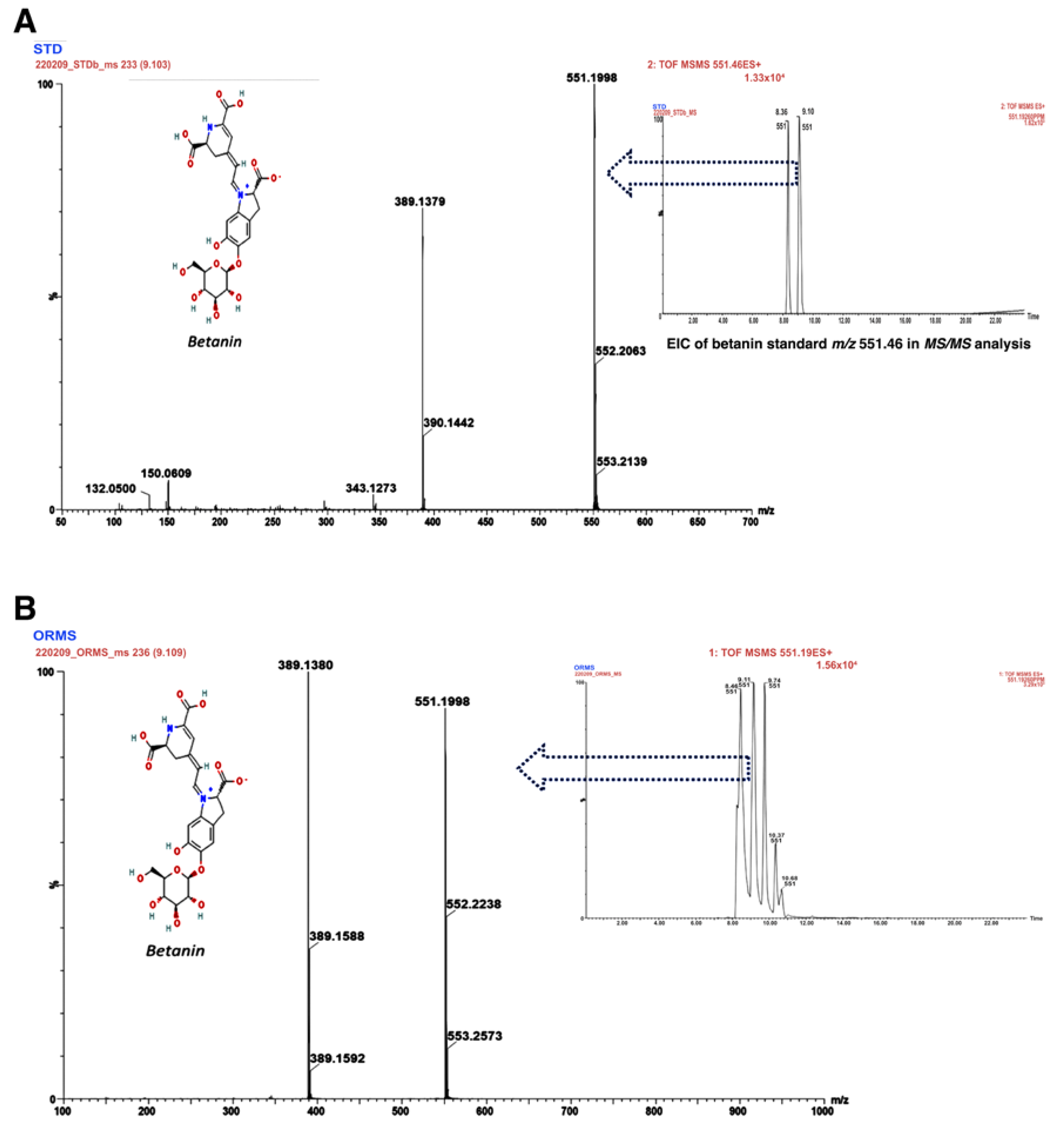
3.2. Identification of the Main Bioactive Compounds in O. robusta Fruit Extract
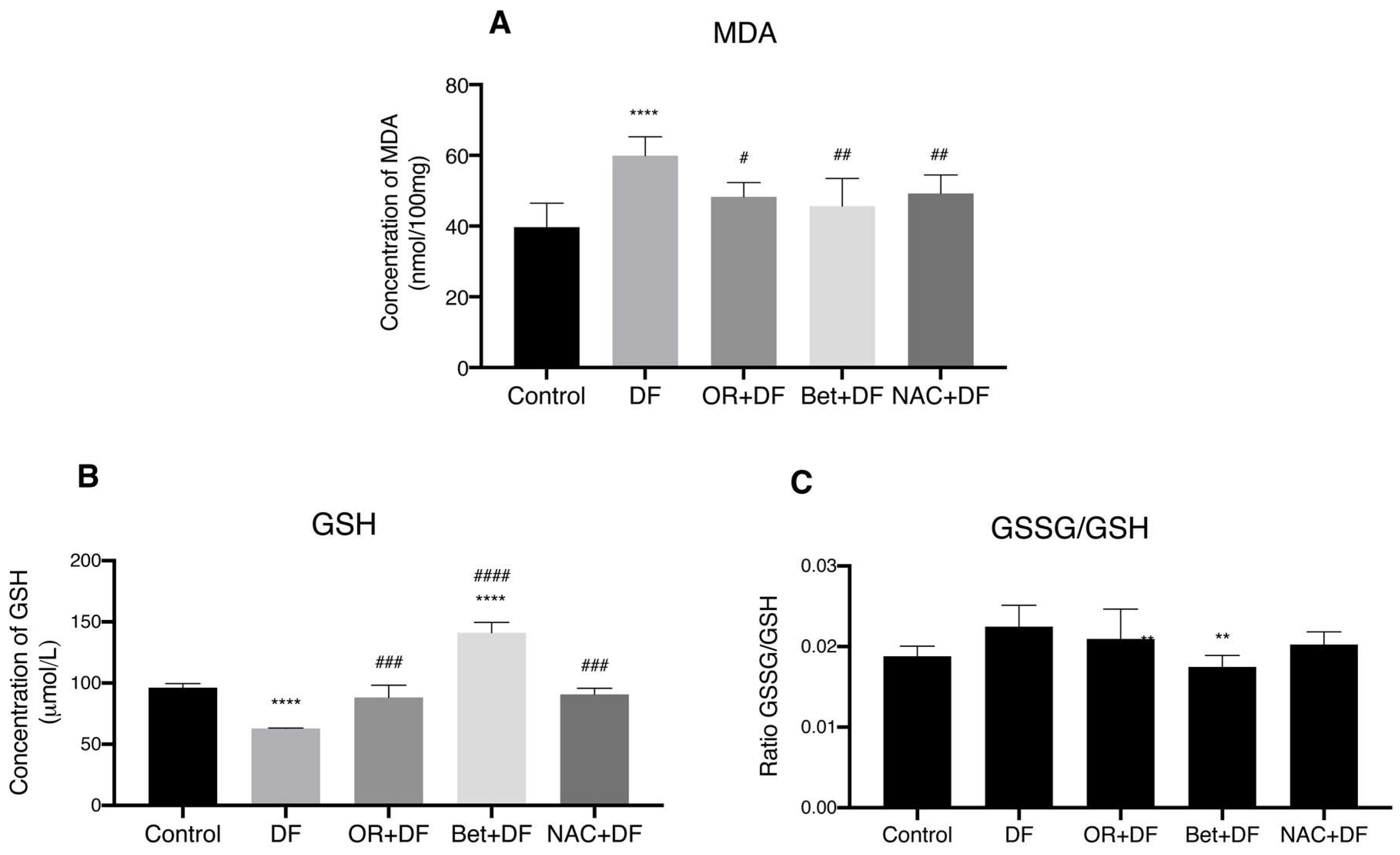
3.3. Biomarkers of Oxidative Stress
3.4. Relative Expression of Genes Related to the Constitutive and Inducible Antioxidant Response
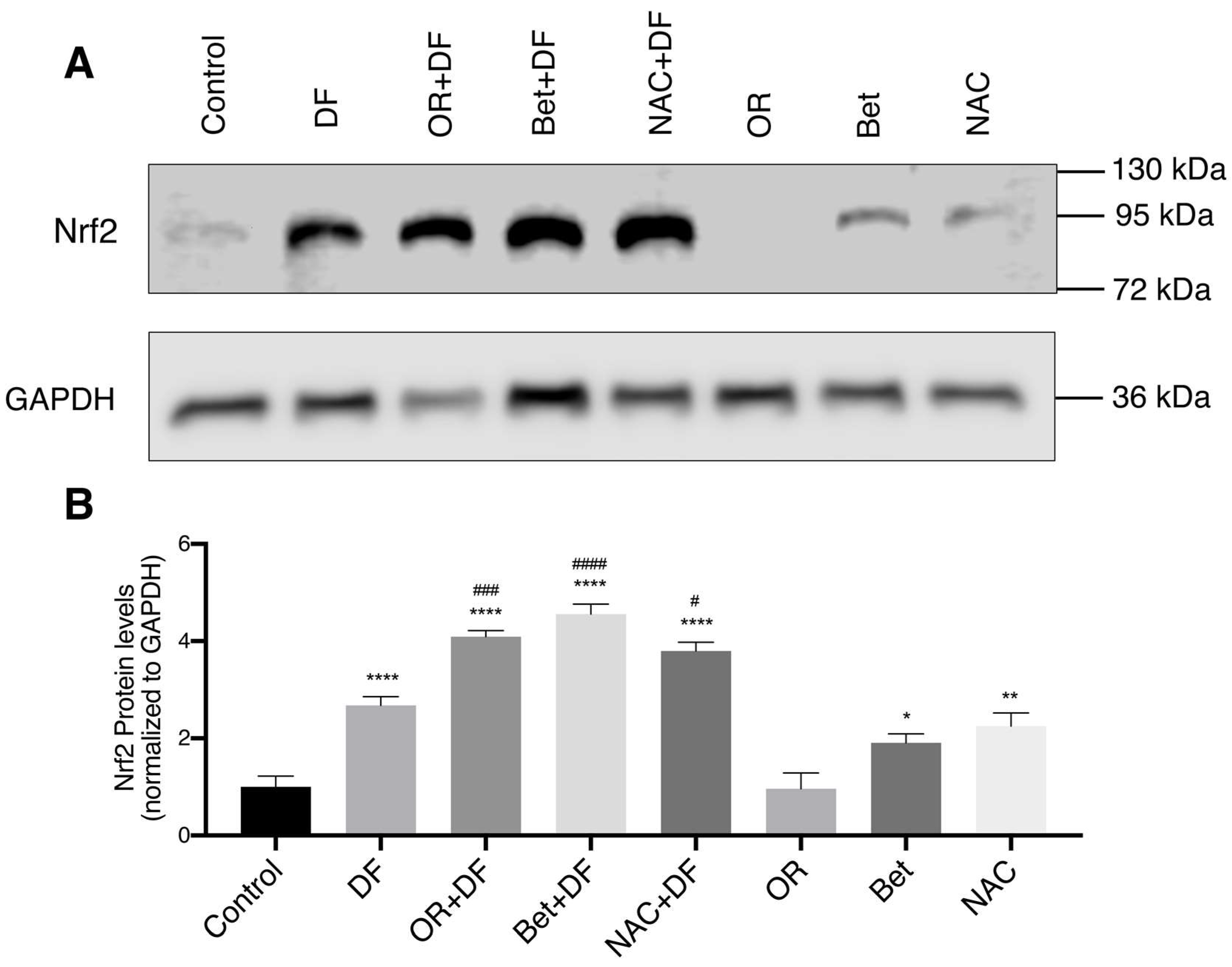
3.5. Nrf2 Protein Expression
3.6. Apoptosis and Active Caspase-3 Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conaculta. People of Corn Mexico’s Ancestral Cuisine; Conaculta: Mexico City, Mexico, 2004. [Google Scholar]
- Zizumbo-Villarreal, D.; Flores-Silva, A.; Colunga-García, P.M. The Archaic Diet in Mesoamerica: Incentive for Milpa Development and Species Domestication. Econ. Bot. 2012, 66, 328–343. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Méndez-Trujillo, V.; Hernández-Delgado, N.C.; Bermúdez-Humarán, L.G.; Reyes-Pavón, D. Looking inside Mexican Traditional Food as Sources of Synbiotics for Developing Novel Functional Products. Fermentation 2022, 8, 123. [Google Scholar] [CrossRef]
- Sipango, N.; Ravhuhali, K.E.; Sebola, N.A.; Hawu, O.; Mabelebele, M.; Mokoboki, H.K.; Moyo, B. Prickly Pear (Opuntia spp.) as an Invasive Species and a Potential Fodder Resource for Ruminant Animals. Sustainability 2022, 14, 3719. [Google Scholar] [CrossRef]
- Illoldi-Rangel, P.; Ciarleglio, M.; Sheinvar, L.; Linaje, M.; Sánchez-Cordero, V.; Sarkar, S. Opuntia in México: Identifying Priority Areas for Conserving Biodiversity in a Multi-Use Landscape. PLoS ONE 2012, 7, e36650. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Santillán, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Mercado-Gonzalez, P.E.; Izquierdo-Vega, J.A.; Vargas-Mendoza, N.; Álvarez-González, I.; Fregoso-Aguilar, T.; Delgado-Olivares, L.; et al. Opuntia genus in Human Health: A Comprehensive Summary on Its Pharmacological, Therapeutic and Preventive Properties. Part 1. Horticulturae 2022, 8, 88. [Google Scholar] [CrossRef]
- Pulido-Hornedo, N.A.; Ventura-Juárez, J.; Guevara-Lara, F.; González-Ponce, H.A.; Sánchez-Alemán, E.; Buist-Homan, M.; Moshage, H.; Martínez-Saldaña, M.C. Hepatoprotective effect of Opuntia robusta fruit biocomponents in a rat model of thioacetamide-induced liver fibrosis. Plants 2022, 10, 1757. [Google Scholar] [CrossRef]
- Castellar, R.; Obón, J.M.; Alacid, M.; Fernández-López, J.A. Color Properties and Stability of Betacyanins from Opuntia Fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Sheth, N.R.; Rathod, I.S.; Suhagia, B.N.; Maradia, R.B. Analysis of betalains from fruits of Opuntia species. Phytochem. Rev. 2012, 12, 35–45. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Vasto, S.; Pintaudi, A.M.; Livrea, M.A.; Allegra, M. Short-term cactus pear [Opuntia ficus-indica (L.) Mill] fruit supplementation ameliorates the inflammatory profile and is associated with improved antioxidant status among healthy humans. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, R.M.; Kritz, H.; Efthimiou, Y.; Stomatopoulos, J.; Sinzinger, H. Effect of prickly pear (Opuntia robusta) on glucose- and lipid-metabolism in non-diabetics with hyperlipidemia-a pilot study. Wien Klin Wochenschr. 2002, 114, 840–846. [Google Scholar]
- Allegra, M.; D’Acquisto, F.; Tesoriere, L.; Attanzio, A.; Livrea, M.A. Pro-oxidant activity of indicaxanthin from Opuntia ficus indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated RAW 264.7 macrophages. Redox Biol. 2014, 2, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.V.T.D.; Baiao, D.D.S.; Ferreira, V.F.; Paschoalin, V.M.F. Betanin as a multipath oxidative stress and inflammation modulator: A beetroot pigment with protective effects on cardiovascular disease pathogenesis. Crit. Rev. Food Sci. Nutr. 2021, 62, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods Hum. Nutr. 2009, 64, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hahm, S.W.; Park, J.; Oh, S.Y.; Lee, C.W.; Park, K.Y.; Kim, H.; Son, Y.S. Anticancer properties of extracts from Opuntia humifusa against human cervical carcinoma cells. J Med. Food. 2015, 18, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; De Cicco, P.; Ercolano, G.; Attanzio, A.; Busà, R.; Cirino, G.; Tesoriere, L.; Livrea, M.A.; Ianaro, A. Indicaxanthin from Opuntia ficus indica (L. Mill) impairs melanoma cell proliferation, invasiveness, and tumor progression. Phytomedicine 2018, 50, 19–24. [Google Scholar] [CrossRef] [Green Version]
- González-Ponce, H.A.; Martínez-Saldaña, M.C.; Tepper, P.G.; Quax, W.J.; Buist-Homan, M.; Faber, K.N.; Moshage, H. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res. Int. 2020, 137, 09461. [Google Scholar] [CrossRef]
- Tsafantakis, N.; Katsanou, E.S.; Kyriakopoulou, K.; Psarou, E.-C.; Raptaki, I.; Skaltsounis, A.L.; Audebert, M.; Machera, K.A.; Fokialakis, N. Comparative UHPLC-HRMS Profiling, Toxicological Assessment, and Protection Against H2O2-Induced Genotoxicity of Different Parts of Opuntia ficus indica. J. Med. Food 2019, 22, 1280–1293. [Google Scholar] [CrossRef]
- Walters, M.; Figueiredo, E.; Crouch, N.; Winter, P.; Smith, G.; Zimmermann, H.; Mashope-Potgieter, B. Naturalised and Invasive Succulents of Southern Africa; SANBI: Cape Town, South Africa, 2011. [Google Scholar]
- Kıvrak, Ş.; Kıvrak, İ.; Karababa, E. Analytical evaluation of phenolic compounds and minerals of Opuntia robusta J.C. Wendl. and Opuntia ficus-barbarica A. Berger. Int. J. Food Prop. 2018, 21, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Stintzing, F.C.; Schieber, A.; Carle, R. Identification of Betalains from Yellow Beet (Beta vulgaris L.) and Cactus Pear [Opuntia ficus-indica (L.) Mill.] by High-Performance Liquid Chromatography−Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2002, 50, 2302–2307. [Google Scholar] [CrossRef]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Conaghan, P.G. A turbulent decade for NSAIDs: Update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol. Int. 2011, 32, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Freytag, A.; Quinzler, R.; Freitag, M.; Bickel, H.; Fuchs, A.; Hansen, H.; Hoefels, S.; König, H.-H.; Mergenthal, K.; Riedel-Heller, S.; et al. Gebrauch und potenzielle Risiken durch nicht verschreibungspflichtige Schmerzmittel. Schmerz 2014, 28, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Grillo, M.P.; Ma, J.; Teffera, Y.; Waldon, D.J. A Novel Bioactivation Pathway for 2-[2-(2,6-Dichlorophenyl)aminophenyl]ethanoic Acid (Diclofenac) Initiated by Cytochrome P450-Mediated Oxidative Decarboxylation. Drug Metab. Dispos. 2008, 36, 1740–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjarnason, I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int. J. Clin. Pract. 2013, 67, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Chalasani, N.; Björnsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Prim. 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoi, D.; Benesic, A.; Pichler, G.; Kulak, N.A.; Bartsch, H.S.; Gerbes, A.L. Proteomics Analysis of Monocyte-Derived Hepatocyte-Like Cells Identifies Integrin Beta 3 as a Specific Biomarker for Drug-Induced Liver Injury by Diclofenac. Front. Pharmacol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Teschke, R. Top-ranking drugs out of 3312 drug-induced liver injury cases evaluated by the Roussel Uclaf Causality Assessment Method. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1169–1187. [Google Scholar] [CrossRef]
- Teschke, R.; Danan, G. Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993–Mid 2020: A Comprehensive Analysis. Medicines 2020, 7, 62. [Google Scholar] [CrossRef]
- Björnsson, E.; Olsson, R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005, 42, 481–489. [Google Scholar] [CrossRef]
- Andrade, R.J.; Lucena, M.I.; Fernández, M.C.; Pelaez, G.; Pachkoria, K.; García-Ruiz, E.; García-Muñoz, B.; González-Grande, R.; Pizarro, A.; Durán, J.A.; et al. Drug-Induced Liver Injury: An Analysis of 461 Incidences Submitted to the Spanish Registry Over a 10-Year Period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef]
- Andrade, R.J.; Lucena, M.I.; Kaplowitz, N.; García-Muņoz, B.; Borraz, Y.; Pachkoria, K.; García-Cortés, M.; Fernández, M.C.; Pelaez, G.; Rodrigo, L.; et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology 2006, 44, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- González-Ponce, H.A.; Rincón-Sánchez, A.R.; Jaramillo-Juárez, F.; Moshage, H. Natural Dietary Pigments: Potential Mediators against Hepatic Damage Induced by Over-The-Counter Non-Steroidal Anti-Inflammatory and Analgesic Drugs. Nutrients 2018, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelsterli, U.A. Diclofenac-induced liver injury: A paradigm of idiosyncratic drug toxicity. Toxicol. Appl. Pharmacol. 2003, 192, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Skonberg, C.; Hansen, S.H. Mitochondrial toxicity of diclofenac and its metabolites via inhibition of oxidative phosphorylation (ATP synthesis) in rat liver mitochondria: Possible role in drug induced liver injury (DILI). Toxicol. Vitr. 2016, 31, 93–102. [Google Scholar] [CrossRef]
- Tang, W. The Metabolism of Diclofenac-Enzymology and Toxicology Perspectives. Curr. Drug Metab. 2003, 4, 319–329. [Google Scholar] [CrossRef]
- González-Ponce, H.A.; Martínez-Saldaña, M.C.; Rincón-Sánchez, A.R.; Sumaya-Martínez, M.T.; Buist-Homan, M.; Faber, K.N.; Moshage, H.; Jaramillo-Juárez, F. Hepatoprotective Effect of Opuntia robusta and Opuntia streptacantha Fruits against Acetaminophen-Induced Acute Liver Damage. Nutrients 2016, 8, 607. [Google Scholar] [CrossRef] [Green Version]
- Villa-Jaimes, G.S.; Aguilar-Mora, F.A.; González-Ponce, H.A.; Avelar-González, F.J.; Martínez Saldaña Ma, C.; Buist-Homan, M.; Moshage, H. Biocomponents from Opuntia robusta and Opuntia streptacantha fruits protect against diclofenac-induced acute liver damage in vivo and in vitro. J. Funct. Foods 2022, 89, 104960. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Phytochemical indicaxanthin suppresses 7-ketocholesterol-induced THP-1 cell apoptosis by preventing cytosolic Ca2+ increase and oxidative stress. Br. J. Nutr. 2013, 110, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Krajka-Kuźniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Esquivel, O.; Moreno, A.O.; Álvarez, V.B.; Dorantes-Álvarez, L.; Giusti, M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- Sumaya-Martínez, M.T.; Cruz-Jaime, S.; Madrigal-Santillán, E.; García-Paredes, J.D.; Cariño-Cortés, R.; Cruz-Cansino, N.; Valadez-Vega, C.; Martinez-Cardenas, L.; Alanís-García, E. Betalain, Acid Ascorbic, Phenolic Contents and Antioxidant Properties of Purple, Red, Yellow and White Cactus Pears. Int. J. Mol. Sci. 2011, 12, 6452–6468. [Google Scholar] [CrossRef]
- Yap, C.H.; Junit, S.M.; Aziz, A.A.; Kong, K.W. Multiple extraction conditions to produce phytochemical- and antioxidant-rich Alternanthera sessilis (red) extracts that attenuate lipid accumulation in steatotic HepG2 cells. Food Biosci. 2019, 32, 100489. [Google Scholar] [CrossRef]
- Ramlagan, P.; Rondeau, P.; Planesse, C.; Neergheen-Bhujun, V.S.; Fawdar, S.; Bourdon, E.; Bahorun, T. Punica granatum L. mesocarp suppresses advanced glycation end products (AGEs)- and H 2 O 2 -induced oxidative stress and pro-inflammatory biomarkers. J. Funct. Foods 2017, 29, 115–126. [Google Scholar] [CrossRef]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Motawi, T.K.; Ahmed, S.A.; El-Boghdady, N.A.; Metwally, N.S.; Nasr, N.N. Protective effects of betanin against paracetamol and diclofenac induced neurotoxicity and endocrine disruption in rats. Biomarkers 2019, 24, 645–651. [Google Scholar] [CrossRef]
- Nissar, A.U.; Farrukh, M.R.; Kaiser, P.J.; Rafiq, R.A.; Afnan, Q.; Bhushan, S.; Adil, H.S.; Subhash, B.C.; Tasduq, S.A. Effect of N-acetyl cysteine (NAC), an organosulfur compound from Allium plants, on experimentally induced hepatic prefibrogenic events in wistar rat. Phytomedicine 2013, 20, 828–833. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Munro, B. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. Pathology 1971, 3, 249. [Google Scholar] [CrossRef]
- Patil, B.S.; Jayaprakasha, G.K.; Murthy, K.N.C.; Vikram, A. Bioactive Compounds: Historical Perspectives, Opportunities, and Challenges. J. Agric. Food Chem. 2009, 57, 8142–8160. [Google Scholar] [CrossRef]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Mabrouki, L.; Boutheina, Z.; Bendhifi, M.; Borgi, M.A. Evaluation of antioxidant capacity, phenol and flavonoid contents of Opuntia streptacantha and Opuntia ficus indica fruits pulp. Nat. Technol. 2015, 13, 2. [Google Scholar]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Bargougui, A.; Tag, H.M.; Bouaziz, M.; Triki, S. Antimicrobial, Antioxidant, Total Phenols and Flavonoids Content of Four Cactus (Opuntia ficus-indica) Cultivars. Biomed. Pharmacol. J. 2019, 12, 1353–1368. [Google Scholar] [CrossRef]
- Shutenko, Z.; Henry, Y.; Pinard, E.; Seylaz, J.; Potier, P.; Berthet, F.; Girard, P.; Sercombe, R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol. 1998, 57, 199–208. [Google Scholar] [CrossRef]
- Miguel, M.G. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Esatbeyoglu, T.; Wagner, A.E.; Schiniatbeyo, V.B.; Rimbach, G. Betanin-A food colorant with biological activity. Mol. Nutr. Food Res. 2014, 59, 36–47. [Google Scholar] [CrossRef]
- Czapski, J.; Mikołajczyk-Bator, K.; Kaczmarek, M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Pol. J. Food Nutr. Sci. 2009, 59, 119–122. [Google Scholar]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Purification and Antiradical Properties of the Structural Unit of Betalains. J. Nat. Prod. 2012, 75, 1030–1036. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant Activity of Betalains from Plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- BButera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, C.; Tesoriere, L.; Allegra, M.; A Livrea, M.; D’Alessio, P. Antioxidant Betalains from Cactus Pear (Opuntia ficus-indica) Inhibit Endothelial ICAM-1 Expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, Betalain Pattern, and Antioxidant Properties of Cactus Pear (Opuntia spp.) Clones. J. Agric. Food Chem. 2004, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Corke, H. Identification and Distribution of Simple and Acylated Betacyanins in the Amaranthaceae. J. Agric. Food Chem. 2001, 49, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Santiago, E.; Yahia, E.M. Identification and Quantification of Betalains from the Fruits of 10 Mexican Prickly Pear Cultivars by High-Performance Liquid Chromatography and Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.V.T.; Dos Santos Baião, D.; De Oliveira Silva, F.; Alves, G.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef] [Green Version]
- Taira, J.; Tsuchida, E.; Katoh, M.C.; Uehara, M.; Ogi, T. Antioxidant capacity of betacyanins as radical scavengers for peroxyl radical and nitric oxide. Food Chem. 2015, 166, 531–536. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Allegra, M.; Gentile, C.; Livrea, M.A. Betacyanins as phenol antioxidants. Chemistry and mechanistic aspects of the lipoperoxyl radical-scavenging activity in solution and liposomes. Free Radic. Res. 2009, 43, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawicki, T.; Topolska, J.; Romaszko, E.; Wiczkowski, W. Profile and Content of Betalains in Plasma and Urine of Volunteers after Long-Term Exposure to Fermented Red Beet Juice. J. Agric. Food Chem. 2018, 66, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Van Kuijk, F.J.G.M.; Sevanian, A.; Handelman, G.J.; Dratz, E.A. A new role for phospholipase A2: Protection of membranes from lipid peroxidation damage. Trends Biochem. Sci. 1987, 12, 31–34. [Google Scholar] [CrossRef]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic. Biol. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef]
- Pehlivan, F. Vitamin C: An Antioxidant Agent. Vitamin C 2017, 2, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Popko, J.L.; Umezawa, T.; Chiang, V.L. 5-Hydroxyconiferyl Aldehyde Modulates Enzymatic Methylation for Syringyl Monolignol Formation, a New View of Monolignol Biosynthesis in Angiosperms. J. Biol. Chem. 2000, 275, 6537–6545. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Mol. Cell. Biochem. 1997, 168, 117–123. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Ali, Y.; Jannat, S.; Jung, H.-A.; Choi, J.-S. Structural Bases for Hesperetin Derivatives: Inhibition of Protein Tyrosine Phosphatase 1B, Kinetics Mechanism and Molecular Docking Study. Molecules 2021, 26, 7433. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.A.; Almela, L.; Obón, J.M.; Castellar, R. Determination of Antioxidant Constituents in Cactus Pear Fruits. Plant Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-H.; Wang, J.-J.; Gu, X.-Z.; Gu, H.-P.; Kang, W.-Y. Antioxidant and a-glucosidase inhibitory activity of red raspberry (Harrywaters) fruits in vitro. Afr. J. Pharm. Pharmacol. 2012, 6, 3118–3123. [Google Scholar] [CrossRef]
- Islas-Flores, H.; Gómez-Oliván, L.M.; Galar-Martínez, M.; Colín-Cruz, A.; Neri-Cruz, N.; García-Medina, S. Diclofenac-induced oxidative stress in brain, liver, gill and blood of common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2013, 92, 32–38. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [Green Version]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free. Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanim, B.; Ahmad, M.; Abdallah, Q.; Qatouseh, L.; Qinna, N. Modulation of NRF2/ARE pathway- and cell death-related genes during drug-induced liver injury. Hum. Exp. Toxicol. 2021, 40, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lin, K.; Zhou, M.; Xiong, Q.; Li, C.; Ru, Q. Polysaccharides from Opuntia milpa alta alleviate alloxan-induced INS-1 cells apoptosis via reducing oxidative stress and upregulating Nrf2 expression. Nutr. Res. 2020, 77, 108–118. [Google Scholar] [CrossRef]
- Alcaraz, M.; Fernandez, P.; Guillen, M. Anti-Inflammatory Actions of the Heme Oxygenase-1 Pathway. Curr. Pharm. Des. 2003, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Choi, A.M. Heme Oxygenase-1: Molecular Mechanisms of Gene Expression in Oxygen-Related Stress. Antioxid. Redox Signal. 2002, 4, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Kundu, J.K.; Li, M.-H.; Na, H.-K.; Cha, Y.-N. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch. Pharm. Res. 2009, 32, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Regulation of redox glutathione levels and gene transcription in lung inflammation: Therapeutic approaches. Free Radic. Biol. Med. 2000, 28, 1405–1420. [Google Scholar] [CrossRef]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Asp. Med. 2009, 30, 86–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-C.; Bratton, S.B. Regulation of the Intrinsic Apoptosis Pathway by Reactive Oxygen Species. Antioxid. Redox Signal. 2013, 19, 546–558. [Google Scholar] [CrossRef] [Green Version]
- Tamura, R.E.; De Vasconcellos, J.F.; Sarkar, D.; Libermann, T.A.; Fisher, P.B.; Zerbini, L.F. GADD45 Proteins: Central Players in Tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, Q.; Shi, C.; Jiao, F.; Gong, Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol. Med. Rep. 2019, 20, 4081–4090. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Li, S.; Song, Z.; Luo, Q.; Zhang, Y.; Wang, H. The Regulatory Roles of Polysaccharides and Ferroptosis-Related Phytochemicals in Liver Diseases. Nutrients 2022, 14, 2303. [Google Scholar] [CrossRef]
- Zheng, K.; Dong, Y.; Yang, R.; Liang, Y.; Wu, H.; He, Z. Regulation of ferroptosis by bioactive phytochemicals: Implications for medical nutritional therapy. Pharmacol. Res. 2021, 168, 105580. [Google Scholar] [CrossRef]
- Chandramouly, G. Gadd45 in DNA Demethylation and DNA Repair. In Gadd45 Stress Sensor Genes; Zaidi, M.R., Liebermann, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 55–67. [Google Scholar] [CrossRef]
- Zhan, Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat. Res. Mol. Mech. Mutagen. 2005, 569, 133–143. [Google Scholar] [CrossRef]


| MMA Extraction | H2O Extraction | |||
|---|---|---|---|---|
| Total Phenols (mg GAE/100 g dw) | Total Flavonoids (mg CatE/100 g dw) | Betacyanins (mg BE/L) | Betaxanthins (mg IxE/L) | Total Betalains (mg betalains/L) |
| 1330 ± 1.7 | 1090 ± 0.9 | 452.2 ± 9.0 | 188.9 ± 3.7 | 641.1 ± 12.7 |
| Antioxidant Activity of MAA Extract (μmol TE/g dw) | ||
|---|---|---|
| DPPH | ABTS●+ | FRAP |
| 30.9 ± 1.3 | 102.6 ± 5.2 | 95.8 ± 7.3 |
| Compound | Formula | Adduct | m/z | Retention Time (min) |
|---|---|---|---|---|
| Oxanes | ||||
| 1,5-Anhydro-D-fructose | C6H10O5 | M+H-H2O | 145.0488 | 0.919 |
| Cinnamic acids and derivatives | ||||
| Cinnamic acid | C9H8O2 | M+H-H2O | 131.0495 | 14.037 |
| Phenylpropanoic acids | ||||
| Hydrocinnamic acid | C9H10O2 | M+H-H2O | 133.0641 | 16.460 |
| Benzene and derivatives | ||||
| 3,4-O-Dimethylgallic acid | C9H10O5 | M+NH4 | 216.0867 | 5.119 |
| Indanes | ||||
| 1-Indanone | C9H8O | M+H | 133.0634 | 11.692 |
| Vitamins | ||||
| Vitamin C | C6H8O6 | M+H | 177.0397 | 5.197 |
| Carboxylic acids and derivatives | ||||
| Betaine | C5H11NO2 | M+H | 118.0821 | 0.919 |
| Betalains | ||||
| Betalamic acid | C9H9NO5 | M+H | 212.055 | 1.048 |
| Indicaxanthin | C14H16N2O6 | M+H | 309.0984 | 8.830 |
| Neobetanin | C24H24N2O13 | M+H | 549.1382 | 11.613 |
| Gomphrenin-I | C24H26N2O13 | M+H | 551.1633 | 10.764 |
| Betanin | C24H26N2O13 | M+H | 551.1498 | 9.887 |
| Compound | Formula | Adduct | m/z | Retention Time (min) |
|---|---|---|---|---|
| Lactones | ||||
| D-Glucaro-1,4-lactone | C6H8O7 | M-H | 191.0223 | 1.616 |
| Cinnamic acids and derivatives | ||||
| 1-O-Sinapoylglucose | C17H22O10 | M-H | 385.1205 | 11.743 |
| Benzene and derivatives | ||||
| Vanillic acid | C8H8O4 | M-H | 167.0382 | 7.284 |
| 2-O-Galloyl-1,4-galactarolactone | C13H12O11 | M+K-2H | 380.9824 | 21.898 |
| Carboxylic acids and derivatives | ||||
| 2-O-Caffeoylhydroxycitric acid | C15H14O11 | M+Na-2H | 391.0282 | 1.486 |
| Organooxygen compounds | ||||
| Cis-5-Caffeoylquinic acid | C16H18O9 | M+K-2H | 391.0376 | 1.099 |
| trans-o-Coumaric acid 2-glucoside | C15H18O8 | M+H-H2O | 309.0968 | 6.176 |
| trans-p-Coumaric acid 4-glucoside | C15H18O8 | M+H-H2O | 309.0969 | 6.176 |
| 6-Caffeoylsucrose | C21H28O14 | M-H | 503.138 | 7.902 |
| Gentiobiosyl 2-methyl-6-oxo-2E,4E-heptadienoate | C20H30O13 | M-H | 477.159 | 9.398 |
| Glucocaffeic acid | C15H18O9 | M-H | 341.0926 | 10.455 |
| Coumarins and derivatives | ||||
| Rutaretin 9-rutinoside | C26H34O14 | M+FA-H | 615.202 | 6.665 |
| Fatty acyls | ||||
| 1-Hexanol arabinosylglucoside | C17H32O10 | M+Na-2H | 417.171 | 7.902 |
| Flavonoids | ||||
| Hesperetin 5-O-glucoside | C22H24O11 | M-H | 463.1337 | 9.038 |
| Phenol lipids | ||||
| Caryoptosidic acid | C16H24O11 | M-H2O-H | 373.119 | 9.578 |
| Hydroxyisonobilin | C20H26O6 | M-H | 361.1717 | 17.079 |
| Furanoid lignans | ||||
| Divanillyltetrahydrofuran ferulate | C30H32O8 | M+FA-H | 565.197 | 16.022 |
| Phenols | ||||
| 5-Hydroxyconiferyl alcohol | C10H12O4 | M-H | 195.0701 | 16.382 |
| Betalains | ||||
| Betalamic acid | C9H9NO5 | 2M-H | 211.0525 | 4.321 |
| Vulgaxanthin I | C14H17N3O7 | M+FA-H | 384.1014 | 6.744 |
| Betanin | C24H26N2O13 | 2M-H | 1099.287 | 7.852 |
| Betalamic acid | C9H9NO5 | M-H2O-H | 192.0344 | 8.909 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Jaimes, G.S.; Moshage, H.; Avelar-González, F.J.; González-Ponce, H.A.; Buist-Homan, M.; Guevara-Lara, F.; Sánchez-Alemán, E.; Martínez-Hernández, S.L.; Ventura-Juárez, J.; Muñoz-Ortega, M.H.; et al. Molecular and Antioxidant Characterization of Opuntia robusta Fruit Extract and Its Protective Effect against Diclofenac-Induced Acute Liver Injury in an In Vivo Rat Model. Antioxidants 2023, 12, 113. https://doi.org/10.3390/antiox12010113
Villa-Jaimes GS, Moshage H, Avelar-González FJ, González-Ponce HA, Buist-Homan M, Guevara-Lara F, Sánchez-Alemán E, Martínez-Hernández SL, Ventura-Juárez J, Muñoz-Ortega MH, et al. Molecular and Antioxidant Characterization of Opuntia robusta Fruit Extract and Its Protective Effect against Diclofenac-Induced Acute Liver Injury in an In Vivo Rat Model. Antioxidants. 2023; 12(1):113. https://doi.org/10.3390/antiox12010113
Chicago/Turabian StyleVilla-Jaimes, Gloria Stephanie, Han Moshage, Francisco Javier Avelar-González, Herson Antonio González-Ponce, Manon Buist-Homan, Fidel Guevara-Lara, Esperanza Sánchez-Alemán, Sandra Luz Martínez-Hernández, Javier Ventura-Juárez, Martín Humberto Muñoz-Ortega, and et al. 2023. "Molecular and Antioxidant Characterization of Opuntia robusta Fruit Extract and Its Protective Effect against Diclofenac-Induced Acute Liver Injury in an In Vivo Rat Model" Antioxidants 12, no. 1: 113. https://doi.org/10.3390/antiox12010113
APA StyleVilla-Jaimes, G. S., Moshage, H., Avelar-González, F. J., González-Ponce, H. A., Buist-Homan, M., Guevara-Lara, F., Sánchez-Alemán, E., Martínez-Hernández, S. L., Ventura-Juárez, J., Muñoz-Ortega, M. H., & Martínez-Saldaña, M. C. (2023). Molecular and Antioxidant Characterization of Opuntia robusta Fruit Extract and Its Protective Effect against Diclofenac-Induced Acute Liver Injury in an In Vivo Rat Model. Antioxidants, 12(1), 113. https://doi.org/10.3390/antiox12010113








