Redox Remodeling by Nutraceuticals for Prevention and Treatment of Acute and Chronic Inflammation
Abstract
:1. Introduction
2. Diabetes as an Archetypical Auto-Inflammatory Disease
3. Experimental Study Models of DM in the Context of Redox and Immunomodulatory Effects of Nutraceuticals
4. M1 Macrophages Initiate and Generate Oxidative Stress and Cell Damage
5. Glucose and Oxidative Stress Determine the Metabolic Signature of T Cell Function
6. Role of Nutraceuticals in Redox-Remodeling, Anti-Inflammatory Response, and Glucose Control to Prevent Diabetes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, R. Lifestyle and Mental Health. Am. Psychol. 2011, 66, 579–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Ah Morano, A.E.; Dorneles, G.P.; Peres, A.; Lira, F.S. The Role of Glucose Homeostasis on Immune Function in Response to Exercise: The Impact of Low or Higher Energetic Conditions. J. Cell Physiol. 2020, 235, 3169–3188. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Defining “Nutraceuticals”: Neither Nutritious nor Pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.J. The Glucose/Insulin System and Vitamin C: Implications in Insulin-Dependent Diabetes Mellitus. J. Am. Coll. Nutr. 1998, 17, 105–108. [Google Scholar] [CrossRef]
- Applegate, E.A.; Grivetti, L.E. Search for the Competitive Edge: A History of Dietary Fads and Supplements. J. Nutr. 1997, 127, 869S–873S. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Debnath, P.; Debnath, P.K. Ayurnutrigenomics: Ayurveda-Inspired Personalized Nutrition from Inception to Evidence. J. Tradit. Complement. Med. 2015, 5, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. Molecular Sciences The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Delmastro, M.M.; Piganelli, J.D. Oxidative Stress and Redox Modulation Potential in Type 1 Diabetes. Clin. Dev. Immunol. 2011, 2011, 593863. [Google Scholar] [CrossRef]
- Primavera, M.; Giannini, C.; Chiarelli, F. Prediction and Prevention of Type 1 Diabetes. Front. Endocrinol. 2020, 11, 248. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2011, 34, S62–S69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incani, M.; Serafini, C.; Satta, C.; Perra, L.; Scano, F.; Frongia, P.; Ricciardi, R.; Ripoli, C.; Soro, M.; Strazzera, A.; et al. High Prevalence of Diabetes-Specific Autoimmunity in First-Degree Relatives of Sardinian Patients with Type 1 Diabetes. Diabetes Metab. Res. Rev. 2017, 33, e2864. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, U.; Liu, X.; Yang, J.; Aronsson, C.A.; Hummel, S.; Butterworth, M.; Lernmark, Å.; Rewers, M.; Hagopian, W.; She, J.X.; et al. Association of Early Exposure of Probiotics and Islet Autoimmunity in the TEDDY Study. JAMA Pediatr. 2016, 170, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [CrossRef]
- Huang, X.; Han, Y.; Jang, K.; Kim, M. Early Prediction for Prediabetes and Type 2 Diabetes Using the Genetic Risk Score and Oxidative Stress Score. Antioxidants 2022, 11, 1196. [Google Scholar] [CrossRef]
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T.; Choi, O.; Luck, H.; Woo, M.; Dunn, S.E.; et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018, 28, 922–934.e4. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Rao, X.; Zhong, J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J. Diabetes Res. 2017, 2017, 6494795. [Google Scholar] [CrossRef] [Green Version]
- Maddaloni, E.; Moretti, C.; Mignogna, C.; Buzzetti, R. Adult-Onset Autoimmune Diabetes in 2020: An Update. Maturitas 2020, 137, 37–44. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Onodera, T.; Fukuda, S.; Kita, S.; Fukuhara, A.; Shimomura, I. SARS-CoV-2 Infection Impairs the Insulin/IGF Signaling Pathway in the Lung, Liver, Adipose Tissue, and Pancreatic Cells via IRF1. Metabolism 2022, 133, 155236. [Google Scholar] [CrossRef]
- Dedrick, S.; Sundaresh, B.; Huang, Q.; Brady, C.; Yoo, T.; Cronin, C.; Rudnicki, C.; Flood, M.; Momeni, B.; Ludvigsson, J.; et al. The Role of Gut Microbiota and Environmental Factors in Type 1 Diabetes Pathogenesis. Front. Endocrinol. 2020, 11, 78. [Google Scholar] [CrossRef]
- Jang, S.M.; Yee, S.T.; Choi, J.; Choi, M.S.; Do, G.M.; Jeon, S.M.; Yeo, J.; Kim, M.J.; Seo, K.; Lee, M.K. Ursolic Acid Enhances the Cellular Immune System and Pancreatic β-Cell Function in Streptozotocin-Induced Diabetic Mice Fed a High-Fat Diet. Int. Immunopharmacol. 2009, 9, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Jayaraman, S. An Update on β-Sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Verhagen, J.; Burton, B.R.; Britton, G.J.; Shepard, E.R.; Anderton, S.M.; Wraith, D.C. Modification of the FoxP3 Transcription Factor Principally Affects Inducible T Regulatory Cells in a Model of Experimental Autoimmune Encephalomyelitis. PLoS ONE 2013, 8, e61334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, S.E.; O’Neill, L.A.J. HIF1α and Metabolic Reprogramming in Inflammation. J. Clin. Investig. 2016, 126, 3699. [Google Scholar] [CrossRef] [Green Version]
- Zirpel, H.; Roep, B.O. Islet-Resident Dendritic Cells and Macrophages in Type 1 Diabetes: In Search of Bigfoot’s Print. Front. Endocrinol. 2021, 12, 290. [Google Scholar] [CrossRef]
- Palmer, C.S.; Ostrowski, M.; Balderson, B.; Christian, N.; Crowe, S.M. Glucose Metabolism Regulates T Cell Activation, Differentiation, and Functions. Front. Immunol. 2015, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Taroncher, M.; Vila-Donat, P.; Tolosa, J.; Ruiz, M.J.; Rodríguez-Carrasco, Y. Biological Activity and Toxicity of Plant Nutraceuticals: An Overview. Curr. Opin. Food Sci. 2021, 42, 113–118. [Google Scholar] [CrossRef]
- Messina, J.P.; Lawrence, D.A. Cell Cycle Progression of Glutathione-Depleted Human Peripheral Blood Mononuclear Cells Is Inhibited at S Phase. J. Immunol. 1989, 143, 1974–1981. [Google Scholar] [CrossRef]
- Muri, J.; Kopf, M. Redox Regulation of Immunometabolism. Nat. Rev. Immunol. 2021, 21, 363–381. [Google Scholar] [CrossRef]
- Do, M.H.; Wang, X.; Zhang, X.; Chou, C.; Nixon, B.G.; Capistrano, K.J.; Peng, M.; Efeyan, A.; Sabatini, D.M.; Li, M.O. Nutrient MTORC1 Signaling Underpins Regulatory T Cell Control of Immune Tolerance. J. Exp. Med. 2020, 217, e20190848. [Google Scholar] [CrossRef]
- Yan, Z.; Banerjee, R. Redox Remodeling as an Immunoregulatory Strategy. Biochemistry 2010, 49, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patwardhan, R.S.; Singh, B.; Pal, D.; Checker, R.; Bandekar, M.; Sharma, D.; Sandur, S.K. Redox Regulation of Regulatory T-Cell Differentiation and Functions. Free Radic. Res. 2020, 54, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Gibson, D.L.; Zhang, Y.; Sham, H.P.; Vallance, B.A.; Dutz, J.P. Gut Barrier Disruption by an Enteric Bacterial Pathogen Accelerates Insulitis in NOD Mice. Diabetologia 2010, 53, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorini, C.; Cosorich, I.; Lo Conte, M.; De Giorgi, L.; Facciotti, F.; Lucianò, R.; Rocchi, M.; Ferrarese, R.; Sanvito, F.; Canducci, F.; et al. Loss of Gut Barrier Integrity Triggers Activation of Islet-Reactive T Cells and Autoimmune Diabetes. Proc. Natl. Acad. Sci. USA 2019, 116, 15140–15149. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia Muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 3520. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, J.C.; Im, S.H.; Lee, M.S. Amelioration of Autoimmune Diabetes of NOD Mice by Immunomodulating Probiotics. Front. Immunol. 2020, 11, 1832. [Google Scholar] [CrossRef]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut Microbial Metabolites Limit the Frequency of Autoimmune T Cells and Protect against Type 1 Diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 2702. [Google Scholar] [CrossRef]
- Bi, X.; Li, F.; Liu, S.; Jin, Y.; Zhang, X.; Yang, T.; Dai, Y.; Li, X.; Zhao, A.Z. ω-3 Polyunsaturated Fatty Acids Ameliorate Type 1 Diabetes and Autoimmunity. J. Clin. Investig. 2017, 127, 1757–1771. [Google Scholar] [CrossRef]
- Gülden, E.; Wong, F.S.; Wen, L. The Gut Microbiota and Type 1 Diabetes. Clin. Immunol. 2015, 159, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal Mucosa of Patients With Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef] [Green Version]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut Microbiota in Children with Type 1 Diabetes Differs from That in Healthy Children: A Case-Control Study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 2022, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; la Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2020, 25, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Orescanin-Dusic, Z.; Blagojevic, D.; Stosic-Grujicic, S.; Tzakos, A.G.; Stojanovic, I. Methanolic Extract of Origanum Vulgare Ameliorates Type 1 Diabetes through Antioxidant, Anti-Inflammatory and Anti-Apoptotic Activity. Br. J. Nutr. 2015, 113, 770–782. [Google Scholar] [CrossRef] [Green Version]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia Melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.S.; Lee, E.B.; Lee, S.J.; Lee, S.P.; Boby, N.; Suk, K.; Birhanu, B.T.; Park, S.C. Aronia Melanocarpa Extract Fermented by Lactobacillus Plantarum EJ2014 Modulates Immune Response in Mice. Antioxidants 2021, 10, 1276. [Google Scholar] [CrossRef]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia Melanocarpa Juice as Part of the Dietary Regimen in Patients with Diabetes Mellitus. Folia Med. 2002, 44, 20–23. [Google Scholar]
- Tasic, N.; Jakovljevic, V.L.J.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black Chokeberry Aronia Melanocarpa Extract Reduces Blood Pressure, Glycemia and Lipid Profile in Patients with Metabolic Syndrome: A Prospective Controlled Trial. Mol. Cell Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norris, J.M.; Yin, X.; Lamb, M.M.; Barriga, K.; Seifert, J.; Hoffman, M.; Orton, H.D.; Barón, A.E.; Clare-Salzler, M.; Chase, H.P.; et al. Omega-3 Polyunsaturated Fatty Acid Intake and Islet Autoimmunity in Children at Increased Risk for Type 1 Diabetes. JAMA 2007, 298, 1420–1428. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, W.; Hong, H.J.; Guan, J.; Kim, D.G.; Yang, E.J.; Koh, G.; Park, D.; Han, C.H.; Lee, Y.J.; Lee, D.H. Resveratrol Improves Insulin Signaling in a Tissue-Specific Manner under Insulin-Resistant Conditions Only: In Vitro and In Vivo Experiments in Rodents. Metabolism 2012, 61, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [Green Version]
- Sartore, G.; Ragazzi, E.; Antonello, G.; Cosma, C.; Lapolla, A. Effect of a New Formulation of Nutraceuticals as an Add-On to Metformin Monotherapy for Patients with Type 2 Diabetes and Suboptimal Glycemic Control: A Randomized Controlled Trial. Nutrients 2021, 13, 2373. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Bae, J.S. Anti-Inflammatory Effects of Aspalathin and Nothofagin from Rooibos (Aspalathus linearis) In Vitro and In Vivo. Inflammation 2015, 38, 1502–1516. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Martin, S. Health Effects of Coffee: Mechanism Unraveled? Nutrients 2020, 12, 1842. [Google Scholar] [CrossRef]
- Masilamani, M.; Wei, J.; Sampson, H.A. Regulation of the Immune Response by Soybean Isoflavones. Immunol. Res. 2012, 54, 95–110. [Google Scholar] [CrossRef]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and Cancer: Mechanisms of Action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. In Vitro Evaluation of the Antioxidant, Cytoprotective, and Antimicrobial Properties of Essential Oil from Pistacia Vera L. Variety Bronte Hull. Int. J. Mol. Sci. 2017, 18, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santarsiero, A.; Onzo, A.; Pascale, R.; Acquavia, M.A.; Coviello, M.; Convertini, P.; Todisco, S.; Marsico, M.; Pifano, C.; Iannece, P.; et al. Pistacia Lentiscus Hydrosol: Untargeted Metabolomic Analysis and Anti-Inflammatory Activity Mediated by NF-κ B and the Citrate Pathway. Oxid. Med. Cell Longev. 2020, 2020, 4264815. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Zirak, M.R.; Rezaee, S.A.; Karimi, G.; Hosseinzadeh, H. Immunoregulatory and Anti-Inflammatory Properties of Crocus Sativus (Saffron) and Its Main Active Constituents: A Review. Iran. J. Basic Med. Sci. 2019, 22, 334. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Meira, C.S.; do Espírito Santo, R.F.; dos Santos, T.B.; Orge, I.D.; Silva, D.K.C.; Guimarães, E.T.; de Aragão França, L.S.; Barbosa-Filho, J.M.; Moreira, D.R.M.; Soares, M.B.P. Betulinic Acid Derivative BA5, a Dual NF-KB/Calcineurin Inhibitor, Alleviates Experimental Shock and Delayed Hypersensitivity. Eur. J. Pharmacol. 2017, 815, 156–165. [Google Scholar] [CrossRef]
- Nair, R.V.R.; Jayasree, D.V.; Biju, P.G.; Baby, S. Anti-Inflammatory and Anticancer Activities of Erythrodiol-3-Acetate and 2,4-Di-Tert-Butylphenol Isolated from Humboldtia Unijuga. Nat. Prod. Res. 2018, 34, 2319–2322. [Google Scholar] [CrossRef]
- Saito-Sasaki, N.; Sawada, Y.; Nakamura, M. Maresin-1 and Inflammatory Disease. Int. J. Mol. Sci. 2022, 23, 1367. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Hu, K.; Xu, Q.; Wen, X.; Cheng, K.; Chen, C.; Yuan, H.; Dai, L.; Sun, H. Synthesis and Anti-Inflammatory Activity of Saponin Derivatives of δ-Oleanolic Acid. Eur. J. Med. Chem. 2021, 209, 112932. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Toledo, E.; Gaforio, J.J. Squalene Stimulates a Key Innate Immune Cell to Foster Wound Healing and Tissue Repair. Evid. Based Complement. Alternat. Med. 2018, 2018, 9473094. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Kusano, K.; Ebara, S.; Tachibana, K.; Nishimura, T.; Sato, S.; Kuwaki, T.; Taniyama, T. A Potential Therapeutic Role for Small Nonpeptidyl Compounds That Mimic Human Granulocyte Colony-Stimulating Factor. Blood 2004, 103, 836–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canani, R.B.; di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Fontenelle, B.; Gilbert, K.M. N-Butyrate Anergized Effector CD4+ T Cells Independent of Regulatory T Cell Generation or Activity. Scand. J. Immunol. 2012, 76, 457–463. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Yu, M.; Darby, T.M.; Vaccaro, C.; Li, J.Y.; Owens, J.A.; Hsu, E.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131.e7. [Google Scholar] [CrossRef] [Green Version]
- Häselbarth, L.; Ouwens, D.M.; Teichweyde, N.; Hochrath, K.; Merches, K.; Esser, C. The Small Chain Fatty Acid Butyrate Antagonizes the TCR-Stimulation-Induced Metabolic Shift in Murine Epidermal Γδ T Cells. EXCLI J. 2020, 19, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Masuelli, L.; Benvenuto, M.; Focaccetti, C.; Ciuffa, S.; Fazi, S.; Bei, A.; Miele, M.T.; Piredda, L.; Manzari, V.; Modesti, A.; et al. Targeting the Tumor Immune Microenvironment with “Nutraceuticals”: From Bench to Clinical Trials. Pharmacol. Ther. 2021, 219, 107700. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune Modulation by Curcumin: The Role of Interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, Against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in Saponin-Based Adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [Green Version]
- Mahn, A.; Castillo, A. Potential of Sulforaphane as a Natural Immune System Enhancer: A Review. Molecules 2021, 26, 752. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, S.; Yang, P.; Guo, H.; Zhang, S.; Zhang, X.; Zhu, F.; Li, Y.; Xiang, S.; Yang, P.; Zhang, S.; et al. Green Tea Makes Polyphenol Nanoparticles with Radical-Scavenging Activities. Macromol. Rapid Commun. 2017, 38, 1700446. [Google Scholar] [CrossRef]
- Fu, Q.Y.; Li, Q.S.; Lin, X.M.; Qiao, R.Y.; Yang, R.; Li, X.M.; Dong, Z.B.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; et al. Antidiabetic Effects of Tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugham, L.N.; Castellani, M.L.; Salini, V.; Falasca, K.; Vecchiet, J.; Conti, P.; Petrarca, C. Relevance of Plant Lectins in Human Cell Biology and Immunology. Riv. Biol. 2006, 99, 227–249. [Google Scholar] [PubMed]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.C.; Carle, R.; Muñoz, E. Chokeberry (Aronia Melanocarpa (Michx.) Elliot) Concentrate Inhibits NF-ΚB and Synergizes with Selenium to Inhibit the Release of pro-Inflammatory Mediators in Macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef]
- Kelepouri, D.; Mavropoulos, A.; Bogdanos, D.P.; Sakkas, L.I. The Role of Flavonoids in Inhibiting Th17 Responses in Inflammatory Arthritis. J. Immunol. Res. 2018, 2018, 9324357. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L. Metabolism as a Driver of Immunity. Nat. Rev. Immunol. 2021, 21, 618–619. [Google Scholar] [CrossRef]
- MacIver, N.J.; Jacobs, S.R.; Wieman, H.L.; Wofford, J.A.; Coloff, J.L.; Rathmell, J.C. Glucose Metabolism in Lymphocytes Is a Regulated Process with Significant Effects on Immune Cell Function and Survival. J. Leukoc. Biol. 2008, 84, 949. [Google Scholar] [CrossRef]
- Allegra, A.; Petrarca, C.; di Gioacchino, M.; Casciaro, M.; Musolino, C.; Gangemi, S. Modulation of Cellular Redox Parameters for Improving Therapeutic Responses in Multiple Myeloma. Antioxidants 2022, 11, 455. [Google Scholar] [CrossRef]
- Sheu, S.S.; Nauduri, D.; Anders, M.W. Targeting Antioxidants to Mitochondria: A New Therapeutic Direction. Biochim. Biophys. Acta 2006, 1762, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A. A Clinical Perspective of IL-1β as the Gatekeeper of Inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Kroger, C.J.; Clark, M.; Tisch, R.M. Evolving Antibody Therapies for the Treatment of Type 1 Diabetes. Front. Immunol. 2021, 11, 3949. [Google Scholar] [CrossRef]
- Petrarca, C.; Lanuti, P.; Petrosino, M.I.; di Pillo, S.; Mistrello, G.; Compalati, E.; Otzuki, T.; Marchisio, M.; Pierdomenico, L.; Paganelli, R.; et al. Peripheral Effector Memory Regulatory T Cells Are Incremented and Functionally Enhanced in Successful Mite Monomeric Allergoid Sublingual Immunotherapy. Allergy 2021, 76, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- di Giampaolo, L.; Zaccariello, G.; Benedetti, A.; Vecchiotti, G.; Caposano, F.; Sabbioni, E.; Groppi, F.; Manenti, S.; Niu, Q.; Poma, A.M.G.; et al. Genotoxicity and Immunotoxicity of Titanium Dioxide-Embedded Mesoporous Silica Nanoparticles (TiO2@msn) in Primary Peripheral Human Blood Mononuclear Cells (Pbmc). Nanomaterials 2021, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Philippou, E.; Petersson, S.D.; Rodomar, C.; Nikiphorou, E. Rheumatoid Arthritis and Dietary Interventions: Systematic Review of Clinical Trials. Nutr. Rev. 2021, 79, 410–428. [Google Scholar] [CrossRef]
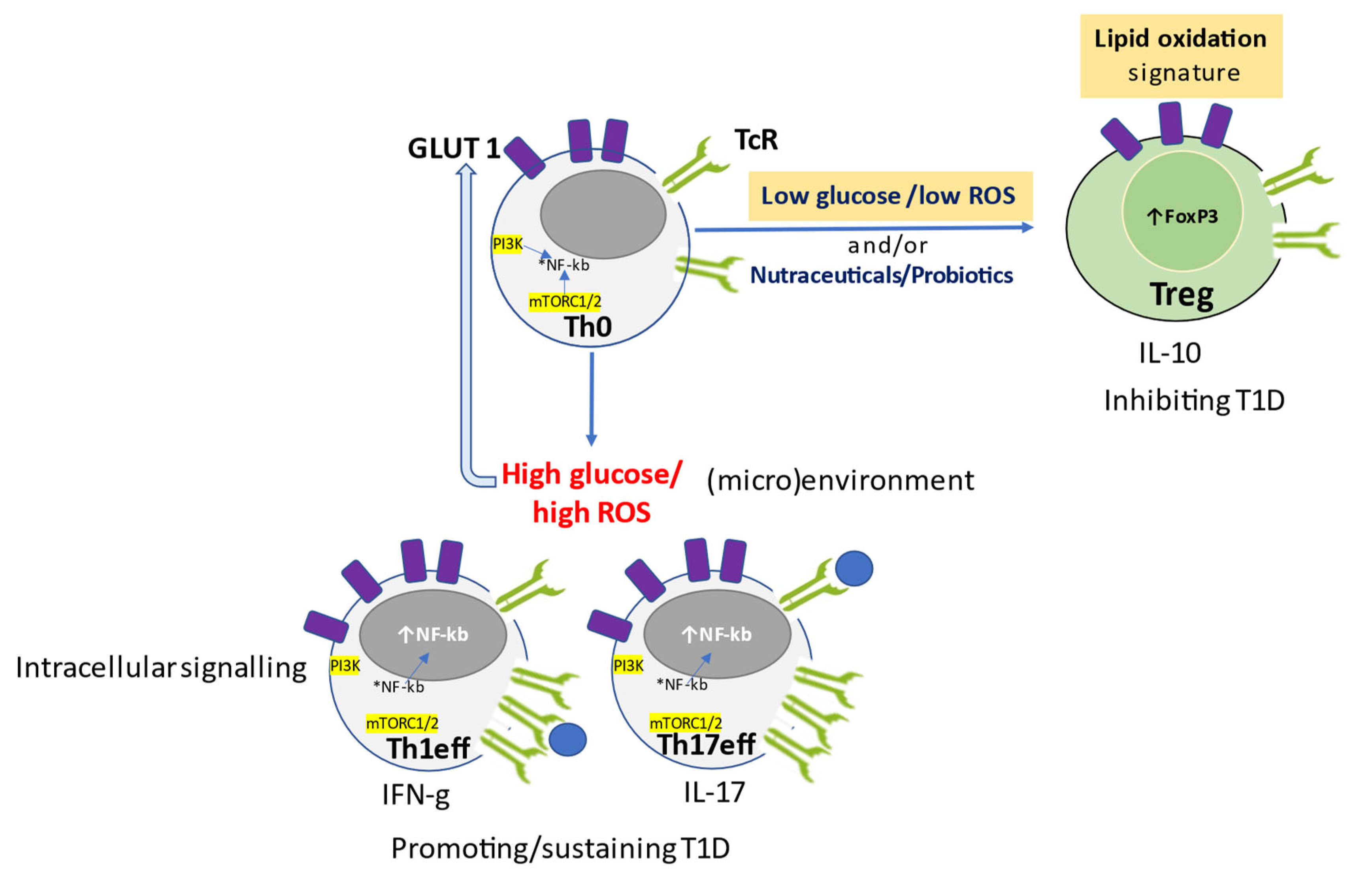

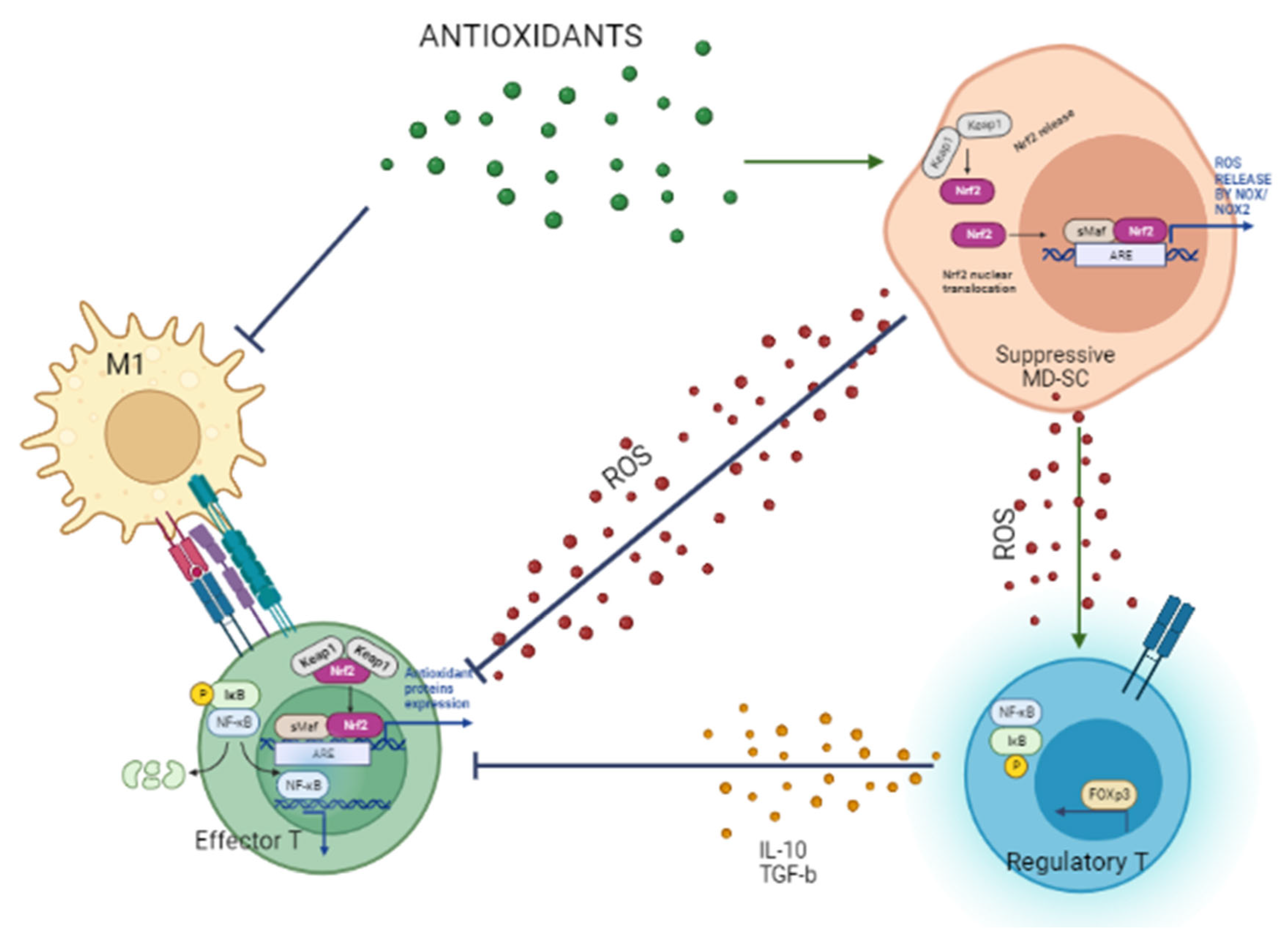
| Immune Cell Type | Subset | Metabolic Pathway | Redox Potential (Extracellular) | Redox Potential (Intracellular) | ROS-Sensor Genes | Antioxidant Enzymes | Nutraceuticals |
|---|---|---|---|---|---|---|---|
| MACROPHAGES (innate immunity) | M1 |
| Inflammation/ ROS | High RNS |
|
|
|
| M2 |
| No influence | No free radicals | Itaconate (anti-inflammatory metabolite) | None |
| |
| T CELLS (adaptive immunity) | NAIVE (Th0) |
| Quiescent status | Not described, yet | |||
| MEMORY (mTh1) |
| No influence | Low ROS |
|
| Not described, yet | |
| EFFECTOR (helper, Th1eff) |
| Low redox potential ▲ GSH: GSSG |
| ||||
| REGULATORY (Treg) |
| High redox potential ▼GSH: GSSG | High ROS |
| NADP |
| |
| Anti-Diabetic and Immunomodulatory Activities | |||||||
|---|---|---|---|---|---|---|---|
| Compound/ Active Component | Natural (Edible) Source | Study Model Cells In Vitro, Animal, Clinical, Epidemiol. | Glycemia | Oxidative Stress | Proinflammatory | Anti-Inflammatory/ Regulatory | References |
| Epicatechins (ECG, EGCG) | Tea (green, black, others) Leaves | NOD | Hypoglycemic | Antioxidant | NOT DESCRIBED | ▼ M1 ▲ M2 ▲ Treg ▲ IL-10 | [44,45,46] |
| Methanolic oregano extract (MOE) | Origanum vulgare | NOD | Hypoglycemic | Antioxidant | NOT FOUND | ▲ Treg ▲ Th2EFF ▼ Th17 EFF | [47] |
| Polyphenols | Aronia Melanocarpa Chockeberry | Human monocytes SZT | Hypoglycemic | Antioxidant | NOT DESCRIBED | ▼ NF-κB ▼ IL-6, IL-8, TNF-α ▼ PGE2 ▼ actMo | [48,49,50,51] |
| DHA/EPA/ARA (Polyunsaturated fatty acids omega-3/-6, PUFAs) (potential therapeutic modality) | Olive oil Fish Cod liver oil Red meat | Cell line Lymphocytes ex vivo TD1 patients DAISY epidemiological study (longitudinal) NOD Hu tumor grafts in mice | ▼ T1D Incidence, severity (long-term assumption) | Antioxidant | NOT DESCRIBED | ▼ NF-κB ▼ IL-6, IL-8, TNF-α, IFN-γ ▼ PGE2 ▼ M1 ▼ Th1EFF / Th17EFF ▲ IL-10, IL-4 ▼ M2 ▲ Th2EFF ▲ Treg | [40,52,53] |
| Resveratrol | Grapes Red wine | Cell line NOD | ▼ T1D incidence, severity | Antioxidant | NOT DESCRIBED | ▼ NF-κB ▼ Th17EFF ▼ TNF-α, IL-6, IL-1β, IL-17 ▲ IL-10 | [45,54,55] |
| Ursolic acid (UA) | Plants Epicuticular wax | SZT | Hypoglycemic | Antioxidant | NOT FOUND | ▼ NF-κB ▼ Th1EFF ▼ Th2EFF | [21,56] |
| Berberin (isoquinoline alkaloid) | Berberis spp. | Cell line NOD / Clinical Trial (combination treatment with metformin) | Hypoglycemic | Antioxidant | NOT DESCRIBED | Unknown | [57] |
| Aspalathin | Aspalathus linearis Rooibos | Cell line NOD | unknown | Antioxidant | NOT DESCRIBED | ▼ NF-κB ▼ TNF-α, IL-6 | [58] |
| Polyphenols, phenolic acids | Coffee beans | Prospective human cohort studies | normal | Weak radical scavenger ▼ T2D risk of disease ▲ Nrf2⟶antioxidant enzymes (+detox, repair) | NOT DESCRIBED | LOW activity | [59] |
| Isoflavons | Soybeans | Immune cells (cell line) Hu tumor grafts in mice | unknown | Antioxidant | NOT FOUND | ▼ NF-κB ▼ IL-6 ▼ M1 | [60] |
| Mangiferin | Mango tree | Hu tumor grafts in mice | unknown | Antioxidant | NOT DESCRIBED | ▼ M1 ▼ NF-κB | [61] |
| Pistacia (oil/hydrosol) | Pistacia vera/P. lentiscus Aromatic tree | Cell line | unknown | Antioxidant | NOT FOUND | ▼ NF-κB (citrate) ▼ TNFα, IL-6, IL-1β | [62,63] |
| Saffron | Crocus sativus | PBMC ex vivo Hu lymphocytes ex vivo NOD Other murine models | unknown | Antioxidant | NOT FOUND | ▲ IL-10 ▲ M2 ▼ TNF-α, IL-6 and IL-1β | [64] |
| Thymol | Lippia thymoides Essential oil | Cell line Hu tumor grafts in mice | unknown | Antioxidant | NOT FOUND | ▼ TNFα, IL-6 ▲ Th2EFF | [65] |
| Betulinic acid (pentacyclic triterpene) | Lycopus lucidus | Immune cells (cell line) Hu tumor grafts in mice | unknown | unknown | NOT DESCRIBED | ▼ TNF-α, IL-2, IFN-γ ▼ Th1EFF ▲ Th2EFF | [66] |
| Erythrodiol (triterpene) | Humboldtia unijuga | Immune cells (cell line) | unknown | unknown | NOT DESCRIBED | ▼ Th1EFF ▼TNFα, IL-6 and IL-1β ▲ Th2EFF | [67] |
| Maresin 1 (docosahexaenoic acid, DHA-derived) | Macrophage pro-resolving bioactive lipid mediator | Immune cells (cell line) Hu tumor grafts in mice | unknown | unknown | NOT DESCRIBED | ▲ M2 ▲ Treg ▲ IL10 ▼ M1 ▼Th1EFF/Th2EFF/Th17EFF | [68] |
| Oleanolic acid | Medicinal herbs | Immune cells (cell line) NOD | unknown | unknown | NOT DESCRIBED | ▲ AMPK | [69] |
| Squalene (2,6,10,15,19,23-hexamethyl-2,6,10,14,18,20-tetracosahexane) | Virgin olive oil (VOO) (non-saponifiable fraction) | Hu tumor grafts in mice | unknown | unknown | NOT FOUND | ▼ M1 ▲ Th2EFF ▲ IL-10, IL-4, IL-13 | [70] |
| Quercetin | Grapes Onion | Cell line NOD | Hypoglycemic | Antioxidant | ▲ NK | ▼ DC1 ▼ IL-6, IL-1β | [71] |
| Adenosine-based (non-peptidyl compounds) | Fungi Plants | NOD | unknown | ROS scavengers | NOT DESCRIBED | ▲ DCreg ▲ Treg ▲ DC2 ▲ Th2EFF ▼ Th1EFF | [72] |
| Bacteroides, Lactobacilli, Bifidobacteria and certain Clostridia | Probiotics (supplements, food microbic flora) | NOD / Allergic inflam. in BALB/c mice Clinical Study TEDDY | unknown | unknown | NOT FOUND (described for other strains and other inflammatory settings) | ▼Insulitis ▼ Th1EFF ▼ Th2EFF ▲ Treg ▲ IL-10 ▼ Eosinophils ▼ Th17EFF | [10,37,39] |
| Acetate Butyrate Propionate (SCFA) | Postbiotics produced by fermentation of indigested carbo by probiotics (supplements, food microbic flora) | 7–17 DETC murine cell line (activated proinflammatory innate epidermal γδT cells) Skin NOD Obese mice* | *Butyrate paradox ▲ insulin (resistance) ▲ glucose uptake ▲FAS | ▼Glycolisys/ OXPHOS▲ Antioxidant | NOT DESCRIBED | ▼ Th1EFF ▼ IFN-γ ▲ Treg ▲ IL-10 ▲CD69 immunoregulatory surface receptor | [73,74,75,76,77] |
| β-sitosterol | Nuts and other seeds Legumes Virgin olive oil (VOO) | High fat diet + sucrose-induced T2D in rats Hu tumor grafts in mice | Hypoglycemic | Antioxidant | ▲ Th1EFF | ▼ M1 ▲ M2 ▲ Th2EFF ▼ Th2EFF | [22,78] |
| Curcumin | Curcuma longa | Hu tumors grafts in mice NOD | unknown | Antioxidant | ▲ M1 ▲ Th1EFF | ▼ M2 ▲ Treg ▲ IL-10 ▼ Th1EFF ▲ Th2EFF | [45,79,80] |
| δ-Oleanolic acid (pentacyclic triterpenoid) Other saponins (adjuvants) | Plants (saponins) | Macrophages Activated T and B cells Splenocytes Hu tumor grafts in mice Clinical trials | unknown | Antioxidant | ▲ Th1EFF ▲ Fc receptor ▲ IgA, G1, G2a, G2b ▲ IL-1, IL-2, IL-12 | ▼ M1 ▼ IL-6, IL-8, TNF-α | [69,81] |
| Spirulina | Arthrospira platensis Arthrospira maxima Cyanobacteriaceae (High protein supplement) | Cell line NOD | unknown | Antioxidant | ▲ IL-1β, IL-4, and INF-γ | ▼TNF-α, IL-6, IL-1β | [82] |
| Sulphoraphane (SFN) | Brassicaceae spp. Vegetables | Hu tumor grafts in mice | Hypoglycemic | unknown | ▲ Th1EFF | ▼ M1 ▲ M2 ▼ Th1EFF ▼ Th2EFF | [83] |
| Vitamin C | Fruits, vegetables (micronutrient) | Immune Cells (cell line) | unknown | Antioxidant | ▲ Th1EFF ▲ Neutrophils | unknown | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrarca, C.; Viola, D. Redox Remodeling by Nutraceuticals for Prevention and Treatment of Acute and Chronic Inflammation. Antioxidants 2023, 12, 132. https://doi.org/10.3390/antiox12010132
Petrarca C, Viola D. Redox Remodeling by Nutraceuticals for Prevention and Treatment of Acute and Chronic Inflammation. Antioxidants. 2023; 12(1):132. https://doi.org/10.3390/antiox12010132
Chicago/Turabian StylePetrarca, Claudia, and Davide Viola. 2023. "Redox Remodeling by Nutraceuticals for Prevention and Treatment of Acute and Chronic Inflammation" Antioxidants 12, no. 1: 132. https://doi.org/10.3390/antiox12010132








