NADPH Oxidase-Mediated Testicular Oxidative Imbalance Regulates the TXNIP/NLRP3 Inflammasome Axis Activation after Ischemia Reperfusion Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rat Model of tIRI and Apocynin Treatment
2.2. Histological Analysis
2.3. Western Blot
2.4. Biochemical Assays
2.4.1. Antioxidant Enzymes and Molecules Concentrations
2.4.2. Inflammation Markers Levels
2.4.3. Caspases Activity
2.4.4. NADP/NADPH Levels
2.5. RNA Extraction, Reverse Transcription, and Real-Time PCR
2.6. Statistical Analysis
3. Results
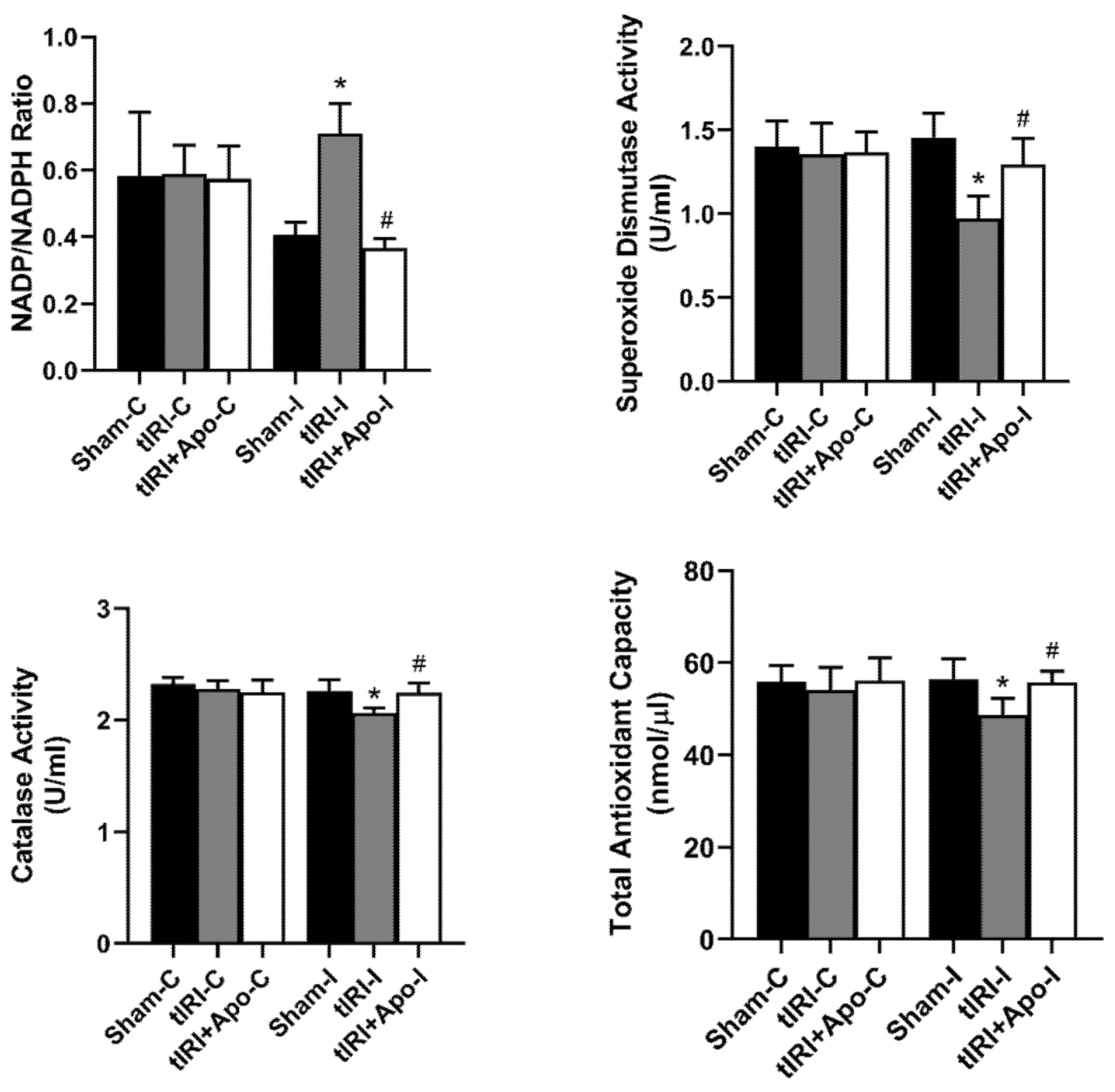
3.1. Antioxidant Effect of NOX Inhibition
3.2. NOX Activates the Expression of the NLRP3 Inflammasome
3.3. NOX-Activated NLRP3 Triggers Germ Cell Inflammatory Response
3.4. NOX Induces Spermatogenic Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, C.; Ko, E.Y. Oxidative stress in the pathophysiology of male infertility. Andrologia 2021, 53, e13581. [Google Scholar] [CrossRef] [PubMed]
- Elshaari, F.A.; Elfagih, R.I.; Sheriff, D.S.; Barassi, I.F. Oxidative and antioxidative defense system in testicular torsion/detorsion. Indian J. Urol. 2011, 27, 479–484. [Google Scholar] [PubMed]
- Musset, B.; Clark, R.A.; DeCoursey, T.E.; Petheo, G.L.; Geiszt, M.; Chen, Y.; Cornell, J.E.; Eddy, C.A.; Brzyski, R.G.; El Jamali, A. NOX5 in human spermatozoa: Expression, function, and regulation. J. Biol. Chem. 2012, 287, 9376–9388. [Google Scholar]
- Vatannejad, A.; Tavilani, H.; Sadeghi, M.R.; Karimi, M.; Lakpour, N.; Amanpour, S.; Shabani Nashtaei, M.; Doosti, M. Evaluation of the NOX5 protein expression and oxidative stress in sperm from asthenozoospermic men compared to normozoospermic men. J. Endocrinol. Investig. 2019, 42, 1181–1189. [Google Scholar] [CrossRef]
- Ghani, E.; Keshtgar, S.; Habibagahi, M.; Ghannadi, A.; Kazeroni, M. Expression of NOX5 in human teratozoospermia compared to normozoospermia. Andrologia 2013, 45, 351–356. [Google Scholar]
- Xu, M.; Dai, D.Z.; Zhang, Q.; Cheng, Y.S.; Dai, Y. Upregulated NADPH oxidase contributes to diabetic testicular complication and is relieved by strontium fructose 1,6-diphosphate. Exp. Clin. Endocrinol. Diabetes 2010, 118, 459–465. [Google Scholar] [CrossRef]
- Al-Saleh, F.; Khashab, F.; Fadel, F.; Al-Kandari, N.; Al-Maghrebi, M. Inhibition of NADPH oxidase alleviates germ cell apoptosis and ER stress during testicular ischemia reperfusion injury. Saudi J. Biol. Sci. 2020, 27, 2174–2184. [Google Scholar]
- Yingze, Y.; Zhihong, J.; Tong, J.; Yina, L.; Zhi, Z.; Xu, Z.; Xiaoxing, X.; Lijuan, G. NOX2-mediated reactive oxygen species are double-edged swords in focal cerebral ischemia in mice. J. Neuroinflamm. 2022, 14, 184. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Tavalaee, M.; Rahmani, M.; Drevet, J.R.; Nasr-Esfahani, M.H. The NLRP3 inflammasome: Molecular activation and regulation in spermatogenesis and male infertility; a systematic review. Basic Clin. Androl. 2022, 32, 8. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Al-Kandari, N.; Fadel, F.; Al-Saleh, F.; Khashab, F.; Al-Maghrebi, M. The Thioredoxin System is Regulated by the ASK-1/JNK/p38/Survivin Pathway During Germ Cell Apoptosis. Molecules 2019, 24, 3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minutoli, L.; Antonuccio, P.; Irrera, N.; Rinaldi, M.; Bitto, A.; Marini, H.; Pizzino, G.; Romeo, C.; Pisani, A.; Santoro, G.; et al. NLRP3 Inflammasome Involvement in the Organ Damage and Impaired Spermatogenesis Induced by Testicular Ischemia and Reperfusion in Mice. J. Pharmacol. Exp. Ther. 2015, 355, 370–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxidative Med. Cell. Longev. 2016, 2016, 2183026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsen, S.G. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2022, 8, 799294. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Morimoto, H.; Iwata, K.; Ogonuki, N.; Inoue, K.; Atsuo, O.; Kanatsu-Shinohara, M.; Morimoto, T.; Yabe-Nishimura, C.; Shinohara, T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell 2013, 12, 774–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, H.; Kanatsu-Shinohara, M.; Shinohara, T. ROS-Generating Oxidase Nox3 Regulates the Self-Renewal of Mouse Spermatogonial Stem Cells. Biol. Reprod. 2015, 92, 147. [Google Scholar] [CrossRef] [PubMed]
- Galardo, M.N.; Regueira, M.; Riera, M.F.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B. Lactate regulates rat male germ cell function through reactive oxygen species. PLoS ONE 2014, 9, e88024. [Google Scholar] [CrossRef] [Green Version]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar]
- Aitken, R.J.; Baker, M.A. Oxidative stress and male reproductive biology. Reprod. Fertil. Dev. 2004, 16, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular aptitudes. Mediat. Inflamm. 2008, 2008, 106507. [Google Scholar] [CrossRef] [Green Version]
- Bakr, A.G.; Hassanein, E.H.M.; Ali, F.E.M.; El-Shoura, E.A.M. Combined apocynin and carvedilol protect against cadmium-induced testicular damage via modulation of inflammatory response and redox-sensitive pathways. Life Sci. 2022, 311, 121152. [Google Scholar] [CrossRef]
- Yücel, A.; Aydogan, M.S.; Ucar, M.; Sarıcı, K.B.; Karaaslan, M.G. Effects of Apocynin on Liver Ischemia-Reperfusion Injury in Rats. Transplant. Proc. 2019, 51, 1180–1183. [Google Scholar] [CrossRef]
- Kapoor, M.; Sharma, N.; Sandhir, R.; Nehru, B. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion hippocampus injury in rat brain. Biomed. Pharmacother. 2018, 97, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.C.; Braga, P.C.; Martins, A.D.; Silva, B.M.; Alves, M.G.; Oliveira, P.F. Antioxidants Present in Reproductive Tract Fluids and Their Relevance for Fertility. Antioxidants 2021, 10, 1441. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Catalase: A tetrameric enzyme with four tightly bound molecules of NADPH. Proc. Natl. Acad. Sci. USA 1984, 81, 4343–4347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montllor-Albalate, C.; Kim, H.; Thompson, A.E.; Jonke, A.P.; Torres, M.P.; Reddi, A.R. Sod1 integrates oxygen availability to redox regulate NADPH production and the thiol redoxome. Proc. Natl. Acad. Sci. USA 2022, 119, e2023328119. [Google Scholar] [CrossRef]
- Xia, W.; Wang, Z.; Wang, Q.; Han, J.; Zhao, C.; Hong, Y.; Zeng, L.; Tang, L.; Ying, W. Roles of NAD(+) / NADH and NADP(+) / NADPH in cell death. Curr. Pharm. Des. 2009, 15, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Liu, Z.; Zhuan, L.; Wang, T.; Guo, S.; Wang, S.; Liu, J.; Ye, Z. Effects of apocynin on oxidative stress and expression of apoptosis-related genes in testes of diabetic rats. Mol. Med. Rep. 2013, 7, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghari, A.; Akbari, G.; Meghdadi, A.; Mortazavi, P. Protective effect of metformin on testicular ischemia/reperfusion injury in rats. Acta Cir. Bras. 2016, 31, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Ozbek, O.; Altintas, R.; Polat, A.; Vardi, N.; Parlakpinar, H.; Sagir, M.; Duran, Z.R.; Yildiz, A. The protective effect of apocynin on testicular ischemia-reperfusion injury. J. Urol. 2015, 193, 1417–1422. [Google Scholar] [CrossRef]
- Rubartelli, A. Redox control of NLRP3 inflammasome activation in health and disease. J. Leukoc. Biol. 2012, 92, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.N.; Wang, C.; Willette-Brown, J.; Herjan, T.; Gulen, M.F.; Zhou, H.; Bulek, K.; Franchi, L.; Sato, T.; Alnemri, E.S.; et al. IKKα negatively regulates ASC-dependent inflammasome activation. Nat. Commun. 2014, 5, 4977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhul Amin, A.R.; Senga, T.; Oo, M.L.; Thant, A.A.; Hamaguchi, M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: A role for the dual signalling pathways, Akt and Erk. Genes Cells 2003, 8, 515–523. [Google Scholar] [CrossRef]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Gurung, P.; Kanneganti, T.D. Novel roles for caspase-8 in IL-1β and inflammasome regulation. Am. J. Pathol. 2015, 185, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Heid, M.E.; Keyel, P.A.; Kamga, C.; Shiva, S.; Watkins, S.C.; Salter, R.D. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol. 2013, 191, 5230–5238. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Hou, L.; Zhai, X.; Ruan, Z.; Sun, W.; Zhang, D.; Zhao, X.; Wang, Q. 2,5-hexanedione induces NLRP3 inflammasome activation and neurotoxicity through NADPH oxidase-dependent pathway. Free. Radic. Biol. Med. 2021, 162, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Xin, R.; Sun, X.; Wang, Z.; Yuan, W.; Jiang, W.; Wang, L.; Xiang, Y.; Zhang, H.; Li, X.; Hou, Y.; et al. Apocynin inhibited NLRP3/XIAP signalling to alleviate renal fibrotic injury in rat diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Abdel-Aziz, A.M.; Hamouda, A.A.H. Diacerein Downregulates NLRP3/Caspase-1/IL-1β and IL-6/STAT3 Pathways of Inflammation and Apoptosis in a Rat Model of Cadmium Testicular Toxicity. Biol. Trace Elem. Res. 2020, 195, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.Z.; He, K.X.; Cheng, F.; Li, W.; Yu, W.M.; Li, H.Y.; Rao, T.; Ruan, Y. Long Non-coding RNA MEG3 Promotes Pyroptosis in Testicular Ischemia-Reperfusion Injury by Targeting MiR-29a to Modulate PTEN Expression. Front. Cell Dev. Biol. 2021, 9, 671613. [Google Scholar] [CrossRef]
- Mu, Y.; Yin, T.L.; Zhang, Y.; Yang, J.; Wu, Y.T. Diet-induced obesity impairs spermatogenesis: The critical role of NLRP3 in Sertoli cells. Inflamm. Regen. 2022, 42, 24. [Google Scholar] [CrossRef]
- Sano, M. NLRP3 inflammasome is involved in testicular inflammation induced by lipopolysaccharide in mice. Am. J. Reprod. Immunol. 2022, 87, e13527. [Google Scholar] [CrossRef]





| Primary Antibody | Dilution | Manufacturer |
|---|---|---|
| NLRP3 (MBS9127062) | 1:500 | My Bio Source (San Diego, CA, USA) |
| GAPDH (MBS9131201) | 1:10,000 | |
| ASC (sc-514414) | 1:500 | |
| Caspase 1 (sc-56036) | 1:500 | Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) |
| GSDMDC1 (sc-393581) | 1:500 | |
| MMP-9 (sc-393859) | 1:500 | |
| VDUP1 (sc-166234) | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almarzouq, D.; Al-Maghrebi, M. NADPH Oxidase-Mediated Testicular Oxidative Imbalance Regulates the TXNIP/NLRP3 Inflammasome Axis Activation after Ischemia Reperfusion Injury. Antioxidants 2023, 12, 145. https://doi.org/10.3390/antiox12010145
Almarzouq D, Al-Maghrebi M. NADPH Oxidase-Mediated Testicular Oxidative Imbalance Regulates the TXNIP/NLRP3 Inflammasome Axis Activation after Ischemia Reperfusion Injury. Antioxidants. 2023; 12(1):145. https://doi.org/10.3390/antiox12010145
Chicago/Turabian StyleAlmarzouq, Duaah, and May Al-Maghrebi. 2023. "NADPH Oxidase-Mediated Testicular Oxidative Imbalance Regulates the TXNIP/NLRP3 Inflammasome Axis Activation after Ischemia Reperfusion Injury" Antioxidants 12, no. 1: 145. https://doi.org/10.3390/antiox12010145
APA StyleAlmarzouq, D., & Al-Maghrebi, M. (2023). NADPH Oxidase-Mediated Testicular Oxidative Imbalance Regulates the TXNIP/NLRP3 Inflammasome Axis Activation after Ischemia Reperfusion Injury. Antioxidants, 12(1), 145. https://doi.org/10.3390/antiox12010145





