Evidence of Increased Oxidative Stress in the Placental Tissue of Women Who Suffered an Episode of Psychosis during Pregnancy
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Tissue Samples
2.3. Gene Analysis
2.4. Protein Studies
2.5. Evaluation of Histopathological Expression and Statistical Analysis
3. Results
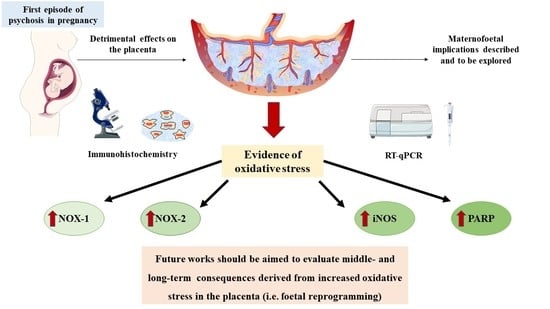
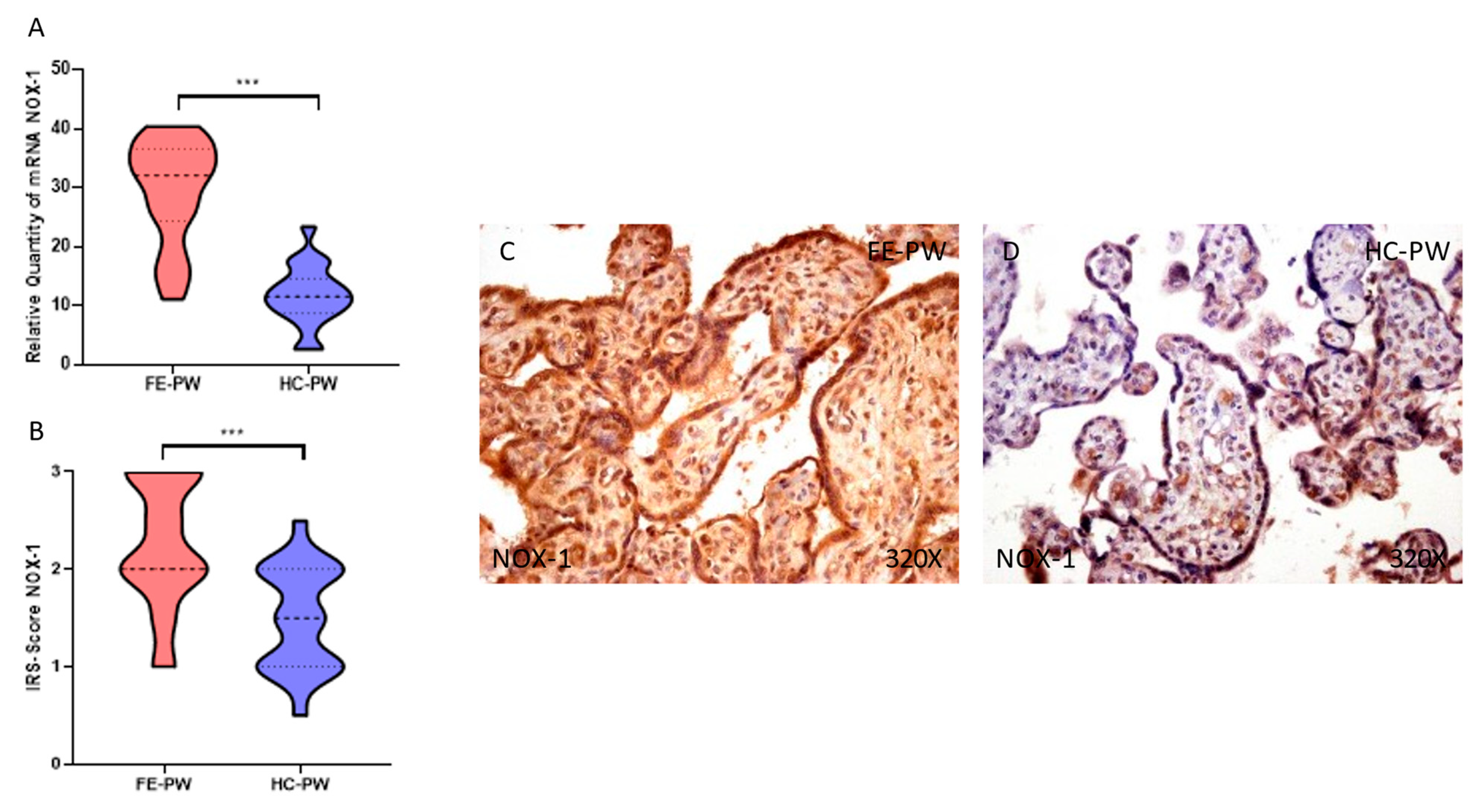
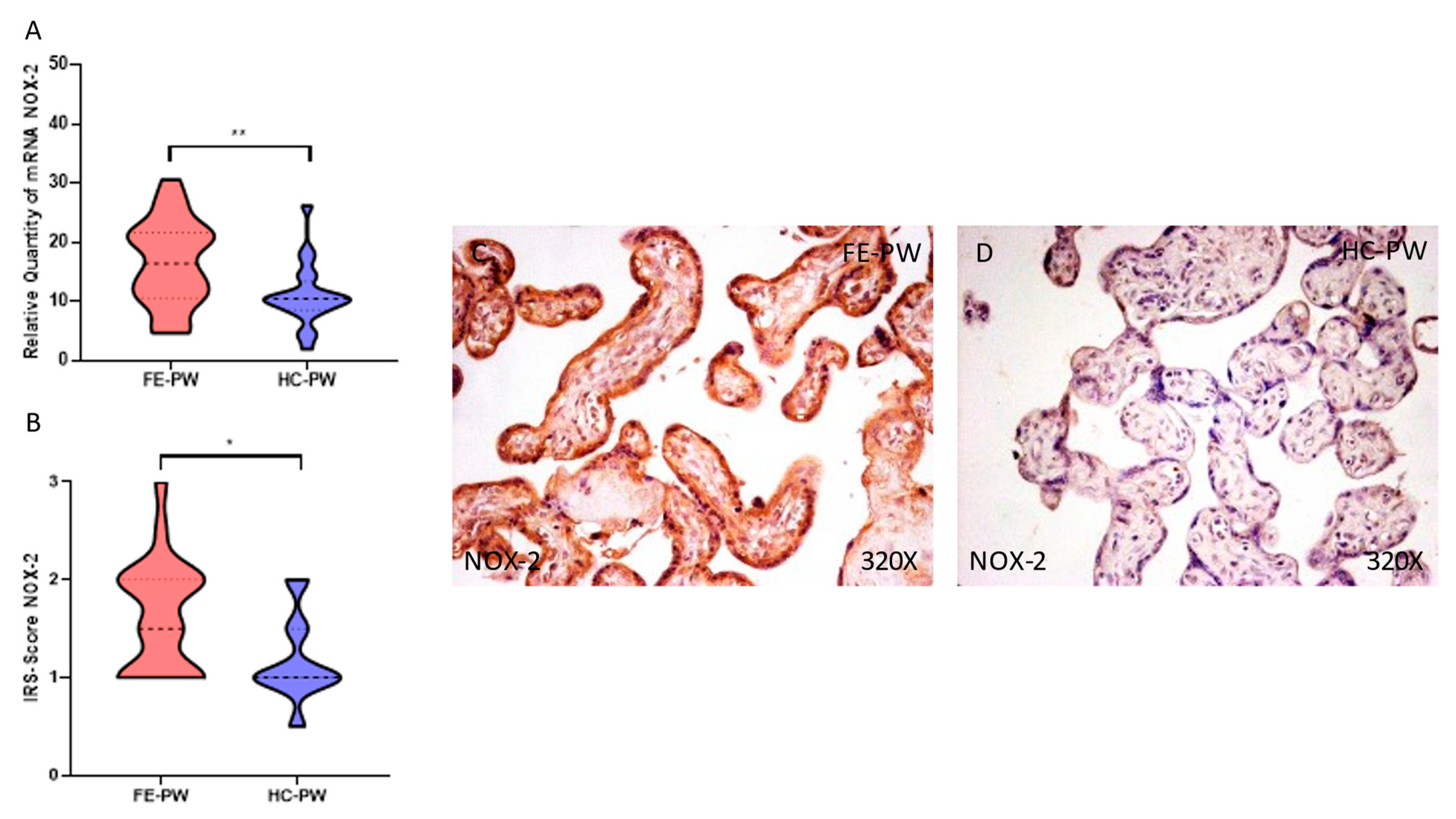
3.1. Placentas of Women with a First Episode of Psychosis during Pregnancy Exhibited Increased Protein and Gene Expression of NOX-1 and NOX-2
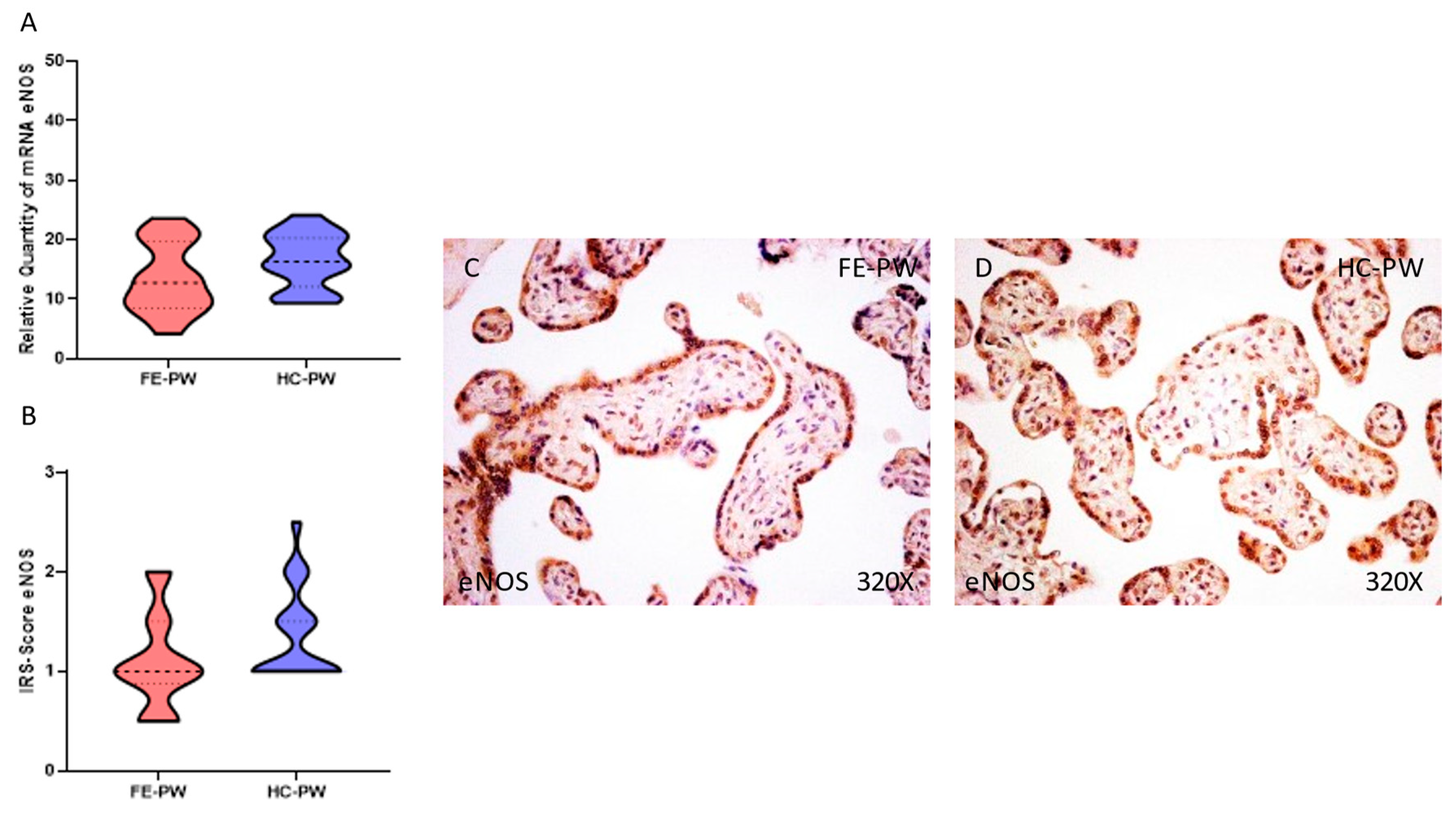
3.2. Placentas of Women with a First Episode of Psychosis during Pregnancy Displayed Augmented Immunohistochemical and Gene Expression of iNOS but Normal eNOS
3.3. Placentas of Women with a First Episode of Psychosis during Pregnancy Showed Increased Protein and Gene Expression of PARP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calabrese, J.; Khalili, Y. Al Psychosis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Carpenter, W.T.; Tandon, R. Psychotic Disorders in DSM-5: Summary of Changes. Asian J. Psychiatr. 2013, 6, 266–268. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Griswold, K.S.; Del Regno, P.A.; Berger, R.C. Recognition and Differential Diagnosis of Psychosis in Primary Care. Am. Fam. Physician 2015, 91, 856–863. [Google Scholar] [PubMed]
- Gaebel, W.; Zielasek, J. Focus on Psychosis. Dialogues Clin. Neurosci. 2015, 17, 9. [Google Scholar] [CrossRef]
- Tan, E.K.; Tan, E.L. Alterations in Physiology and Anatomy during Pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Watkins, M.E.; Newport, D.J. Psychosis in Pregnancy. Obstet. Gynecol. 2009, 113, 1349–1353. [Google Scholar] [CrossRef]
- Mondelli, V. From Stress to Psychosis: Whom, How, When and Why? Epidemiol. Psychiatr. Sci. 2014, 23, 215. [Google Scholar] [CrossRef] [Green Version]
- Scandurra, C.; Zapparella, R.; Policastro, M.; Continisio, G.I.; Ammendola, A.; Bochicchio, V.; Maldonato, N.M.; Locci, M. Obstetric Violence in a Group of Italian Women: Socio-Demographic Predictors and Effects on Mental Health. Cult. Health Sex. 2021, 24, 1466–1480. [Google Scholar] [CrossRef]
- Malvasi, A.; Zaami, S.; Tinelli, A.; Trojano, G.; Montanari Vergallo, G.; Marinelli, E. Kristeller Maneuvers or Fundal Pressure and Maternal/Neonatal Morbidity: Obstetric and Judicial Literature Review. J. Matern.-Fetal Neonatal Med. 2018, 32, 2598–2607. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Maymon, R. The Condemned Fundal Pressure Maneuver: Time to Reconsider? Arch. Gynecol. Obstet. 2022, 306, 1953–1957. [Google Scholar] [CrossRef]
- Zaami, S.; Stark, M.; Beck, R.; Malvasi, A.; Marinelli, E. Does Episiotomy Always Equate Violence in Obstetrics? Routine and Selective Episiotomy in Obstetric Practice and Legal Questions. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1847–1854. [Google Scholar] [CrossRef]
- Zhong, Q.Y.; Gelaye, B.; Fricchione, G.L.; Avillach, P.; Karlson, E.W.; Williams, M.A. Adverse Obstetric and Neonatal Outcomes Complicated by Psychosis among Pregnant Women in the United States. BMC Pregnancy Childbirth 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Howard, L.M.; Goss, C.; Leese, M.; Appleby, L.; Thornicroft, G. The Psychosocial Outcome of Pregnancy in Women with Psychotic Disorders. Schizophr. Res. 2004, 71, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kratimenos, P.; Penn, A.A. Placental Programming of Neuropsychiatric Disease. Pediatr. Res. 2019, 86, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, M.; Chatterjee, S.; Zeng, X.; Joseph, P.; Tekola-Ayele, F. Impact of Depression and Stress on Placental DNA Methylation in Ethnically Diverse Pregnant Women. Epigenomics 2021, 13, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Dahlerup, B.R.; Egsmose, E.L.; Siersma, V.; Mortensen, E.L.; Hedegaard, M.; Knudsen, L.E.; Mathiesen, L. Maternal Stress and Placental Function, a Study Using Questionnaires and Biomarkers at Birth. PLoS ONE 2018, 13, e0207184. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Maurer, S.V.; Stevens, H.E. Future Horizons for Neurodevelopmental Disorders: Placental Mechanisms. Front. Pediatr. 2021, 9, 243. [Google Scholar] [CrossRef]
- Barron, H.; Hafizi, S.; Andreazza, A.C.; Mizrahi, R. Neuroinflammation and Oxidative Stress in Psychosis and Psychosis Risk. Int. J. Mol. Sci. 2017, 18, 651. [Google Scholar] [CrossRef] [Green Version]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative Stress: Normal Pregnancy versus Preeclampsia. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Fraguas, D.; Díaz-Caneja, C.M.; Ayora, M.; Hernández-Álvarez, F.; Rodríguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2019, 45, 742–751. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tian, F.J.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. Biomed Res. Int. 2015, 2015, 293271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.O.; Ball, S.S.R.; Bowater, R.P.; Wormstone, I.M. PARP-1 Inhibition Influences the Oxidative Stress Response of the Human Lens. Redox Biol. 2016, 8, 354–362. [Google Scholar] [CrossRef] [Green Version]
- First, M.; Williams, J.; Karg, R.; Spitzer, R. Structured Clinical Interview for DSM-5 Disorders–Research Version (SCID-5-RV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Saez, M.A.; Álvarez-Mon, M.A.; Gómez-Lahoz, A.M.; Bravo, C.; De León Luis, J.A.; Sainz, F.; Coca, S.; Asúnsolo, Á.; et al. Abnormal Proinflammatory and Stressor Environmental with Increased the Regulatory Cellular IGF-1/PAPP-A/STC and Wnt-1/β-Catenin Canonical Pathway in Placenta of Women with Chronic Venous Disease during Pregnancy. Int. J. Med. Sci. 2021, 18, 2814–2827. [Google Scholar] [CrossRef]
- Ortega, M.A.; Sánchez-Trujillo, L.; Bravo, C.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Alvarez-Mon, M.A.; Sainz, F.; Alvarez-Mon, M.; Bujan, J.; et al. Newborns of Mothers with Venous Disease during Pregnancy Show Increased Levels of Lipid Peroxidation and Markers of Oxidative Stress and Hypoxia in the Umbilical Cord. Antioxidants 2021, 10, 980. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Vallone, P.M.; Butler, J.M. AutoDimer: A Screening Tool for Primer-Dimer and Hairpin Structures. Biotechniques 2004, 37, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.J.; Jeon, R.H.; Kim, H.D.; Hwang, J.C.; Lee, H.J.; Bae, S.G.; Lee, S.L.; Rho, G.J.; Kim, S.J.; Lee, W.J. TATA Box Binding Protein and Ribosomal Protein 4 Are Suitable Reference Genes for Normalization during Quantitative Polymerase Chain Reaction Study in Bovine Mesenchymal Stem Cells. Asian-Australas. J. Anim. Sci. 2020, 33, 2021. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Funes Moñux, R.M.; Rodriguez-Martín, S.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Saez, M.A.; Guijarro, L.G.; et al. The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis. Biomolecules 2023, 13, 120. [Google Scholar] [CrossRef]
- Ortega, M.A.; Chaowen, C.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Cruza, I.; Pereda-Cerquella, C.; Alvarez-Mon, M.A.; Guijarro, L.G.; Fatych, Y.; et al. Chronic Venous Disease in Pregnant Women Causes an Increase in ILK in the Placental Villi Associated with a Decrease in E-Cadherin. J. Pers. Med. 2022, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Sáez, M.A.; Fraile-Martínez, O.; Álvarez-Mon, M.A.; García-Montero, C.; Guijarro, L.G.; Asúnsolo, Á.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; et al. Overexpression of Glycolysis Markers in Placental Tissue of Pregnant Women with Chronic Venous Disease: A Histological Study. Int. J. Med. Sci. 2022, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Karakasi, M.V.; Markopoulou, M.; Tentes, I.K.; Tsikouras, P.N.; Vasilikos, E.; Pavlidis, P. Prepartum Psychosis and Neonaticide: Rare Case Study and Forensic-Psychiatric Synthesis of Literature. J. Forensic Sci. 2017, 62, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Lazarides, C.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Voelkle, M.C.; Buss, C.; Simhan, H.N.; Wadhwa, P.D.; Entringer, S. Maternal Pro-Inflammatory State during Pregnancy and Newborn Leukocyte Telomere Length: A Prospective Investigation. Brain. Behav. Immun. 2019, 80, 419–426. [Google Scholar] [CrossRef]
- Rambaud, V.; Marzo, A.; Chaumette, B. Oxidative Stress and Emergence of Psychosis. Antioxidants 2022, 11, 1870. [Google Scholar] [CrossRef]
- Ruano, C.S.M.; Miralles, F.; Méhats, C.; Vaiman, D. The Impact of Oxidative Stress of Environmental Origin on the Onset of Placental Diseases. Antioxidants 2022, 11, 106. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative Stress in Placental Pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, Oxidative Stress and Inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- García-Honduvilla, N.; Ortega, M.A.; Asúnsolo, Á.; Álvarez-Rocha, M.J.; Romero, B.; De León-Luis, J.; Álvarez-Mon, M.; Buján, J. Placentas from Women with Pregnancy-Associated Venous Insufficiency Show Villi Damage with Evidence of Hypoxic Cellular Stress. Hum. Pathol. 2018, 77, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Martínez-Vivero, C.; Sainz, F.; Bravo, C.; De León-Luis, J.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Pregnancy-Associated Venous Insufficiency Course with Placental and Systemic Oxidative Stress. J. Cell. Mol. Med. 2020, 24, 4157–4170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarafdar, A.; Pula, G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myatt, L. Reactive Oxygen and Nitrogen Species and Functional Adaptation of the Placenta. Placenta 2010, 31, S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, I.; Fournier, T.; Chissey, A.; Therond, P.; Slama, A.; Beaudeux, J.L.; Zerrad-Saadi, A. NADPH Oxidase Is the Major Source of Placental Superoxide in Early Pregnancy: Association with MAPK Pathway Activation. Sci. Rep. 2019, 9, 13962. [Google Scholar] [CrossRef] [Green Version]
- Santana-Garrido, Á.; Reyes-Goya, C.; Espinosa-Martín, P.; Sobrevia, L.; Beltrán, L.M.; Vázquez, C.M.; Mate, A. Oxidative and Inflammatory Imbalance in Placenta and Kidney of SFlt1-Induced Early-Onset Preeclampsia Rat Model. Antioxidants 2022, 11, 1608. [Google Scholar] [CrossRef]
- Raijmakers, M.T.M.; Peters, W.H.M.; Steegers, E.A.P.; Poston, L. NAD(P)H Oxidase Associated Superoxide Production in Human Placenta from Normotensive and Pre-Eclamptic Women. Placenta 2004, 25, S85–S89. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Anavi, S.; Tirosh, O. INOS as a Metabolic Enzyme under Stress Conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef]
- Du, L.; He, F.; Kuang, L.; Tang, W.; Li, Y.; Chen, D. ENOS/INOS and Endoplasmic Reticulum Stress-Induced Apoptosis in the Placentas of Patients with Preeclampsia. J. Hum. Hypertens. 2017, 31, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; Liu, Z.; Xu, X.; Liu, Q.; Zhang, X.; Kong, B.; Wei, J.J.; Gong, Y.; Shao, C. Increased Oxidative Stress Mediates the Antitumor Effect of PARP Inhibition in Ovarian Cancer. Redox Biol. 2018, 17, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Erdélyi, K.; Bakondi, E.; Gergely, P.; Szabó, C.; Virág, L. Pathophysiologic Role of Oxidative Stress-Induced Poly(ADP-Ribose) Polymerase-1 Activation: Focus on Cell Death and Transcriptional Regulation. Cell. Mol. Life Sci. 2005, 62, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Abán, C.; Martinez, N.; Carou, C.; Albamonte, I.; Toro, A.; Seyahian, A.; Franchi, A.; Leguizamón, G.; Trigubo, D.; Damiano, A.; et al. Endocannabinoids Participate in Placental Apoptosis Induced by Hypoxia Inducible Factor-1. Apoptosis 2016, 21, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Skepper, J.N.; Charnock-Jones, D.S.; Burton, G.J. Hypoxia-Reoxygenation: A Potent Inducer of Apoptotic Changes in the Human Placenta and Possible Etiological Factor in Preeclampsia. Circ. Res. 2002, 90, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Magee, T.R.; Ross, M.G.; Wedekind, L.; Desai, M.; Kjos, S.; Belkacemi, L. Gestational Diabetes Mellitus Alters Apoptotic and Inflammatory Gene Expression of Trophobasts from Human Term Placenta. J. Diabetes Complicat. 2014, 28, 448–459. [Google Scholar] [CrossRef] [Green Version]
- Belkacemi, L.; Kjos, S.; Nelson, D.M.; Desai, M.; Ross, M.G. Reduced Apoptosis in Term Placentas from Gestational Diabetic Pregnancies. J. Dev. Orig. Health Dis. 2013, 4, 256–265. [Google Scholar] [CrossRef]
- Hemberger, M.; Nozaki, T.; Winterhager, E.; Yamamoto, H.; Nakagama, H.; Kamada, N.; Suzuki, H.; Ohta, T.; Ohki, M.; Masutani, M.; et al. Parp1-Deficiency Induces Differentiation of ES Cells into Trophoblast Derivatives. Dev. Biol. 2003, 257, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative Stress-Mediated Aging during the Fetal and Perinatal Periods. Oxid. Med. Cell. Longev. 2014, 2014, 358375. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; Carmen González, M.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]


| FE-PW (n = 22) | HC-PW (n = 20) | |
|---|---|---|
| Median age (IQR), years | 33.5 (21–42) | 33.5 (25–39) |
| Median gestational age (IQR), weeks | 40 (38–41) | 40 (39–42) |
| C-section delivery, n (%) | 3 (13.6) | 2 (10.0) |
| Previous pregnancies, n (%) | 8 (36.4) | 9 (45.0) |
| Previous abortions, n (%) | 1 (4.5) | 2 (10.0) |
| Regular menstrual cycles, n (%) | 17 (77.3) | 16 (80.0) |
| PANSS Mean (SD) | Positive 18.8 (6.3) | ----- |
| Negative 25.7 (7.9) |
| GENE | SEQUENCE Fwd (5′→3′) | SEQUENCE Rev (5′→3′) | Temp |
|---|---|---|---|
| TBP | TGCACAGGAGCCAAGAGTGAA | CACATCACAGCTCCCCACCA | 60 °C |
| NOX-1 | GTTTTACCGCTCCCAGCAGAA | GGATGCCATTCCAGGAGAGAG | 55 °C |
| NOX-2 | TCCGCATCGTTGGGGACTGGA | CCAAAGGGCCCATCAACCGCT | 60 °C |
| iNOS | CCTTACGAGGCGAAGAAGGACAG | CAGTTTGAGAGAGGAGGCTCCG | 61 °C |
| eNOS | AAGAGGAAGGAGTCCAGTAACACAGA | ACG AGC AAA GGC GCA GAA | 60 °C |
| PARP | CCAGGATGAAGAGGCAGTGAAG | TTCTGAAGGTCGATCTCATACTCC | 58 °C |
| Antigen | Species | Dilution | Provider | Protocol Specifications |
|---|---|---|---|---|
| NOX 1 | Rabbit polyclonal | 1:250 | Abcam (ab78016) | 10 mM sodium citrate pH = 6 before incubation with blocking solution |
| NOX 2 | Goat polyclonal | 1:500 | Abcam (ab111175) | 0.1% Triton X-100 in PBS, 10 min, before incubation with blocking solution |
| iNOS | Rabbit polyclonal | 1:350 | Abcam (ab95866) | 10 mM sodium citrate pH = 6 before incubation with blocking solution |
| eNOS | Rabbit polyclonal | 1:50 | Abcam (ab66127) | EDTA pH = 9 before incubation with blocking solution |
| PARP | Mouse monoclonal | 1:1000 | Abcam (ab110915) | 10 mM sodium citrate pH = 6 before incubation with blocking solution |
| IgG (Rabbit) | Mouse | 1:1000 | Sigma–Aldrich (RG-96/B5283) | ------ |
| IgG (Goat) | Mouse | 1:100 | Sigma–Aldrich [GT-4/B3148] | ------ |
| IgG (Mouse) | Goat | 1:300 | Sigma–Aldrich (F2012/045K6072) | ------ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Rodriguez-Martín, S.; Funes Moñux, R.M.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Saez, M.A.; Guijarro, L.G.; et al. Evidence of Increased Oxidative Stress in the Placental Tissue of Women Who Suffered an Episode of Psychosis during Pregnancy. Antioxidants 2023, 12, 179. https://doi.org/10.3390/antiox12010179
Ortega MA, Fraile-Martinez O, García-Montero C, Rodriguez-Martín S, Funes Moñux RM, Bravo C, De Leon-Luis JA, Saz JV, Saez MA, Guijarro LG, et al. Evidence of Increased Oxidative Stress in the Placental Tissue of Women Who Suffered an Episode of Psychosis during Pregnancy. Antioxidants. 2023; 12(1):179. https://doi.org/10.3390/antiox12010179
Chicago/Turabian StyleOrtega, Miguel A., Oscar Fraile-Martinez, Cielo García-Montero, Sonia Rodriguez-Martín, Rosa M. Funes Moñux, Coral Bravo, Juan A. De Leon-Luis, Jose V. Saz, Miguel A. Saez, Luis G. Guijarro, and et al. 2023. "Evidence of Increased Oxidative Stress in the Placental Tissue of Women Who Suffered an Episode of Psychosis during Pregnancy" Antioxidants 12, no. 1: 179. https://doi.org/10.3390/antiox12010179