Extract of Wheatgrass and Aronia Mixture Ameliorates Atopic Dermatitis-Related Symptoms by Suppressing Inflammatory Response and Oxidative Stress In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of TAAR Extract
2.3. High Performance Liquid Chromatography (HPLC)
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Real-Time Polymerase Chain Reaction (PCR)
2.7. Western Blotting Analysis
2.8. Animals
2.9. Induction of AD-like Skin Lesion in Mice
2.10. Measurement of AD Clinical Score, Dorsal Skin Thickness and Moisture Contents
2.11. Measurement of Serum IgE, IL-4, and Inflammatory Markers
2.12. Histological Analysis
2.13. Immunohistochemistry (IHC) Staining
2.14. Statistical Analysis
3. Results
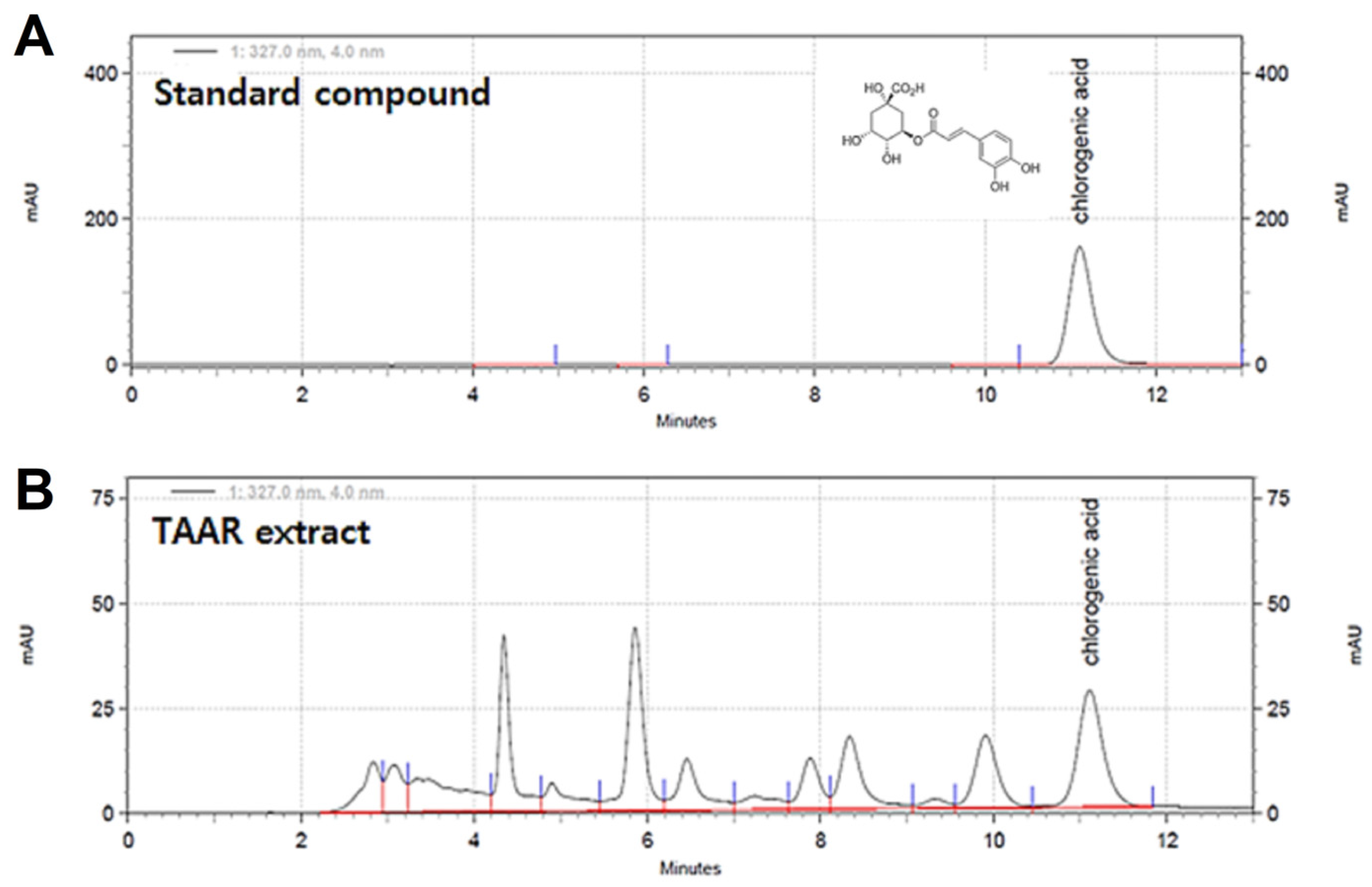
3.1. HPLC Analysis of the TAAR Extract
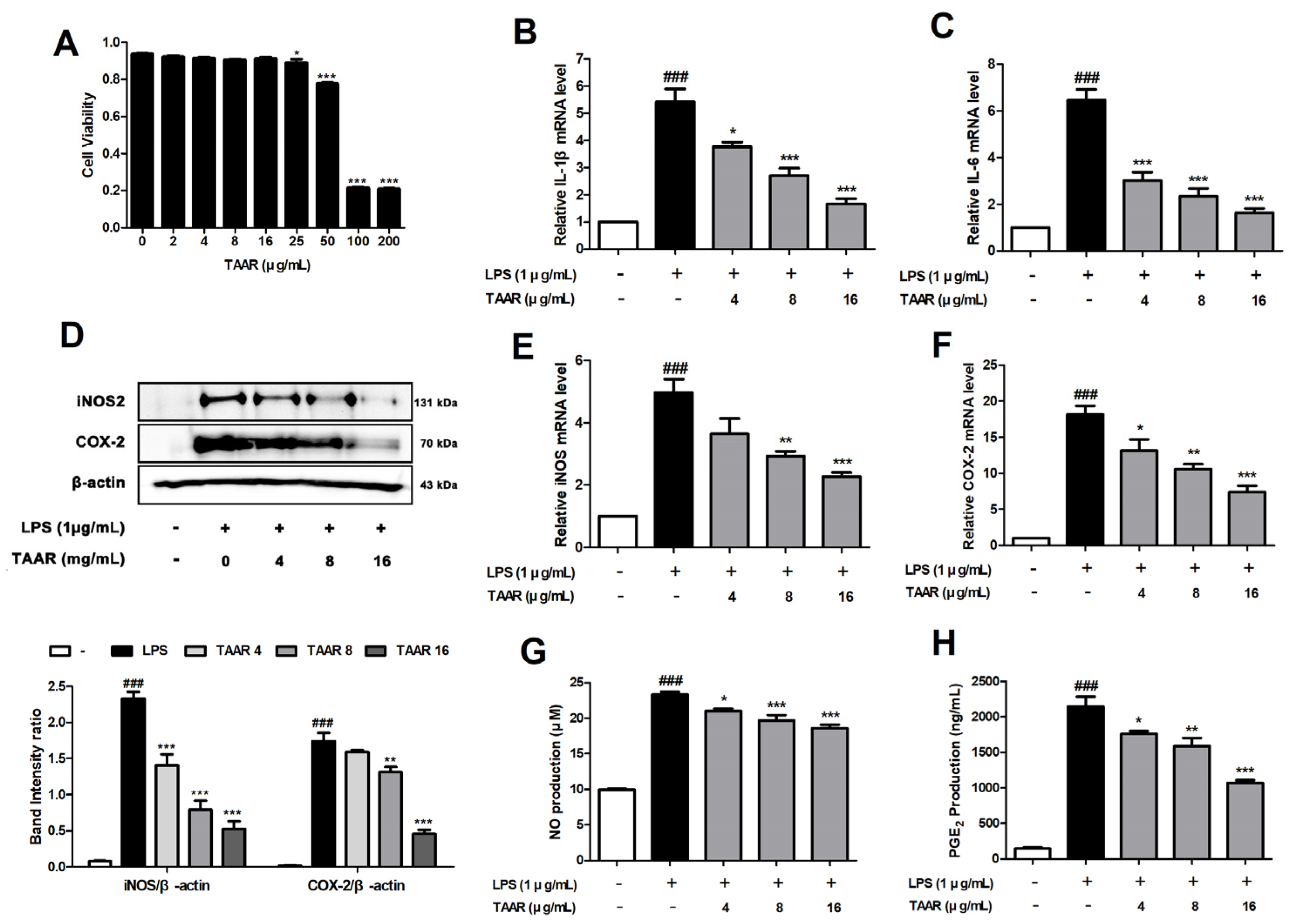
3.2. TAAR Extract Attenuates LPS-Induced Pro-Inflammatory Cytokines and Inflammatory Mediators in RAW 264.7 Cells
3.3. TAAR Extract Suppresses the Expression of TNF-α/IFN-γ-Induced Pro-Inflammatory Cytokines and Chemokines in HaCaT Cells
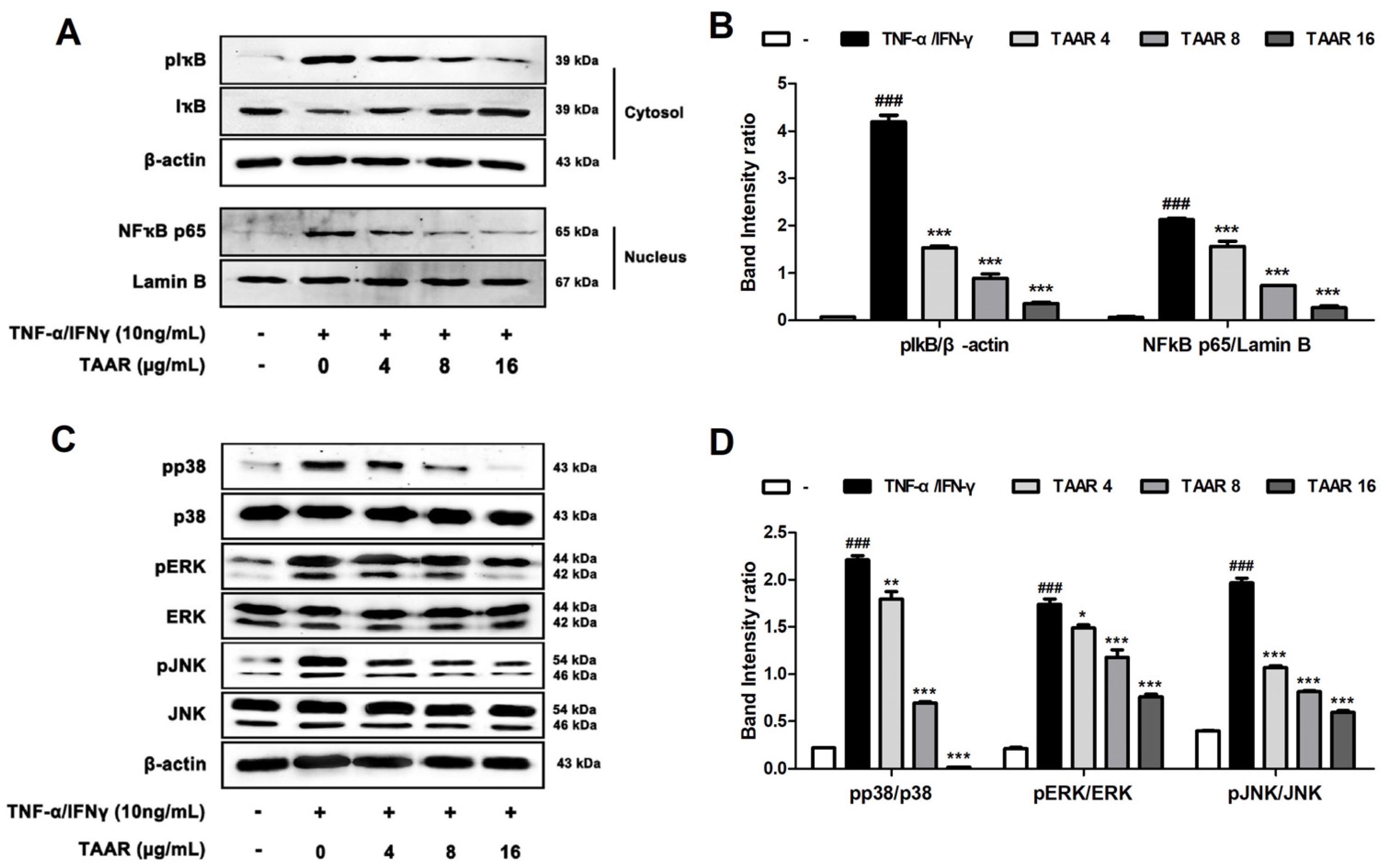
3.4. TAAR Extract Inhibits Inflammation through the Regulation of NFκB and MAPKs Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Cells
3.5. TAAR Extract Activates Nrf2/HO-1/NQO1 Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Cells
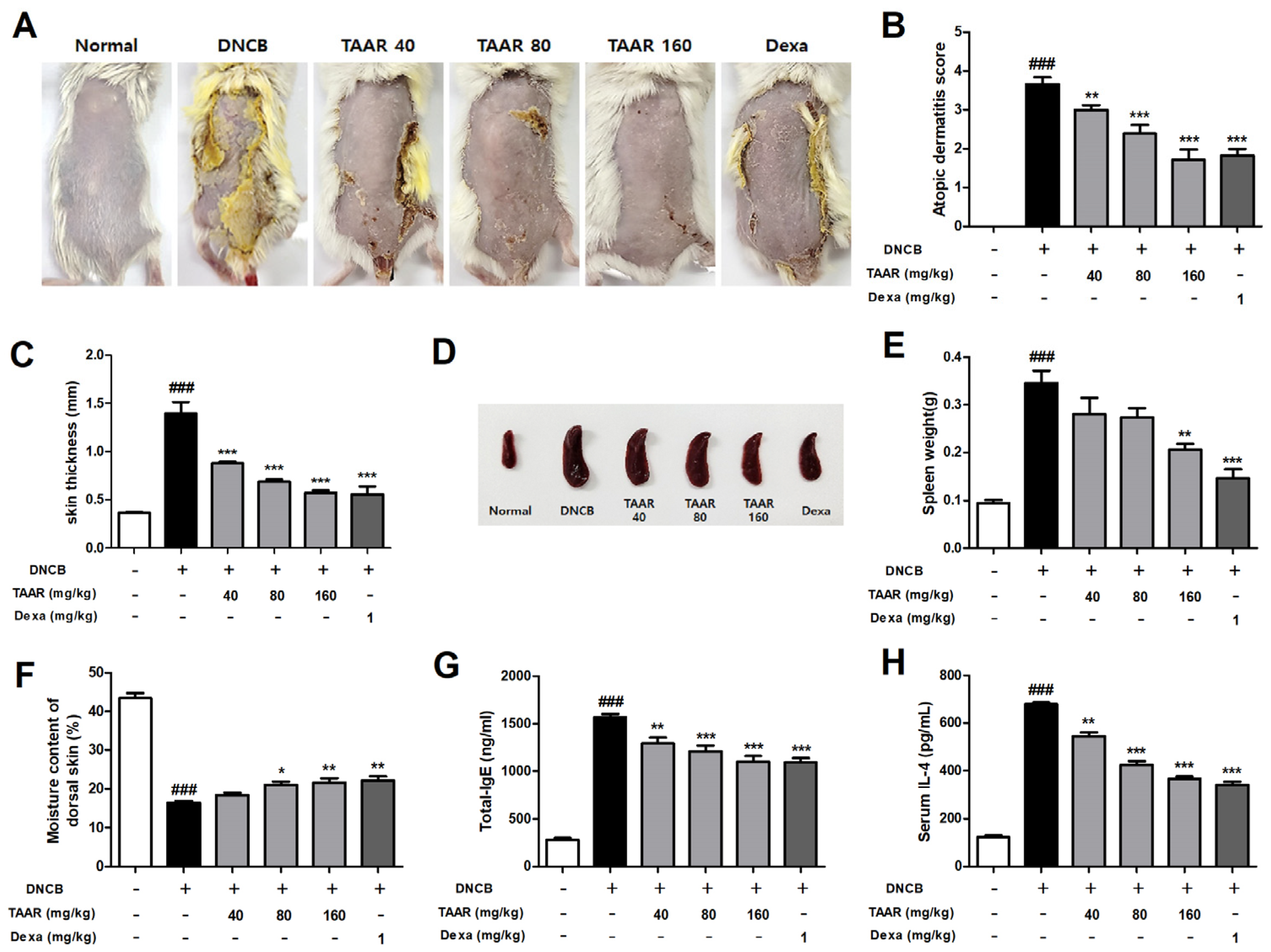
3.6. TAAR Extract Alleviates AD-like Symptoms in DNCB-Induced Mice
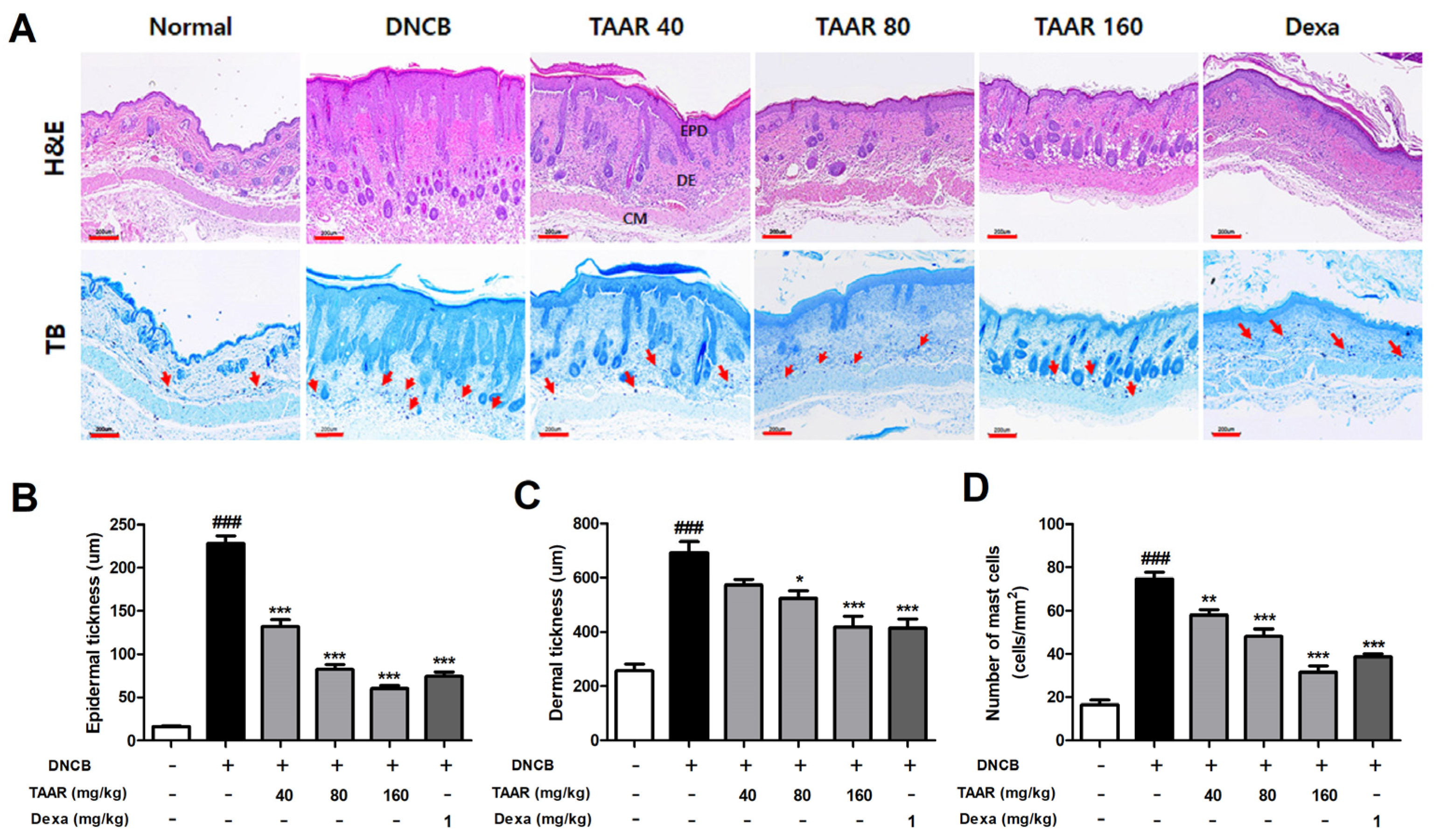
3.7. TAAR Extract Decreases Epidermal Thickness and Mast Cell Infiltration in Mice with DNCB-Induced AD-like Skin Lesions
3.8. TAAR Extract Reduces the Expression of DNCB-Induced Pro-Inflammatory Cytokines and Chemokines in Mice
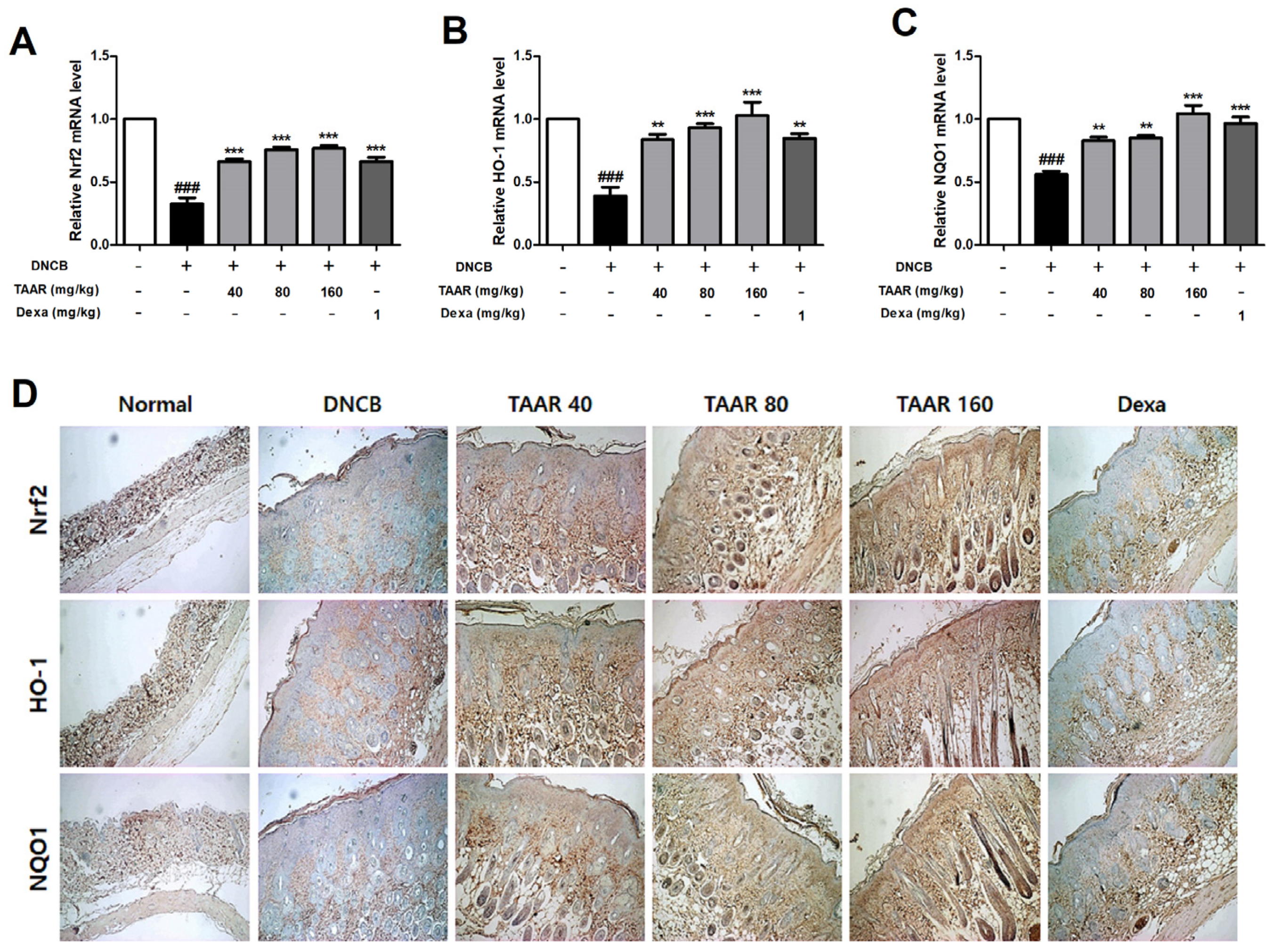
3.9. TAAR Extract Promoted Nrf2/HO-1/NQO1 Pathway Activation in Mice with DNCB-Induced AD-like Skin Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2021, 21, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Avena-Woods, C. Overview of Atopic Dermatitis. Am. J. Manag. Care 2017, 23, 115–123. [Google Scholar]
- Peng, W.; Novak, N. Pathogenesis of atopic dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Oranje, A.; Deleuran, M.; Simon, D.; Szalai, Z.; Kunz, B.; Svensson, A.; Barbarot, S.; von Kobyletzki, L.; Taieb, A.; et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 729–747. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and Safety of Crisaborole Ointment, a Novel, Nonsteroidal Phosphodiesterase 4 (PDE4) Inhibitor for the Topical Treatment of Atopic Dermatitis (AD) in Children and Adults. J. Am. Acad. Derm. 2016, 75, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: Latest evidence and clinical considerations. Drugs Context 2018, 7, 212530. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.Y.; Zhang, A.L.; Chen, D.; Xue, C.C.; Lenon, G.B. Chinese herbal medicine for atopic dermatitis: A systematic review. J. Am. Acad. Dermatol. 2013, 69, 295–304. [Google Scholar] [CrossRef]
- Hendricks, A.J.; Yosipovitch, G.; Shi, V.Y. Dupilumab use in dermatologic conditions beyond atopic dermatitis—A systematic review. J. Dermatol. Treat. 2021, 32, 19–28. [Google Scholar] [CrossRef]
- Tirgar, P.R.; Thumber, B.L.; Desai, T.R. Isolation, characterization and biological evaluation of iron chelator from Triticum aestivum (wheat grass). Int. J. Pharma. BioSci. 2011, 2, 288–296. [Google Scholar]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int. J. Food. Sci. Nutr. 2012, 63, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Gore, R.D.; Palaskar, S.J.; Bartake, A.R. Wheatgrass: Green blood can help to fight cancer. J. Clin. Diagn. Res. 2017, 11, ZC40–ZC42. [Google Scholar] [CrossRef] [PubMed]
- Ben Arye, E.; Goldin, E.; Wengrower, D.; Stamper, A.; Kohn, R.; Berry, E. Wheat grass juice in the treatment of active distal ulcerative colitis: A randomized double blind placebo controlled trial. Scand. J. Gastroenterol. 2002, 37, 444–449. [Google Scholar] [CrossRef]
- Nepali, S.; Ki, H.H.; Lee, J.H.; Cha, J.Y.; Lee, Y.M.; Kim, D.K. Triticum aestivum sprout derived polysaccharide exerts hepatoprotective effects against ethanol induced liver damage by enhancing the antioxidant system in mice. Int. J. Mol. Med. 2017, 40, 1243–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nepali, S.; Ki, H.H.; Lee, J.H.; Lee, H.Y.; Kim, D.K.; Lee, Y.M. Wheatgrass derived polysaccharide has anti-inflammatory, anti-oxidative and anti-apoptotic effects on LPS induced hepatic injury in mice. Phytother. Res. 2017, 31, 1107–1116. [Google Scholar] [CrossRef]
- Lee, J.H.; Ki, H.H.; Kim, D.K.; Lee, Y.M. Triticum aestivum sprout extract attenuates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice and the expression of chemokines in human keratinocytes. Mol. Med. Rep. 2018, 18, 3461–3468. [Google Scholar] [CrossRef] [Green Version]
- Ki, H.H.; Hwang, S.W.; Lee, J.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. A dichloromethane fraction of Triticum aestivum sprouts reduces allergic immune response through inhibiting Th2 differentiation in ovalbumin immunized mice. Mol. Med. Rep. 2017, 16, 3535–3541. [Google Scholar] [CrossRef] [Green Version]
- Ki, H.H.; Poudel, B.; Lee, J.H.; Lee, Y.M.; Kim, D.K. In vitro and in vivo anti-cancer activity of dichloromethane fraction of Triticum aestivum sprouts. Biomed. Pharmacother. 2017, 96, 120–128. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Gao, J.; Hao, R.; Yang, J.; Wei, J. Effects of simulated digestion on black chokeberry (Aronia melanocarpa (Michx.) Elliot) anthocyanins and intestinal flora. J. Food. Sci. Technol. 2021, 58, 1511–1523. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food. 2010, 3, 255–269. [Google Scholar] [CrossRef] [Green Version]
- King, E.S.; Bolling, B.W. Composition, polyphenol bioavailability, and health benefits of aronia berry: A review. J. Food Bioact. 2020, 11, 13–30. [Google Scholar] [CrossRef]
- Banjari, I.; Misir, A.; Šavikin, K.; Jokić, S.; Molnar, M.; De Zoysa, H.K.S.; Waisundara, V.Y. Antidiabetic effects of Aronia melanocarpa and its other therapeutic properties. Front. Nutr. 2017, 4, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of aronia berry (poly)phenols on vascular function and gut microbiota: A double-blind randomized controlled trial in adult men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, Y.D.; Lee, Y.M.; Kim, D.K. The suppressive effect of puerarin on atopic dermatitis-like skin lesions through regulation of inflammatory mediators in vitro and in vivo. Biochem. Biophys. Res. Commun. 2018, 498, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liang, X.C.; Zhong, Y.L.; He, W.Y.; Wang, Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food Agric. 2015, 95, 1903–1910. [Google Scholar] [CrossRef]
- Luo, J.; He, W.; Li, X.; Ji, X.; Liu, J. Anti-acne vulgaris effects of chlorogenic acid by anti-inflammatory activity and lipogenesis inhibition. Exp. Dermatol. 2021, 30, 865–871. [Google Scholar] [CrossRef]
- Kasraie, S.; Werfel, T. Role of macrophages in the pathogenesis of atopic dermatitis. Mediat. Inflamm. 2013, 2013, 942375. [Google Scholar] [CrossRef] [Green Version]
- Albanesi, C. Keratinocytes in allergic skin diseases. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Werner, Y.; Lindberg, M. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm. Venereol. 1985, 65, 102–105. [Google Scholar]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef]
- Kayama, Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 517–519. [Google Scholar]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramovits, W.; Rivas Bejarano, J.J.; Valdecantos, W.C. Role of interleukin 1 in atopic dermatitis. Dermatol. Clin. 2013, 31, 437–444. [Google Scholar] [CrossRef]
- Qi, X.F.; Kim, D.H.; Yoon, Y.S.; Li, J.H.; Song, S.B.; Jin, D.; Huang, X.Z.; Teng, Y.C.; Lee, K.J. The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF-kappaB in HaCaT keratinocytes. Mol. Immunol. 2009, 46, 1925–1934. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tanga, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; He, J.; Li, S.; Song, L.; Guo, X.; Yao, W.; Zou, D.; Gao, X.; Liu, Y.; Bai, F. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-κB signaling pathway. Int. Immunopharmacol. 2016, 38, 144–152. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Peris, K.; Girolomoni, G. Targeting IL-4 for the Treatment of Atopic Dermatitis. Immunotargets Ther. 2020, 9, 151–156. [Google Scholar] [CrossRef] [PubMed]




| Gene | Gene ID | Forward | Reverse |
|---|---|---|---|
| hTNF-α | 7124 | TTGGAGTGATCGGCCCCCAG | ACAGGCTTGTCACTCGGGGTT |
| hIL-1β | 3553 | CAGCTCTCTCCTTTCAGGGCCA | GGCCGTGGTTTCTGTCAGGC |
| hIL-5 | 3567 | GCTAGCTCTTGGAGCTGCCT | CTTCAGTGCACAGTTGGTGA |
| hIL-6 | 3569 | CTCCACAAGCGCCTTCGGTC | TGTGTGGGGCGGCTACATCT |
| hTSLP | 85480 | GCCATGAAAACTAAGGCTGC | CGCCACAATCCTTGTAATTG |
| hCCL2 | 6347 | CAA ACTGAAGCTCGCACTC | CATTTCCACAATAATATTTTAG |
| hCCL5 | 6352 | CGCTGTCATCCTCATTGCTA | GCACTTGCCACTGGTGTAGA |
| hCCL17 | 6361 | CCATTCCCCTTAGAA AGCTG | CTCTCAAGGCTTTGCAGGTA |
| hCCL22 | 6367 | TGCCGTGATTACGTCCGTTAC | AAGGCCACGGTCATCAGAGTAG |
| hCXCL8 | 3576 | ACCGGAGCACTCCATAAGGCA | AGGCTGCCAAGAGAGCCACG |
| hCXCL10 | 3627 | TTGCTGCCTTATCTTTCTGACTC | ATGGCCTTCGATTCTGGATT |
| miNOS | 18126 | CAGCTGGGCTGTACAAAC | CATTGGAAGTGAAGCGATTCG |
| mCOX-2 | 5912281 | GAAGTCTTTGGTCTGGTGCCTG | GTCTGCTGGTTTGGAATAGTTGC |
| mTNF-α | 21926 | TAGCCAGGAGGGAGAACAGA | TTTTCTGGAGGGAGATGTGG |
| mIL-1β | 16176 | CTCCATGAGCTTTGTACAAGG | TGCTGATGTACCAGTTGGGG |
| mIL-4 | 16189 | ATGGGTCTCAACCCCCAGCTA | TGCATGGCGTCCCTTCTCCT |
| mIL-6 | 16193 | GACAACCACGGCCTTCCCTA | GGTACTCCAGAAGACCAGAGGA |
| mCCL17 | 20295 | GGATGCCATCGTGTTTCTGA | GCCTTCTTCACATGTTTGTCTTTG |
| mCCL22 | 20299 | ATTCTGGGAGTTTCAGGC | ATTCTGAGCCTGCTCCTT |
| mCXCL9 | 17329 | GCAGTGTGGAGTTCGAGGAA | TCTAGGCAGGTTTGATCTCC |
| mCXCL10 | 15945 | CTGAGTGGGACTCAAGGGA | TCGTGGCAATGATCTCAACAC |
| mCXCL11 | 56066 | GGCAGAGATCGAGAAAGCT | ATTGCCTGCATTATGAGGCG |
| mNrf2 | 18024 | ACCAAGGGGCACCATATAAAAG | CTTCGCCGAGTTGCACTC |
| mHO-1 | 15368 | CAGAACCAGCCTGAACTAGC | TGGATGTGTACCTCCTTGGT |
| mNQO1 | 18104 | ACAGGTGAGCTGAAGGACTC | GTTGTCGTACATGGCAGCAT |
| GAPDH | 14433 | CATGGCCTTCCGTGTTC | CCTGGTCCTCAGTGTAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Lim, J.-Y.; Jeon, Y.-D.; Yun, D.-H.; Lee, Y.-M.; Kim, D.-K. Extract of Wheatgrass and Aronia Mixture Ameliorates Atopic Dermatitis-Related Symptoms by Suppressing Inflammatory Response and Oxidative Stress In Vitro and In Vivo. Antioxidants 2023, 12, 27. https://doi.org/10.3390/antiox12010027
Lee J-H, Lim J-Y, Jeon Y-D, Yun D-H, Lee Y-M, Kim D-K. Extract of Wheatgrass and Aronia Mixture Ameliorates Atopic Dermatitis-Related Symptoms by Suppressing Inflammatory Response and Oxidative Stress In Vitro and In Vivo. Antioxidants. 2023; 12(1):27. https://doi.org/10.3390/antiox12010027
Chicago/Turabian StyleLee, Ji-Hyun, Ji-Ye Lim, Yong-Deok Jeon, Dae-Ho Yun, Young-Mi Lee, and Dae-Ki Kim. 2023. "Extract of Wheatgrass and Aronia Mixture Ameliorates Atopic Dermatitis-Related Symptoms by Suppressing Inflammatory Response and Oxidative Stress In Vitro and In Vivo" Antioxidants 12, no. 1: 27. https://doi.org/10.3390/antiox12010027
APA StyleLee, J.-H., Lim, J.-Y., Jeon, Y.-D., Yun, D.-H., Lee, Y.-M., & Kim, D.-K. (2023). Extract of Wheatgrass and Aronia Mixture Ameliorates Atopic Dermatitis-Related Symptoms by Suppressing Inflammatory Response and Oxidative Stress In Vitro and In Vivo. Antioxidants, 12(1), 27. https://doi.org/10.3390/antiox12010027








