Metabolomics Integrated with HPLC–MS Reveals the Crucial Antioxidant Compounds of Muscadine Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Conditions and Materials
2.2. Vinification
2.3. Determination of Physicochemical Indices of Wine
2.4. Oxidation Processes of Wine Samples
2.5. Wine Colour Measurement
2.6. Determination of Antioxidant Indices
2.7. Total Phenolic Content, HPLC–MS, and Metabolomic Analysis
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Muscadine Wines
3.2. Changes in Relevant Attributes during the Oxidation Process
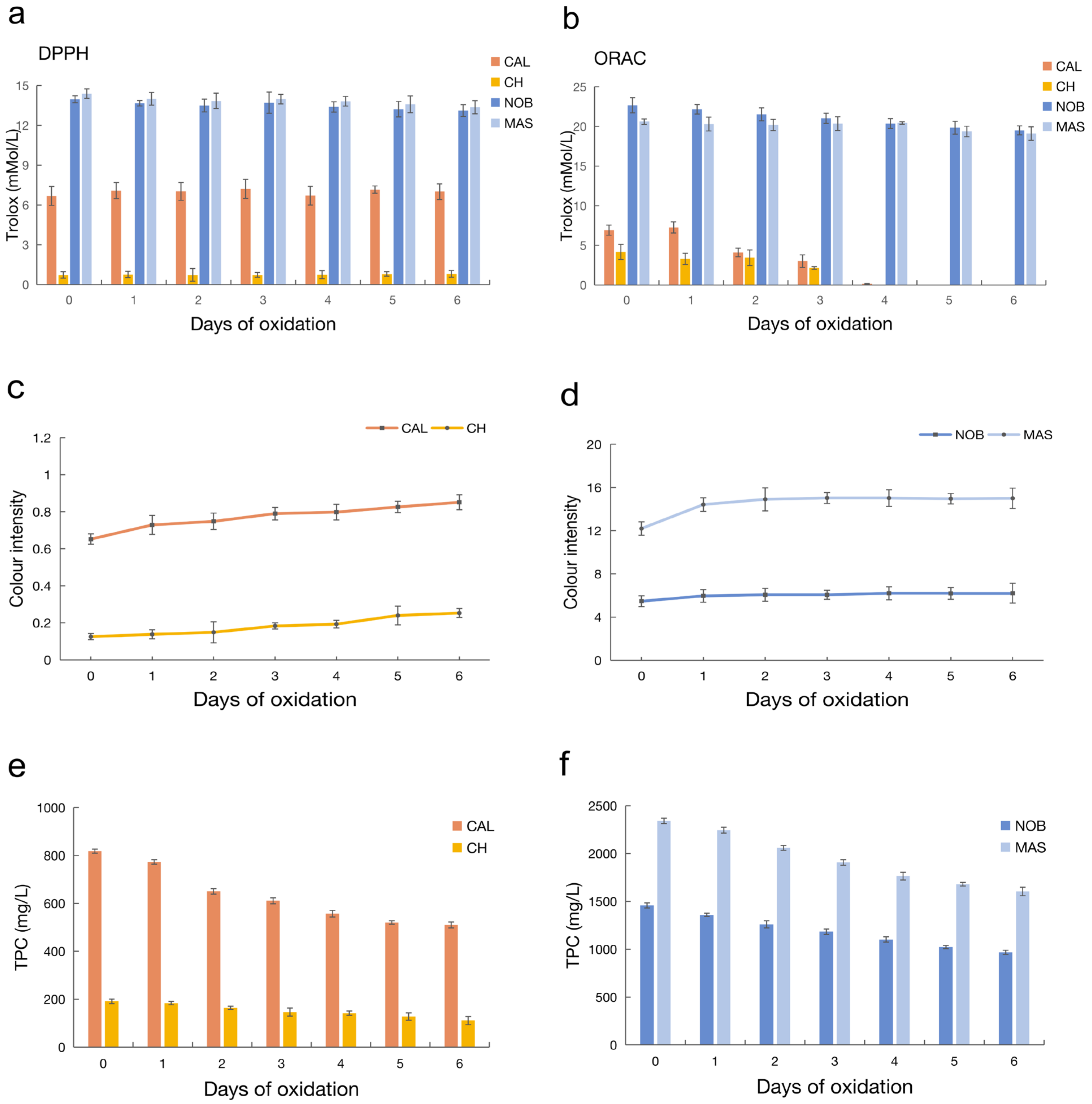
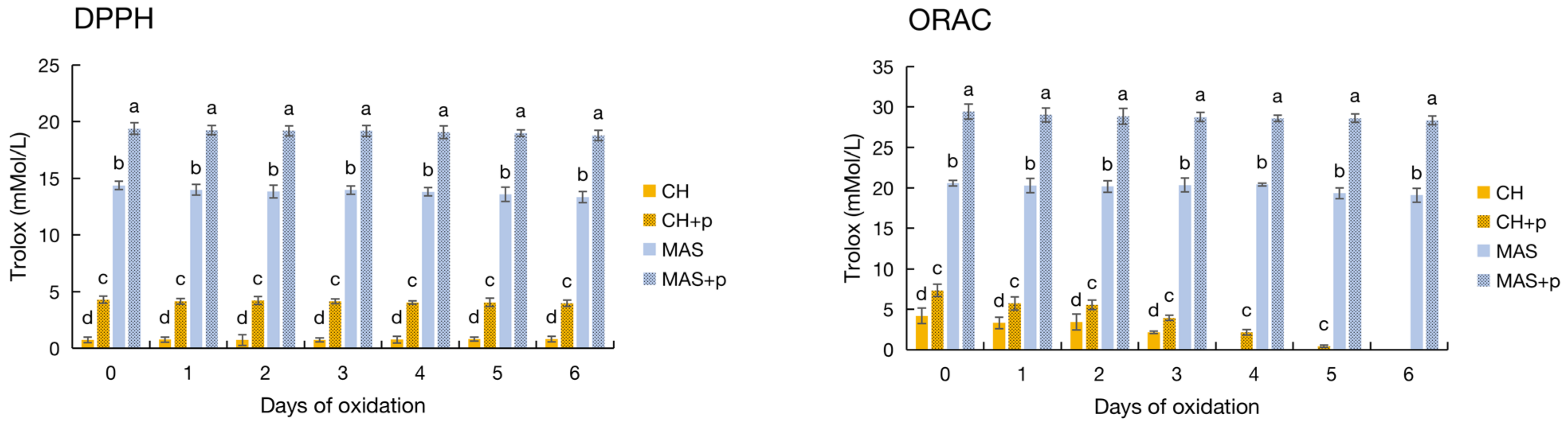
3.2.1. DPPH and ORAC
3.2.2. Color Intensity
3.2.3. Total Phenolic Content
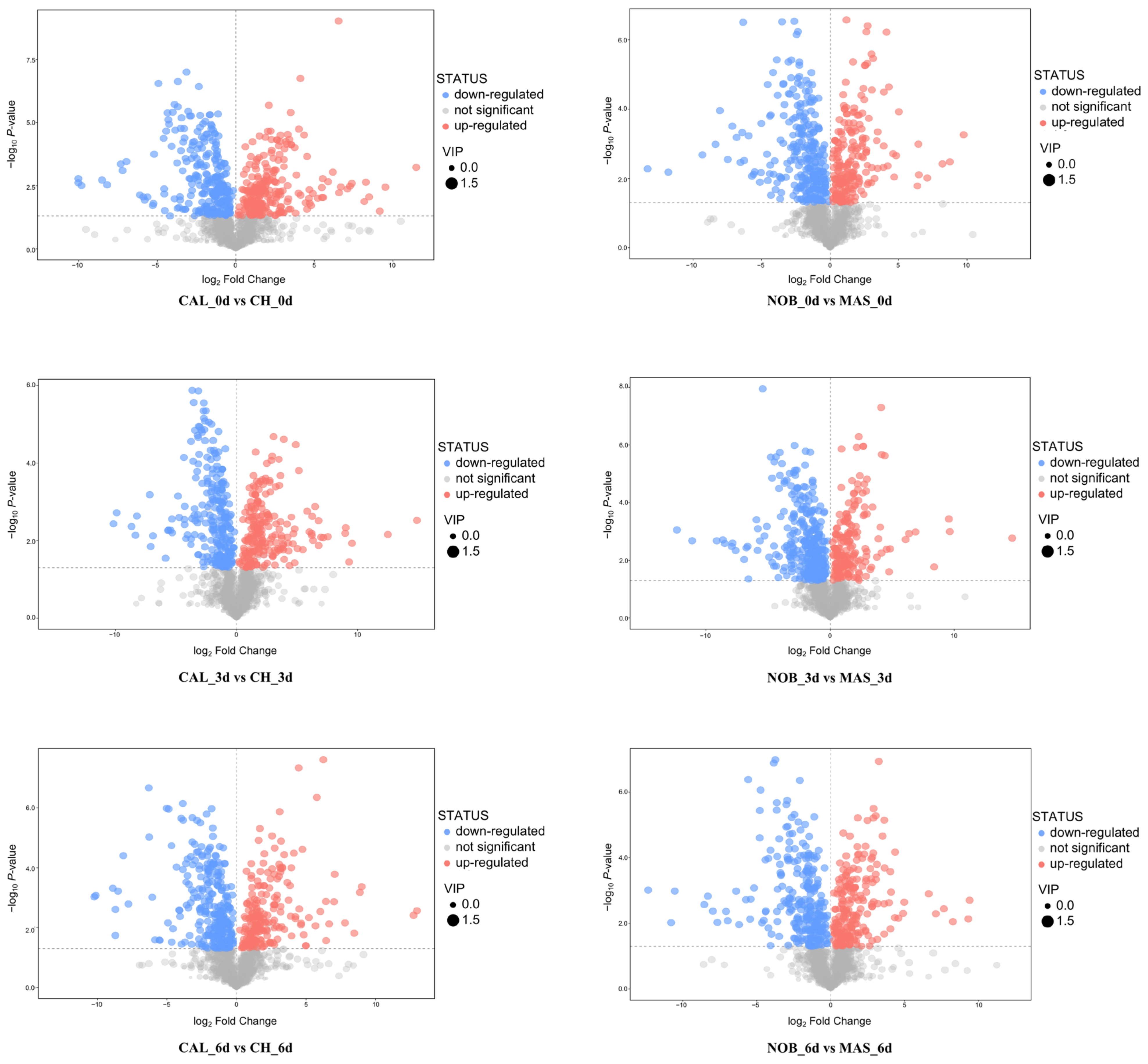
3.3. Metabolomic Analysis of Wine Samples during Oxidation
3.4. Selection of Common SCMs Associated with Antioxidant Capacity in Wine
3.5. Pyrogallol Was a Unique Antioxidant Compound in the Muscadine Wine Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Martinson, T.E.; Liu, R.H. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009, 116, 332–339. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Rangel, L.P.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive compounds and metabolites from grapes and red wine in breast cancer chemoprevention and therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef] [PubMed]
- Mazué, F.; Delmas, D.; Murillo, G.; Saleiro, D.; Limagne, E.; Latruffe, N. Differential protective effects of red wine polyphenol extracts (RWES) on colon carcinogenesis. Food Funct. 2014, 5, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Ferreira, E.; Freitas, V.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Intestinal anti-inflammatory activity of red wine extract: Unveiling the mechanisms in colonic epithelial cells. Food Funct. 2013, 4, 373–383. [Google Scholar] [CrossRef]
- Ju, Y.L.; Yang, B.H.; He, S.; Tu, T.Y.; Min, Z.; Fang, Y.L.; Sun, X.Y. Anthocyanin accumulation and biosynthesis are modulated by regulated deficit irrigation in cabernet sauvignon (Vitis vinifera L.) grapes and wines. Plant Physiol. Biochem. 2019, 135, 469–479. [Google Scholar] [CrossRef]
- OIV. 2019 Statistical Report on World Vitiviniculture. 2019. Available online: https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 1 July 2022).
- Danilewicz, J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of iron and copper. Am. J. Enol. Viticult. 2003, 54, 73–85. [Google Scholar] [CrossRef]
- Oliveira, R.; Marques, J.; Bento, F.; Geraldo, D.; Bettencourt, P. Reducing antioxidant capacity evaluated by means of controlled potential electrolysis. Electroanalysis 2011, 23, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Dimkou, E.; Ugliano, M.; Dieval, J.B.; Vidal, S.; Aagaard, O.; Rauhut, D.; Jung, R. Impact of headspace oxygen and closure on sulfur dioxide, color, and hydrogen sulfide levels in a Riesling wine. Am. J. Enol. Viticult. 2011, 62, 261–269. [Google Scholar] [CrossRef]
- Ugliano, M. Oxygen contribution to wine aroma evolution during bottle aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef]
- Sanchez-Gomez, R.; del Alamo-Sanza, M.; Martinez-Martinez, V.; Nevares, I. Study of the role of oxygen in the evolution of red wine colour under different ageing conditions in barrels and bottles. Food Chem. 2020, 328, 127040. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Pons, A.; Nikolantonaki, M.; Lavigne, V.; Shinoda, K.; Dubourdieu, D.; Darriet, P. New insights into intrinsic and extrinsic factors triggering premature aging in white wines. In Advances in Wine Research; American Chemical Society: Washington, DC, USA, 2015. [Google Scholar]
- Ferreira, A.C.S.; Oliveira, C.; Hogg, T.; de Pinho, P.G. Relationship between potentiometric measurements, sensorial analysis, and some substances responsible for aroma degradation of white wines. J. Agric. Food Chem. 2003, 51, 4668–4672. [Google Scholar] [CrossRef] [PubMed]
- Cullere, L.; Cacho, J.; Ferreira, V. An assessment of the role played by some oxidation-related aldehydes in wine aroma. J. Agric. Food Chem. 2007, 55, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, J. Cytogenetic study of interspecific hybrids between Vitis rotundifolia and Vitis vinifera. In Proceedings of the 26th International Horticultural Congress, Toronto, ON, Canada, 11–17 August 2002. [Google Scholar]
- Talcott, S.T.; Lee, J.H. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J. Agric. Food Chem. 2002, 50, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.A.; Stringer, S.J.; Spiers, J.D. Storage retention of stilbene, ellagic acid, flavonol, and phenolic content of muscadine grape (Vitis rotundifolia Michx.) cultivars. HortScience 2014, 49, S37. [Google Scholar]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Filho, D.W.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Phenolic profile and effect of regular consumption of brazilian red wines on in vivo antioxidant activity. J. Food Compos. Anal. 2013, 31, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Lamikanra, O. Aroma constituents of muscadine wines. J. Food Qual. 1987, 10, 57–66. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis-Oiv. 2021. Available online: https://www.oiv.int/public/medias/2492/oiv-ma-as312-03a-en.pdf (accessed on 1 July 2022).
- Yildirim, H.K. Evaluation of colour parameters and antioxidant activities of fruit wines. Int. J. Food Sci. Nutr. 2006, 57, 47–63. [Google Scholar] [CrossRef]
- Chrzczanowicz, J.; Gawron, A.; Zwolinska, A.; de Graft-Johnson, J.; Krajewski, W.; Krol, M.; Markowski, J.; Kostka, T.; Nowak, D. Simple method for determining human serum 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity–possible application in clinical studies on dietary antioxidants. Clin. Chem. Lab. Med. 2008, 46, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.A.; Villanueva, M.J.; Lissarrague, J.R. Effect of irrigation on changes in berry composition of tempranillo during maturation. Sugars, organic acids, and mineral elements. Am. J. Enol. Viticult. 1999, 50, 418–434. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Z.; Li, Y.; Zhou, Z.; Ye, Y.; Lu, T.; Sun, L.; Qian, H. Comparative molecular and metabolic responses of wheat seedlings (Triticum aestivum L.) to the imazethapyr enantiomers S-IM and R-IM. Sci. Total Environ. 2019, 692, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Chenguang, Z.; Yuanyuan, T.; Gossner, S.; Youfa, L.; Qingyao, S.; Engel, K.H. Impact of crossing parent and environment on the metabolite profiles of progenies generated from a low phytic acid rice (Oryza sativa L.) mutant. J. Agric. Food Chem. 2019, 67, 2396–2407. [Google Scholar]
- Ertekin, B.; Okur, O.D.; Guzel-Seydim, Z. Formation of flavor compounds by amino acid catabolism in cheese. Gida 2009, 34, 43–50. [Google Scholar]
- Procopio, S.; Krause, D.; Hofmann, T.; Becker, T. Significant amino acids in aroma compound profiling during yeast fermentation analyzed by PLS regression. LWT-Food Sci. Technol. 2013, 51, 423–432. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jimenez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Sun, X.Y.; Cheng, X.H.; Zhang, J.Z.; Ju, Y.L.; Que, Z.L.; Liao, X.; Lao, F.; Fang, Y.L.; Ma, T.T. Letting wine polyphenols functional: Estimation of wine polyphenols bioaccessibility under different drinking amount and drinking patterns. Food Res. Int. 2020, 127, 108704. [Google Scholar] [CrossRef]
- Yang, B.H.; Yao, H.; Zhang, J.X.; Li, Y.Q.; Ju, Y.L.; Zhao, X.F.; Sun, X.Y.; Fang, Y.L. Effect of regulated deficit irrigation on the content of soluble sugars, organic acids and endogenous hormones in Cabernet Sauvignon in the Ningxia region of China. Food Chem. 2020, 312, 126020. [Google Scholar] [CrossRef]
- Basha, S.M.; Vasanthaiah, H.K.; Kambiranda, D.M.; Easwaran, K.; Queeley, G. Genetic variation in sugar composition among muscadine, florida hybrid bunch and bunch grape genotypes. Int. J. Wine Res. 2012, 4, 15–23. [Google Scholar]
- Lamikanra, O. Changes in organic acid composition during fermentation and aging of noble muscadine wine. J. Agric. Food Chem. 1997, 45, 935–937. [Google Scholar] [CrossRef]
- Singleton, V.L. Oxygen with phenols and related reactions in musts, wines, and model systems: Observations and practical implications. Am. J. Enol. Viticult. 1987, 38, 69–77. [Google Scholar]
- Kilmartin, P.A.; Zou, H.L.; Waterhouse, A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C.; Wallbridge, P.J. Further studies on the mechanism of interaction of polyphenols, oxygen, and sulfite in wine. Am. J. Enol. Viticult. 2010, 61, 166–175. [Google Scholar] [CrossRef]
- Cheynier, V.; Duenas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Viticult. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Truscott, T.G. Interactions of dietary carotenoids with singlet oxygen (1o2) and free radicals: Potential effects for human health. Acta Biochim. Pol. 2012, 59, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.H.; He, S.; Liu, Y.; Liu, B.C.; Ju, Y.L.; Kang, D.Z.; Sun, X.Y.; Fang, Y.L. Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries. Food Chem. 2020, 314, 126170. [Google Scholar] [CrossRef]
- Paixao, J.; Dinis, T.C.P.; Almeida, L.M. Protective role of malvidin-3-glucoside on peroxynitrite-induced damage in endothelial cells by counteracting reactive species formation and apoptoticmitochondrial pathway. Oxid. Med. Cell. Longev. 2012, 2012, 428538. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Gu, Y.; Peng, T.; Yang, D.; Chang, R.C.-C.; So, K.-F.; Liu, K.; Shen, J. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite-mediated neurotoxicity in cerebral ischemia-reperfusion injury. J. Ethnopharmacol. 2013, 150, 116–124. [Google Scholar] [CrossRef]
- Shin, M.; Park, E.; Lee, H. Plant-inspired pyrogallol-containing functional materials. Adv. Funct. Mater. 2019, 29, 1903022. [Google Scholar] [CrossRef]
- Bita, M.; Preda, M. The effect of polyphenols from Vitis vinifera on the oxidation of coffee lipids. Riv. Ital. Sostanze Gr. 2004, 81, 377–379. [Google Scholar]
- Mendes, V.; Vilaca, R.; de Freitas, V.; Ferreira, P.M.; Mateus, N.; Costa, V. Effect of myricetin, pyrogallol, and phloroglucinol on yeast resistance to oxidative stress. Oxid. Med. Cell. Longev. 2015, 2015, 782504. [Google Scholar] [CrossRef] [PubMed]
- Thi, H.N.; Waterhouse, A.L. Redox cycling of iron: Effects of chemical composition on reaction rates with phenols and oxygen in model wine. Am. J. Enol. Viticult. 2021, 72, 209–216. [Google Scholar]



| Sample | Residue Sugar (g/L) | Total Acidity (g/L) | Alcohol (%) | Total SO2 (mg/L) | Free SO2 (mg/L) | pH |
|---|---|---|---|---|---|---|
| CAL | 1.12 ± 0.09 | 5.39 ± 0.14 | 7.52 ± 0.24 | 78.24 ± 0.56 | 29.08 ± 0.87 | 3.63 ± 0.02 |
| CH | 1.35 ± 0.26 | 6.32 ± 0.08 | 13.53 ± 0.31 | 92.19 ± 1.34 | 32.21 ± 0.69 | 3.32 ± 0.02 |
| NOB | 2.34 ± 0.12 | 5.11 ± 0.17 | 7.04 ± 0.22 | 70.12 ± 1.83 | 23.33 ± 0.28 | 3.68 ± 0.03 |
| MAS | 2.81 ± 0.15 | 5.52 ± 0.11 | 13.98 ± 0.27 | 84.85 ± 1.02 | 30.04 ± 0.35 | 3.51 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, F.; Yang, B.; Fu, P.; Peng, Y.; Lu, J. Metabolomics Integrated with HPLC–MS Reveals the Crucial Antioxidant Compounds of Muscadine Wine. Antioxidants 2023, 12, 55. https://doi.org/10.3390/antiox12010055
Xue F, Yang B, Fu P, Peng Y, Lu J. Metabolomics Integrated with HPLC–MS Reveals the Crucial Antioxidant Compounds of Muscadine Wine. Antioxidants. 2023; 12(1):55. https://doi.org/10.3390/antiox12010055
Chicago/Turabian StyleXue, Fei, Bohan Yang, Peining Fu, Yachun Peng, and Jiang Lu. 2023. "Metabolomics Integrated with HPLC–MS Reveals the Crucial Antioxidant Compounds of Muscadine Wine" Antioxidants 12, no. 1: 55. https://doi.org/10.3390/antiox12010055
APA StyleXue, F., Yang, B., Fu, P., Peng, Y., & Lu, J. (2023). Metabolomics Integrated with HPLC–MS Reveals the Crucial Antioxidant Compounds of Muscadine Wine. Antioxidants, 12(1), 55. https://doi.org/10.3390/antiox12010055






