Sex Differences in X-ray-Induced Endothelial Damage: Effect of Taurine and N-Acetylcysteine
Abstract
:1. Introduction
2. Methods
2.1. Donors
2.2. Cell Isolation and Characterization
2.3. Experimental Procedures
2.4. Cell Viability
2.5. LDH Assay
2.6. Wound Healing Assay
2.7. MDA Determination
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Characteristics of Donors
3.2. Effect of X-rays on HUVECs Viability and Lactate Dehydrogenase (LDH) Release
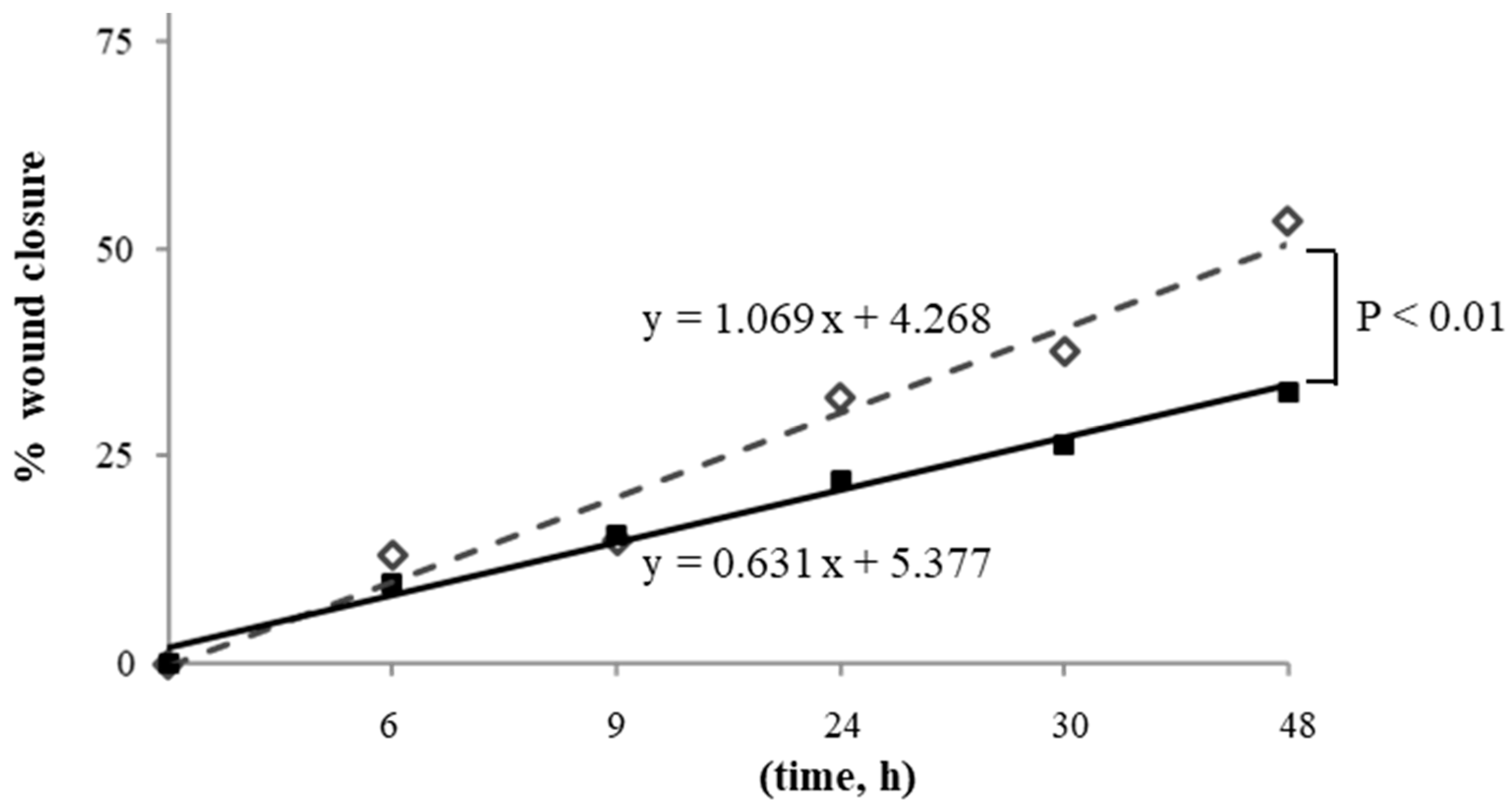
3.3. Effect of X-rays on HUVECs Migration
3.4. Effect of X-rays on Autophagy
3.5. Effect of X-rays on Lipid Peroxidation
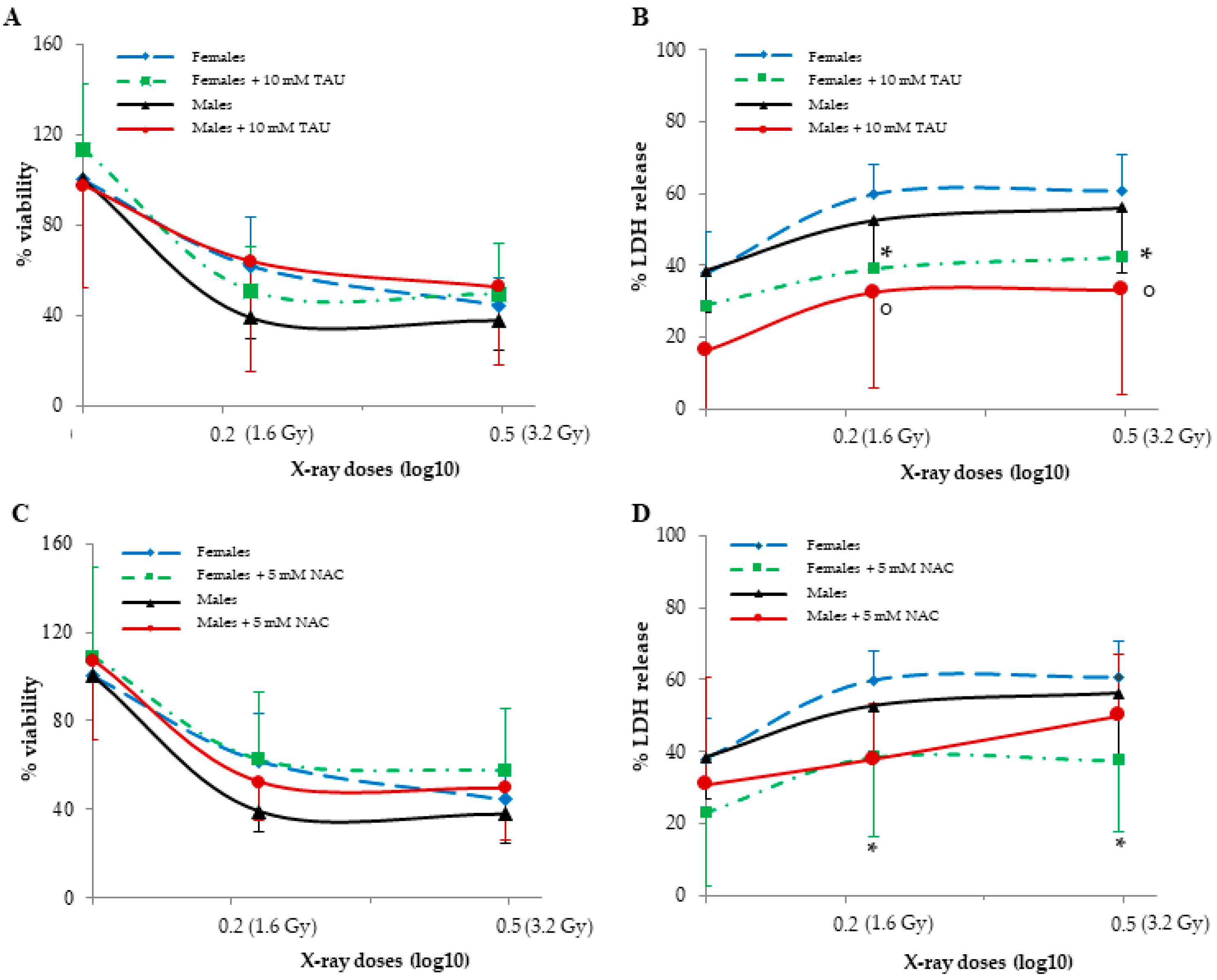
3.6. Effect of Pre-Treatments on HUVECs Viability and LDH Release
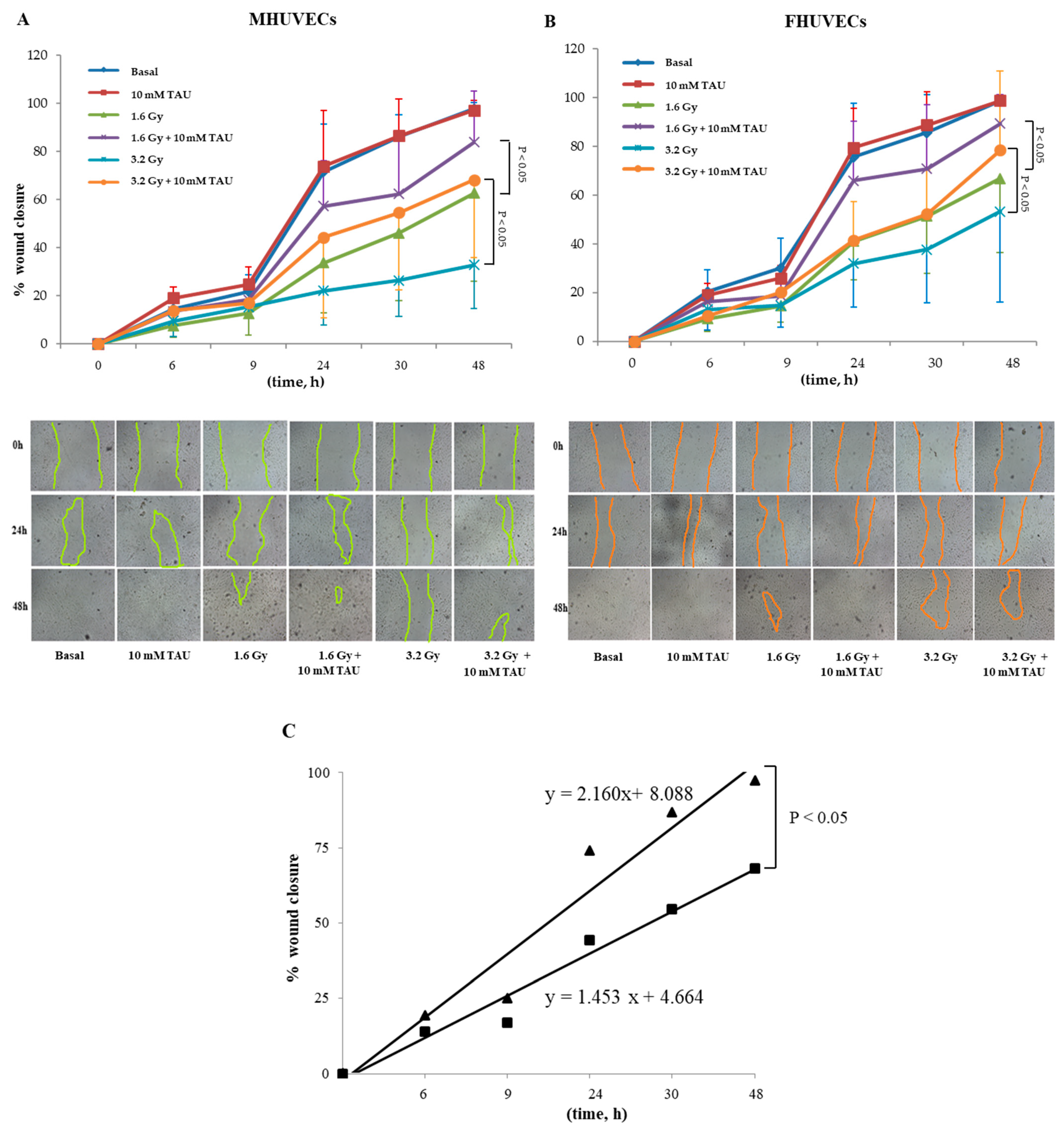
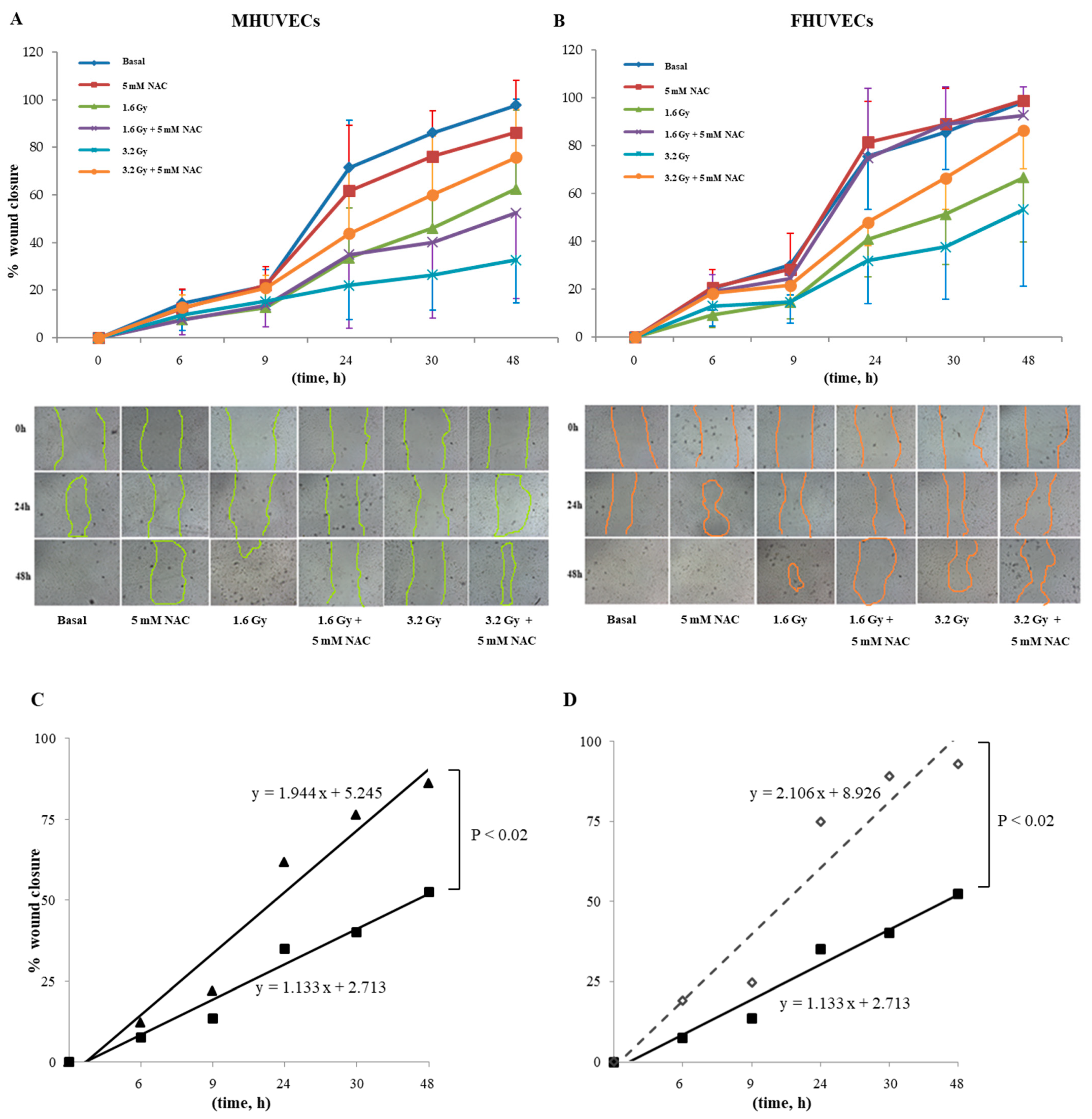
3.7. Effect of Pre-Treatments on HUVECs Migration
3.8. Effect of Pre-Treatments on Autophagy
3.9. Effect of Pre-Treatments on Lipid Peroxidation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Legato, M. Principles of Gender-Specific Medicine, 3rd ed.; Elservier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Campesi, I.; Montella, A.; Seghieri, G.; Franconi, F. The person’s care requires a sex and gender approach. J. Clin. Med. 2021, 10, 4470. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, E.; Domanico, F.; La Russa, D.; Pellegrino, D. Sex differences in oxidative stress biomarkers. Curr. Drug Targets 2014, 15, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.R.; Singh, K.; Yu, Q.; Sen, C.K.; Wang, M. Sex as biological variable in cardiac mitochondrial bioenergetic responses to acute stress. Int. J. Mol. Sci. 2022, 23, 9312. [Google Scholar] [CrossRef]
- Bazan, I.S.; Kim, S.J.; Ardito, T.A.; Zhang, Y.; Shan, P.; Sauler, M.; Lee, P.J. Sex differences and altered mitophagy in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L761–L769. [Google Scholar] [CrossRef]
- Campesi, I.; Romani, A.; Franconi, F. The sex-gender effects in the road to tailored botanicals. Nutrients 2019, 11, 1637. [Google Scholar] [CrossRef] [Green Version]
- Campesi, I.; Marino, M.; Cipolletti, M.; Romani, A.; Franconi, F. Put “gender glasses” on the effects of phenolic compounds on cardiovascular function and diseases. Eur. J. Nutr. 2018, 57, 2677–2691. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhong, Y.; Qin, L.; Li, J. Sex-dimorphic distribution and anti-oxidative effects of selenomethionine and Se-methylselenocysteine supplementation. J. Food Sci. 2021, 86, 5424–5438. [Google Scholar] [CrossRef]
- Wang, L.; Ahn, Y.; Asmis, R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox Biol. 2020, 31, 101410. [Google Scholar] [CrossRef]
- Protecting Workers—Canadian Nuclear Safety Commission. Available online: http://www.nuclearsafety.gc.ca/eng/resources/radiation/introduction-to-radiation/protecting-workers.cfm (accessed on 26 September 2022).
- Langen, B.; Vorontsov, E.; Spetz, J.; Swanpalmer, J.; Sihlbom, C.; Helou, K.; Forssell-Aronsson, E. Age and sex effects across the blood proteome after ionizing radiation exposure can bias biomarker screening and risk assessment. Sci. Rep. 2022, 12, 7000. [Google Scholar] [CrossRef]
- Narendran, N.; Luzhna, L.; Kovalchuk, O. Sex difference of radiation response in occupational and accidental exposure. Front. Genet. 2019, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Biegon, A.; Cohen, S.; Franceschi, D. Modulation of secondary cancer risks from radiation exposure by sex, age and gonadal hormone status: Progress, opportunities and challenges. J. Pers. Med. 2022, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.S. Ionising radiation and cancer risks: What have we learned from epidemiology? Int. J. Radiat. Biol. 2009, 85, 467–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Hatoum, O.A.; Otterson, M.F.; Kopelman, D.; Miura, H.; Sukhotnik, I.; Larsen, B.T.; Selle, R.M.; Moulder, J.E.; Gutterman, D.D. Radiation induces endothelial dysfunction in murine intestinal arterioles via enhanced production of reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Panganiban, R.A.M.; Mungunsukh, O.; Day, R.M. X-irradiation induces ER stress, apoptosis, and senescence in pulmonary artery endothelial cells. Int. J. Radiat. Biol. 2013, 89, 656–667. [Google Scholar] [CrossRef]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell. Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, C.; Aerts, A.; Beck, M.; De Vos, W.H.; Van Oostveldt, P.; Benotmane, M.A.; Baatout, S. Differential response to acute low dose radiation in primary and immortalized endothelial cells. Int. J. Radiat. Biol. 2013, 89, 841–850. [Google Scholar] [CrossRef]
- Kalamida, D.; Karagounis, I.; Giatromanolaki, A.; Koukourakis, M. Important role of autophagy in endothelial cell response to ionizing radiation. PLoS ONE 2014, 9, e102408. [Google Scholar] [CrossRef]
- Guipaud, O.; Jaillet, C.; Clément-Colmou, K.; François, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef]
- Kouam, P.N.; Rezniczek, G.A.; Adamietz, I.A.; Bühler, H. Ionizing radiation increases the endothelial permeability and the transendothelial migration of tumor cells through ADAM10-activation and subsequent degradation of VE-cadherin. BMC Cancer 2019, 19, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, S.; Broese, S.; Baake, J.; Juerß, D.; Kriesen, S.; Hildebrandt, G.; Manda, K. Effect of ionizing radiation on human EA.hy926 endothelial cells under inflammatory conditions and their interactions with A549 tumour cells. J. Immunol. Res. 2019, 2019, 9645481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, R.; Claessens, M.; Cocquyt, E.; Mysara, M.; Decrock, E.; Baatout, S.; Aerts, A.; Leybaert, L. X-irradiation induces acute and early term inflammatory responses in atherosclerosis-prone ApoE-/-mice and in endothelial cells. Mol. Med. Rep. 2021, 23, 399. [Google Scholar] [CrossRef] [PubMed]
- Kuefner, M.A.; Brand, M.; Engert, C.; Schwab, S.A.; Uder, M. Radiation Induced DNA Double-Strand Breaks in Radiology. RoFo Fortschr. Geb. Rontgenstrahlen Bildgeb. Verfahr. 2015, 187, 872–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, N.; Seo, E.; Efferth, T. Prevention from radiation damage by natural products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef]
- Yamashita, T.; Kato, T.; Isogai, T.; Gu, Y.; Ma, N. Protective Effects of Taurine on the Radiation Exposure Induced Cellular Damages in the Mouse Intestine. Adv. Exp. Med. Biol. 2019, 1155, 443–450. [Google Scholar] [CrossRef]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubert, J.; Agrawal, D.K. Radioprotective agents to prevent cellular damage due to ionizing radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef]
- Franconi, F.; Loizzo, A.; Ghirlanda, G.; Seghieri, G. Taurine supplementation and diabetes mellitus. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 32–36. [Google Scholar] [CrossRef]
- Franconi, F.; Di Leo, M.A.S.; Bennardini, F.; Ghirlanda, G. Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem. Res. 2004, 29, 143–150. [Google Scholar] [CrossRef]
- Bianchi, L.; Lari, R.; Anichini, R.; De Bellis, A.; Berti, A.; Napoli, Z.; Seghieri, G.; Franconi, F. Taurine transporter gene expression in peripheral mononuclear blood cells of type 2 diabetic patients. Amino Acids 2012, 42, 2267–2274. [Google Scholar] [CrossRef]
- Katakawa, M.; Fukuda, N.; Tsunemi, A.; Mori, M.; Maruyama, T.; Matsumoto, T.; Abe, M.; Yamori, Y. Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Hypertens. Res. 2016, 39, 848–856. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Moscarella, E.; Caiazza, M.; Gragnano, F.; Ranieri, A.; D’Alicandro, G.; Tinto, N.; et al. Dietary Thiols: A potential supporting strategy against oxidative stress in heart failure and muscular damage during sports activity. Int. J. Environ. Res. Public Health 2020, 17, 9424. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, O. Radiation protection following nuclear power accidents: A survey of putative mechanisms involved in the radioprotective actions of taurine during and after radiation exposure. Microb. Ecol. Health Dis. 2012, 23, 14787. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Maliakkal, J.; Kinzie, J.; Ehrinpreis, M.; Luk, G.; Cejka, J. Taurine deficiency after intensive chemotherapy and/or radiation. Am. J. Clin. Nutr. 1992, 55, 708–711. [Google Scholar] [CrossRef]
- Bkaily, G.; Simon, Y.; Normand, A.; Jazzar, A.; Najibeddine, H.; Khalil, A.; Jacques, D. Short-communication: Short-term treatment with taurine prevents the development of cardiac hypertrophy and early death in hereditary cardiomyopathy of the hamster and is sex-dependent. Nutrients 2022, 14, 3287. [Google Scholar] [CrossRef]
- Vasquez, M.A.; Cruz, G.B.; Cabañas, E.; Joseph, J.N.; Mian, M.; Madhira, S.K.V.; Akintunde, C.A.; Clarke, E.G.; Skeen, J.C.; Bonitto, J.R.; et al. In vivo sex-dependent effects of perinatal Pb2+ exposure on pilocarpine-induced seizure susceptibility and taurine neuropharmacology. Adv. Exp. Med. Biol. 2022, 1370, 481–496. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on human health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, C.Y.; Fan, G.H. Efficacy of N-acetylcysteine in preventing atrial fibrillation after cardiac surgery: A meta-analysis of published randomized controlled trials. BMC Cardiovasc. Disord. 2014, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Eligini, S.; Porro, B.; Aldini, G.; Colli, S.; Banfi, C. N-Acetylcysteine Inhibits Platelet Function through the Regeneration of the Non-Oxidative Form of Albumin. Antioxidants 2022, 11, 445. [Google Scholar] [CrossRef]
- Reliene, R.; Pollard, J.; Sobol, Z.; Trouiller, B.; Gatti, R.; Schiestl, R. N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals. Mutat. Res. 2009, 665, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Hafez, H.; Fahmy, N.; Hanafi, N. Protective effect of N-acetylcysteine against radiation induced DNA damage and hepatic toxicity in rats. Biochem. Pharmacol. 2008, 75, 773–780. [Google Scholar] [CrossRef]
- Demirel, C.; Kilciksiz, S.; Gurgul, S.; Erdal, N.; Yildiz, A. N-acetylcysteine ameliorates γ-radiation-induced deterioration of bone quality in the rat femur. J. Int. Med. Res. 2011, 39, 2393–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafarullah, M.; Li, W.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef]
- Underwood, B.; Imarisio, S.; Fleming, A.; Rose, C.; Krishna, G.; Heard, P.; Quick, M.; Korolchuk, V.; Renna, M.; Sarkar, S.; et al. Antioxidants can inhibit basal autophagy and enhance neurodegeneration in models of polyglutamine disease. Hum. Mol. Genet. 2010, 19, 3413–3429. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Chen, K.; Xia, Y.; Dai, W.; Wang, F.; Shen, M.; Cheng, P.; Wang, J.; Lu, J.; Zhang, Y.; et al. N-acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS ONE 2014, 9, e108855. [Google Scholar] [CrossRef]
- Yang, L.; Tan, P.; Zhou, W.; Zhu, X.; Cui, Y.; Zhu, L.; Feng, X.; Qi, H.; Zheng, J.; Gu, P.; et al. N-acetylcysteine protects against hypoxia mimetic-induced autophagy by targeting the HIF-1α pathway in retinal ganglion cells. Cell. Mol. Neurobiol. 2012, 32, 1275–1285. [Google Scholar] [CrossRef]
- Walther, M.; Kaffenberger, W.; Van Beuningen, D. Influence of clinically used antioxidants on radiation-induced expression of intercellular cell adhesion molecule-1 on HUVEC. Int. J. Radiat. Biol. 1999, 75, 1317–1325. [Google Scholar] [CrossRef]
- Azuma, J.; Sawamura, A.; Awata, N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn. Circ. J. 1992, 56, 95–99. [Google Scholar] [CrossRef]
- Schoeps, V.A.; Graves, J.S.; Stern, W.A.; Zhang, L.; Nourbakhsh, B.; Mowry, E.M.; Henry, R.G.; Waubant, E. N-acetyl cysteine as a neuroprotective agent in progressive multiple sclerosis (NACPMS) trial: Study protocol for a randomized, double-blind, placebo-controlled add-on phase 2 trial. Contemp. Clin. Trials 2022, 122, 106941. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Di Nicola, P.; Varalda, A.; Occhi, L.; Giuliani, F.; Coscia, A. Neonatal growth charts. J. Matern. Neonatal Med. 2012, 25, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Addis, R.; Campesi, I.; Fois, M.; Capobianco, G.; Dessole, S.; Fenu, G.; Montella, A.; Cattaneo, M.G.; Vicentini, L.M.; Franconi, F. Human umbilical endothelial cells (HUVECs) have a sex: Characterisation of the phenotype of male and female cells. Biol. Sex Differ. 2014, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnegan, N.M.; Redmond, H.P.; Bouchier-Hayes, D.J. Taurine attenuates recombinant interleukin-2-activated, lymphocyte-mediated endothelial cell injury. Cancer 1998, 82, 186–199. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, L.; Fennessy, F.; Redmond, H.; Bouchier-Hayes, D. Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. Am. J. Physiol. 1999, 277, C1229–C1238. [Google Scholar] [CrossRef]
- Abebe, W.; Mozaffari, M.S. Role of taurine in the vasculature: An overview of experimental and human studies. Am. J. Cardiovasc. Dis. 2011, 1, 293–311. [Google Scholar]
- Szotowski, B.; Antoniak, S.; Goldin-Lang, P.; Tran, Q.V.; Pels, K.; Rosenthal, P.; Bogdanov, V.Y.; Borchert, H.H.; Schultheiss, H.P.; Rauch, U. Antioxidative treatment inhibits the release of thrombogenic tissue factor from irradiation- and cytokine-induced endothelial cells. Cardiovasc. Res. 2007, 73, 806–812. [Google Scholar] [CrossRef]
- Li, C. N-Acetylcysteine Resists High-Glucose-Induced Injury of Human Umbilical Vein Endothelial Cells by Inhibiting the Leptin/Leptin Receptor. 2020. Available online: https://assets.researchsquare.com/files/rs-18647/v2/42043b9b-6deb-49f6-9fe0-c62387b80a0e.pdf?c=1631832888 (accessed on 12 May 2022).
- Elengoe, A.; Hamdan, S. Evaluation of hyperthermia effect on cell viability using crystal violet staining, LDH and trypan blue assays. Adv. Environ. Biol. 2014, 8, 744–747. [Google Scholar]
- Campesi, I.; Galistu, A.; Carru, C.; Franconi, F.; Fois, M.; Zinellu, A. Glutamyl cycle in the rat liver appears to be sex-gender specific. Exp. Toxicol. Pathol. 2013, 65, 585–589. [Google Scholar] [CrossRef]
- Campesi, I.; Straface, E.; Occhioni, S.; Montella, A.; Franconi, F. Protein oxidation seems to be linked to constitutive autophagy: A sex study. Life Sci. 2013, 93, 145–152. [Google Scholar] [CrossRef]
- Campesi, I.; Occhioni, S.; Tonolo, G.; Cherchi, S.; Basili, S.; Carru, C.; Zinellu, A.; Franconi, F. Ageing/menopausal status in healthy women and ageing in healthy men differently affect cardiometabolic parameters. Int. J. Med. Sci. 2016, 13, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalby, K.N.; Tekedereli, I.; Lopez-Berestein, G.; Ozpolat, B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy 2010, 6, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef] [Green Version]
- Mayer, P.J.; Lange, C.S.; Bradley, M.O.; Nichols, W.W. Gender differences in age-related decline in DNA double-strand break damage and repair in lymphocytes. Ann. Hum. Biol. 1991, 18, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Omori, A.; Kashiwakura, I. Radiosensitivity of human haematopoietic stem/progenitor cells. J. Radiol. Prot. 2013, 33, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Berthold, H.K.; Campesi, I.; Carrero, J.J.; Dakal, S.; Franconi, F.; Gouni-Berthold, I.; Heiman, M.L.; Kautzky-Willer, A.; Klein, S.L.; et al. Sex-and gender-based pharmacological response to drugs. Pharmacol. Rev. 2021, 73, 730–762. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Montella, A.; Sotgiu, G.; Dore, S.; Carru, C.; Zinellu, A.; Palermo, M.; Franconi, F. Combined oral contraceptives modify the effect of smoking on inflammatory cellular indexes and endothelial function in healthy subjects. Eur. J. Pharmacol. 2021, 891, 173762. [Google Scholar] [CrossRef]
- Casey, R.; Gang, C.; Joyce, M.; Bouchier-Hayes, D. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte-endothelial cell interactions and cardiac dysfunction. J. Vasc. Res. 2007, 44, 31–39. [Google Scholar] [CrossRef]
- Wang, J.; Redmond, H.; Watson, R.; Condron, C.; Bouchier-Hayes, D. The beneficial effect of taurine on the prevention of human endothelial cell death. Shock 1996, 6, 331–338. [Google Scholar] [CrossRef]
- Franconi, F.; Giotti, A.; Manzini, S.; Martini, F.; Stendardi, I.; Zilletti, L. The effect of taurine on high potassium-and noradrenaline-induced contraction in rabbit ear artery. Br. J. Pharmacol. 1982, 75, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Jiang, D.; Huang, H.; Jia, S.; Jiang, J.; Hu, C.; Li, Y. Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Vascul. Pharmacol. 2007, 46, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Huang, H.P.; Hsu, J.D.; Lai, Y.R.; Hsiao, Y.P.; Lu, F.J.; Chang, H.R. Topical N-acetylcysteine accelerates wound healing in vitro and in vivo via the PKC/Stat3 pathway. Int. J. Mol. Sci. 2014, 15, 7563–7578. [Google Scholar] [CrossRef] [PubMed]

| Age of Mothers (Years) | Body Weight of Mothers (kg) | Body Weight of Neonates (kg) | |
|---|---|---|---|
| Males (n = 9) | 31.2 ± 5.9 | 65.3 ± 7.0 | 3.3 ± 0.3 |
| Females (n = 10) | 30.6 ± 4.4 | 62.3 ± 6.4 | 3.3 ± 0.4 |
| 6 h | 9 h | 24 h | 30 h | 48 h | ||
|---|---|---|---|---|---|---|
| Basal | MHUVECs | 14.6 ± 5.7 | 21.8 ± 6.8 | 71.5 ± 19.8 | 86.1 ± 9.2 | 97.7 ± 2.6 |
| FHUVECs | 20.2 ± 8.9 | 30.1 ± 12.3 | 75.6 ± 22.1 | 85.6 ± 15.7 | 98.5 ± 1.9 | |
| 1.6 Gy | MHUVECs | 7.7 ± 4.8 ° | 12.7 ± 8.9 | 33.6 ± 20.8 ° | 46.1 ± 28.1 ° | 62.6 ± 36.6 |
| FHUVECs | 9.2 ± 5.2 * | 14.6 ± 6.8 * | 41.0 ± 15.9 * | 51.4 ± 23.6 * | 66.7 ± 30.2 | |
| 3.2 Gy | MHUVECs | 9.5 ± 6.4 | 15.4 ± 3.9 | 22.1 ± 14.3 ° | 26.4 ± 14.8 ° | 32.7 ± 17.9 ° |
| FHUVECs | 13.0 ± 8.3 | 14.8 ± 8.9 * | 31.9 ± 18.0 * | 37.7 ± 21.9 * | 53.3 ± 37.1 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campesi, I.; Brunetti, A.; Capobianco, G.; Galistu, A.; Montella, A.; Ieri, F.; Franconi, F. Sex Differences in X-ray-Induced Endothelial Damage: Effect of Taurine and N-Acetylcysteine. Antioxidants 2023, 12, 77. https://doi.org/10.3390/antiox12010077
Campesi I, Brunetti A, Capobianco G, Galistu A, Montella A, Ieri F, Franconi F. Sex Differences in X-ray-Induced Endothelial Damage: Effect of Taurine and N-Acetylcysteine. Antioxidants. 2023; 12(1):77. https://doi.org/10.3390/antiox12010077
Chicago/Turabian StyleCampesi, Ilaria, Antonio Brunetti, Giampiero Capobianco, Adriana Galistu, Andrea Montella, Francesca Ieri, and Flavia Franconi. 2023. "Sex Differences in X-ray-Induced Endothelial Damage: Effect of Taurine and N-Acetylcysteine" Antioxidants 12, no. 1: 77. https://doi.org/10.3390/antiox12010077
APA StyleCampesi, I., Brunetti, A., Capobianco, G., Galistu, A., Montella, A., Ieri, F., & Franconi, F. (2023). Sex Differences in X-ray-Induced Endothelial Damage: Effect of Taurine and N-Acetylcysteine. Antioxidants, 12(1), 77. https://doi.org/10.3390/antiox12010077










