Optimized Single-Step Recovery of Lipophilic and Hydrophilic Compounds from Raspberry, Strawberry and Blackberry Pomaces Using a Simultaneous Ultrasound-Enzyme-Assisted Extraction (UEAE)
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Pomaces Preparation
2.3. Determination of the Pomace Proximate Compositions
2.3.1. Polyphenol Content
2.3.2. Oil Extraction
2.3.3. Protein and Ashes Contents
2.3.4. Fiber Content Determination
2.3.5. Polyphenol Analyses
2.3.6. Antioxidant Activity
2.3.7. Oil Content Characterization
2.4. Simultaneous Ultrasound-Enzyme-Assisted Extraction (UEAE) Process Development
2.4.1. Enzyme-Assisted Extraction (EAE)
2.4.2. Ultrasound-Enzyme-Assisted Extraction (UEAE)
2.4.3. UEAE Optimization by Experimental Design
2.5. UPLC-DAD-ESI-MS/MS Polyphenols Analysis
2.6. Statistics
3. Results and Discussion
3.1. Pomaces’ Chemical Compositions
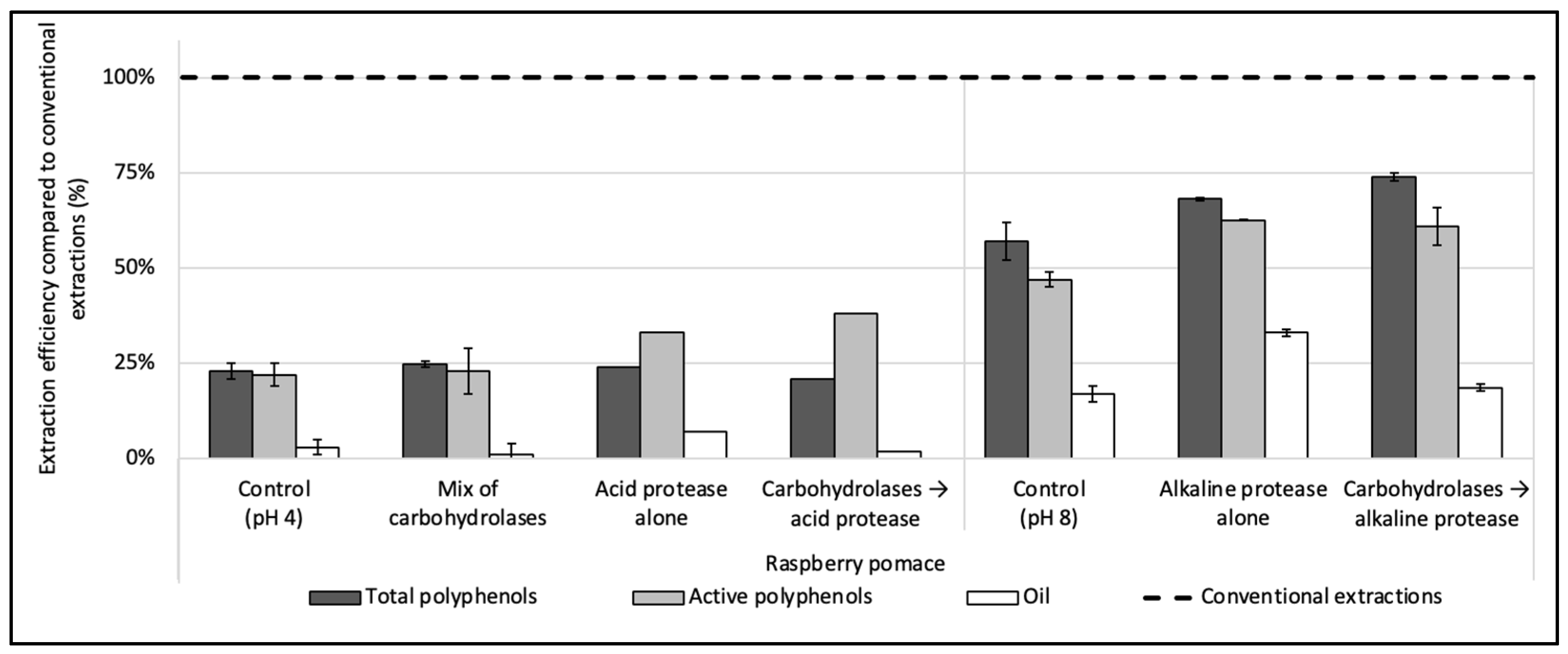
3.2. Enzyme Screening
3.3. UEAE Procedure
3.4. UEAE Optimization by DSD
3.5. UEAE Optimized Parameters Transposition to Strawberry and Blackberry Pomaces
3.6. UEAE Extracts Characterization
3.7. UEAE Phenolics Identification by UPLC-DQD-ESI-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry Pomace—A Review of Processing and Chemical Analysis of Its Polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Hidalgo, G.-I.; Almajano, M. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and Human Health: A Review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef]
- Lei, Z.; Jervis, J.; Helm, R.F. Use of Methanolysis for the Determination of Total Ellagic and Gallic Acid Contents of Wood and Food Products. J. Agric. Food Chem. 2001, 49, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Van Hoed, V.; De Clercq, N.; Echim, C.; Andjelkovic, M.; Leber, E.; Dewettinck, K.; Verhé, R. Berry Seeds: A Source of Specialty Oils with High Content of Bioactives and Nutritional Value. J. Food Lipids 2009, 16, 33–49. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of Raspberry (Rubus idaeus L.) Seed Oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health Benefits of N-3 Polyunsaturated Fatty Acids. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 65, pp. 211–222. ISBN 978-0-12-416003-3. [Google Scholar]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Ferdosh, S.; Akanda, J.H.; Ghafoor, K.; Rukshana, A.H.; Ali, E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.; et al. Techniques for the Extraction of Phytosterols and Their Benefits in Human Health: A Review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L.; Huang, Q.; Wang, J.; Lin, Q.; Liu, M.; Lee, W.Y.; Song, H. Ultrasonic-Assisted Extraction of Raspberry Seed Oil and Evaluation of Its Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. PLoS ONE 2016, 11, e0153457. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures. Foods 2021, 10, 1780. [Google Scholar] [CrossRef] [PubMed]
- Tamkutė, L.; Haddad, J.G.; Diotel, N.; Desprès, P.; Venskutonis, P.R.; El Kalamouni, C. Cranberry Pomace Extract Exerts Antiviral Activity against Zika and Dengue Virus at Safe Doses for Adult Zebrafish. Viruses 2022, 14, 1101. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Louvet, F.; Tarrade, S.; Meudec, E.; Grenier, K.; Landolt, C.; Ouk, T.S.; Bressollier, P. Enzyme-Assisted Extraction of Bioactive Compounds from Raspberry (Rubus idaeus L.) Pomace. J. Food Sci. 2019, 84, 1371–1381. [Google Scholar] [CrossRef]
- Kurtulbaş Şahin, E.; Bilgin, M.; Şahin, S. Recovery of Anthocyanins from Sour Cherry (Prunus cerasus L.) Peels via Microwave Assisted Extraction: Monitoring the Storage Stability. Prep. Biochem. Biotechnol. 2020, 51, 686–696. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Huang, H. Effects of Pulsed Electric Fields on Anthocyanin Extraction Yield of Blueberry Processing By-Products: Anthocyanin Extraction and Optimization by PEF. J. Food Process. Preserv. 2015, 39, 1898–1904. [Google Scholar] [CrossRef]
- Marić, B.; Pavlić, B.; Čolović, D.; Abramović, B.; Zeković, Z.; Bodroža-Solarov, M.; Ilić, N.; Teslić, N. Recovery of High-Content ω–3 Fatty Acid Oil from Raspberry (Rubus idaeus L.) Seeds: Chemical Composition and Functional Quality. LWT 2020, 130, 109627. [Google Scholar] [CrossRef]
- Hu, B.; Wang, H.; He, L.; Li, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhou, K.; Zhang, Q.; Liu, A.; et al. A Method for Extracting Oil from Cherry Seed by Ultrasonic-Microwave Assisted Aqueous Enzymatic Process and Evaluation of Its Quality. J. Chromatogr. A 2019, 1587, 50–60. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Ultrasound-Assisted Enzymatic Extraction of Anthocyanins from Raspberry Wine Residues: Process Optimization, Isolation, Purification, and Bioactivity Determination. Food Anal. Methods 2021, 14, 1369–1386. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary Bioactive Compounds and Their Health Implications: Dietary Bioactive Compounds and Health Implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Ultrasound Assisted Intensification of Enzyme Activity and Its Properties: A Mini-Review. World J. Microbiol. Biotechnol. 2017, 33, 170. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and Bioactivities of Free, Esterified and Insoluble-Bound Phenolics from Berry Seed Meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chu, J.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C.; Zhao, H. Physicochemical and Functional Properties of Dietary Fiber from Foxtail Millet (Setaria italic) Bran. J. Cereal Sci. 2018, 79, 456–461. [Google Scholar] [CrossRef]
- Alba, K.; MacNaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and Characterisation of Dietary Fibre from Blackcurrant Pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Bunzel, M.; Schüßler, A.; Tchetseubu Saha, G. Chemical Characterization of Klason Lignin Preparations from Plant-Based Foods. J. Agric. Food Chem. 2011, 59, 12506–12513. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Giusti, M.M.; Haché, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Martin, S.; Martinsen, S.K.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Šarkinas, A.; Venskutonis, P.R. Phytochemical composition, antioxidant and antimicrobial properties of raspberry fruit, pulp, and marc extracts. CyTA-J. Food 2013, 11, 334–342. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pieszka, M.; Migdał, W.; Gąsior, R.; Rudzińska, M.; Bederska-Łojewska, D.; Pieszka, M.; Szczurek, P. Native Oils from Apple, Blackcurrant, Raspberry, and Strawberry Seeds as a Source of Polyenoic Fatty Acids, Tocochromanols, and Phytosterols: A Health Implication. J. Chem. 2015, 2015, 659541. [Google Scholar] [CrossRef]
- Jones, B.; Nachtsheim, C.J. A Class of Three-Level Designs for Definitive Screening in the Presence of Second-Order Effects. J. Qual. Technol. 2011, 43, 1–15. [Google Scholar] [CrossRef]
- Marić, B.; Abramović, B.; Ilić, N.; Krulj, J.; Kojić, J.; Perović, J.; Bodroža-Solarov, M.; Teslić, N. Valorization of Red Raspberry (Rubus idaeus L.) Seeds as a Source of Health Beneficial Compounds: Extraction by Different Methods. J. Food Process. Preserv. 2020, 44, e14744. [Google Scholar] [CrossRef]
- Kosmala, M.; Jurgoński, A.; Juśkiewicz, J.; Karlińska, E.; Macierzyński, J.; Rój, E.; Zduńczyk, Z. Chemical Composition of Blackberry Press Cake, Polyphenolic Extract, and Defatted Seeds, and Their Effects on Cecal Fermentation, Bacterial Metabolites, and Blood Lipid Profile in Rats. J. Agric. Food Chem. 2017, 65, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Rébufa, C.; Artaud, J.; Le Dréau, Y. Walnut (Juglans regia L.) Oil Chemical Composition Depending on Variety, Locality, Extraction Process and Storage Conditions: A Comprehensive Review. J. Food Compos. Anal. 2022, 110, 104534. [Google Scholar] [CrossRef]
- Kosmala, M.; Zduńczyk, Z.; Juśkiewicz, J.; Jurgoński, A.; Karlińska, E.; Macierzyński, J.; Jańczak, R.; Rój, E. Chemical Composition of Defatted Strawberry and Raspberry Seeds and the Effect of These Dietary Ingredients on Polyphenol Metabolites, Intestinal Function, and Selected Serum Parameters in Rats. J. Agric. Food Chem. 2015, 63, 2989–2996. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Nikiforidis, C.V. Structure and Functions of Oleosomes (Oil bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and Enzymatic Processes for Edible Oil Extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Goula, A.M.; Papatheodorou, A.; Karasavva, S.; Kaderides, K. Ultrasound-Assisted Aqueous Enzymatic Extraction of Oil from Pomegranate Seeds. Waste Biomass Valor. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Varo, M.A.; Jacotet-Navarro, M.; Serratosa, M.P.; Mérida, J.; Fabiano-Tixier, A.-S.; Bily, A.; Chemat, F. Green Ultrasound-Assisted Extraction of Antioxidant Phenolic Compounds Determined by High Performance Liquid Chromatography from Bilberry (Vaccinium myrtillus L.) Juice By-Products. Waste Biomass Valor. 2019, 10, 1945–1955. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Król, B.; Kosmala, M.; Milala, J.; Zduńczyk, Z.; Żary-Sikorska, E. Physiological Properties of Dietary Ellagitannin-Rich Preparations Obtained from Strawberry Pomace Using Different Extraction Methods. Pol. J. Food Nutr. Sci. 2015, 65, 199–209. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential Fatty Acids as Functional Components of Foods- a Review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Guesnet, P.; Alessandri, J.-M.; Astorg, P.; Pifferi, F.; Lavialle, M. Les Rôles Physiologiques Majeurs Exercés Par Les Acides Gras Polyinsaturés (AGPI). OCL 2005, 12, 333–343. [Google Scholar] [CrossRef][Green Version]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef]
- Raczyk, M.; Bryś, J.; Brzezińska, R.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Górska, A. Quality Assessment of Cold-Pressed Strawberry, Raspberry and Blackberry Seed Oils Intended for Cosmetic Purposes. Acta Sci. Pol. Technol. Aliment. 2021, 20, 127–133. [Google Scholar] [CrossRef]
- Mullen, W.; Yokota, T.; Lean, M.E.J.; Crozier, A. Analysis of Ellagitannins and Conjugates of Ellagic Acid and Quercetin in Raspberry Fruits by LC–MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and Accurate Quantification of Rubus Ellagitannins and Ellagic Acid Conjugates Using Direct UPLC-Q-TOF HDMS and HPLC-DAD Analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef]
- Beekwilder, J.; Jonker, H.; Meesters, P.; Hall, R.D.; Van Der Meer, I.M.; Ric De Vos, C.H. Antioxidants in Raspberry: On-Line Analysis Links Antioxidant Activity to a Diversity of Individual Metabolites. J. Agric. Food Chem. 2005, 53, 3313–3320. [Google Scholar] [CrossRef]
- Gođevac, D.; Tešević, V.; Vajs, V.; Milosavljević, S.; Stanković, M. Antioxidant Properties of Raspberry Seed Extracts on Micronucleus Distribution in Peripheral Blood Lymphocytes. Food Chem. Toxicol. 2009, 47, 2853–2859. [Google Scholar] [CrossRef]
- Kool, M.M.; Comeskey, D.J.; Cooney, J.M.; McGhie, T.K. Structural Identification of the Main Ellagitannins of a Boysenberry (Rubus Loganbaccus × baileyanus Britt.) Extract by LC–ESI-MS/MS, MALDI-TOF-MS and NMR Spectroscopy. Food Chem. 2010, 119, 1535–1543. [Google Scholar] [CrossRef]
- Daniel, E.M.; Ratnayake, S.; Kinstle, T.; Stoner, C.D. The Effects of PH and Rat Intestinal Contents on the Liberation of Ellagic Acid from Purified and Crude Ellagitannins. J. Nat. Prod. 1991, 54, 946–952. [Google Scholar] [CrossRef]
- Sójka, M.; Janowski, M.; Grzelak-Błaszczyk, K. Stability and Transformations of Raspberry (Rubus idaeus L.) Ellagitannins in Aqueous Solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Giongo, L.; Mattivi, F.; Viola, R. A Survey of Ellagitannin Content in Raspberry and Blackberry Cultivars Grown in Trentino (Italy). Eur. Food Res. Technol. 2008, 226, 817–824. [Google Scholar] [CrossRef]
- Kula, M.; Głód, D.; Krauze-Baranowska, M. Two-Dimensional Liquid Chromatography (LC) of Phenolic Compounds from the Shoots of Rubus Idaeus ‘Glen Ample’ Cultivar Variety. J. Pharm. Biomed. Anal. 2016, 121, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Guella, G.; Gasperotti, M.; Pojer, E.; Zancato, M.; Mattivi, F. Clarifying the Identity of the Main Ellagitannin in the Fruit of the Strawberry, Fragaria vesca and Fragaria ananassa Duch. J. Agric. Food Chem. 2012, 60, 2507–2516. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Guella, G.; Palmieri, L.; Martinatti, P.; Pojer, E.; Mattivi, F.; Vrhovsek, U. Evolution of Ellagitannin Content and Profile during Fruit Ripening in Fragaria spp. J. Agric. Food Chem. 2013, 61, 8597–8607. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. C. Reaction of Dehydrohexahydroxydiphenic Acid Esters with Bases, and Its Application to the Structure Determination of Pomegranate Tannins, Granatins A and B. Chem. Pharm. Bull. 1990, 38, 2424–2428. [Google Scholar] [CrossRef]
- Buendía, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.M.; Tomás-Barberán, F.A. HPLC-MS Analysis of Proanthocyanidin Oligomers and Other Phenolics in 15 Strawberry Cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef]
- Mertz, C.; Cheynier, V.; Günata, Z.; Brat, P. Analysis of Phenolic Compounds in Two Blackberry Species (Rubus glaucus and Rubus adenotrichus) by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ion Trap Mass Spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin Composition of Blackberry as Determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tachibana, H.; Nonaka, G.; Nishioka, I.; Hsu, F.-L.; Kohda, H.; Tanaka, O. Tannins and Related Compounds. CXXII. New Dimeric, Trimeric and Tetrameric Ellagitannins, Lambertianins A-D, from Rubus Lambertianus SERINGE. Chem. Pharm. Bull. 1993, 41, 1214–1220. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus Berries for the Control of Gastric Inflammation: In Vitro and In Vivo Studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, D.; Li, L.; Ma, H.; Yuan, T.; Chichester, C.O.; Seeram, N.P. Anti-Inflammatory Effects of Polyphenolic-Enriched Red Raspberry Extract in an Antigen-Induced Arthritis Rat Model. J. Agric. Food Chem. 2012, 60, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A. The Antimicrobial Activity of Fruits from Some Cultivar Varieties of Rubus Idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef]
- Ross, H.A.; McDougall, G.J.; Stewart, D. Antiproliferative Activity Is Predominantly Associated with Ellagitannins in Raspberry Extracts. Phytochemistry 2007, 68, 218–228. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Ellagitannins as Active Constituents of Medicinal Plants. Planta Med. 1989, 55, 117–122. [Google Scholar] [CrossRef]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, Flavonoids, and Other Phenolics in Red Raspberries and Their Contribution to Antioxidant Capacity and Vasorelaxation Properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-Inflammatory Properties of a Pomegranate Extract and Its Metabolite Urolithin-A in a Colitis Rat Model and the Effect of Colon Inflammation on Phenolic Metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Milala, J.; Kosmala, M.; Karlińska, E.; Juśkiewicz, J.; Zduńczyk, Z.; Fotschki, B. Ellagitannins from Strawberries with Different Degrees of Polymerization Showed Different Metabolism through Gastrointestinal Tract of Rats. J. Agric. Food Chem. 2017, 65, 10738–10748. [Google Scholar] [CrossRef]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, Ellagic Acid and Vascular Health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of Anthocyanins and Ellagitannins Following Consumption of Raspberries by Healthy Humans and Subjects with an Ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef] [PubMed]



| Pomace | Raspberry | Strawberry | Blackberry |
|---|---|---|---|
| Moisture | 4.7 | 6.4 | 4.9 |
| Ashes | 1.4 ± 0.07 | 2.9 ± 0.6 | 2.3 ± 0.2 |
| Fat | 16 ± 0.3 | 6.3 ± 0.1 | 18 ± 0.3 |
| Proteins | 8 ± 0.4 | 10 ± 2 | 9.5 ± 0.05 |
| Total fibers | 80 ± 3 | 74 ± 0.1 | 62 ± 2 |
| Fibers profile (% of total fibers) | |||
| Pectin | 0.4 ± 0.02 | 8 ± 1 | 4.1 ± 0.1 |
| Soluble lignin | 0.3 ± 0.1 | 0.4 ± 0.01 | 0.4 ± 0.06 |
| Klason lignin | 34 ± 0.4 | 28 ± 2 | 28 ± 0.3 |
| Hemicellulose | 6 ± 0.3 | 9 ± 0.6 | 7 ± 0.5 |
| Cellulose | 79 ± 2 | 57 ± 3 | 67 ± 2 |
| TPC | 4.7 ± 0.2 | 3.8 ± 0.1 | 5.1 ± 0.1 |
| Ellagitannins 1 | 1.4 ± 0.05 | 1.5 ± 0.2 | 2.9 ± 0.3 |
| Anthocyanins 2 | 10 ± 2 | 27 ± 9 | 99 ± 11 |
| Active polyphenol content 3 | 2.0 ± 0.07 | 1.9 ± 0.04 | 2.9 ± 0.2 |
| Independent Factors | Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | |||||||
| U1 | US amplitude (%) | 20 | 45 | 70 | |||||
| U2 | pH | 7 | 8 | 9 | |||||
| U3 | E/S ratio (%, w/w) | 1 | 2 | 3 | |||||
| U4 | S/L ratio (%, w/v) | 3 | 6 | 9 | |||||
| U5 | Extraction time (min) | 30 | 45 | 60 | |||||
| U6 | Temperature (°C) | 40 | 50 | 60 | |||||
| Extraction yields (g/100 g DP) | |||||||||
| Assay | U1 | U2 | U3 | U4 | U5 | U6 | Total polyphenols | Active polyphenols | Oil |
| 1 | 0 | 1 | 1 | 1 | 1 | 1 | 4.9 ± 0.30 | 1.6 ± 0.02 | 11.7 ± 0.02 |
| 2 | 0 | −1 | −1 | −1 | −1 | −1 | 2.2 ± 0.20 | 0.9 ± 0.08 | 10.0 ± 0.01 |
| 3 | 1 | 0 | 1 | −1 | −1 | 1 | 4.4 ± 0.20 | 1.7 ± 0.05 | 13.2 ± 0.20 |
| 4 | −1 | 0 | −1 | 1 | 1 | −1 | 3.2 ± 0.02 | 1.1 ± 0.07 | 11.1 ± 0.01 |
| 5 | 1 | 1 | 0 | 1 | −1 | −1 | 4.3 ± 0.30 | 1.2 ± 0.30 | 11.9 ± 0.20 |
| 6 | −1 | −1 | 0 | −1 | 1 | 1 | 3.6 ± 0.20 | 1.5 ± 0.04 | 12.5 ± 0.60 |
| 7 | 1 | −1 | 1 | 0 | 1 | −1 | 1.9 ± 0.09 | 0.9 ± 0.02 | 8.6 ± 0.30 |
| 8 | −1 | 1 | −1 | 0 | −1 | 1 | 4.5 ± 0.10 | 1.6 ± 0.06 | 12.3 ± 0.20 |
| 9 | 1 | −1 | −1 | 1 | 0 | 1 | 2.3 ± 0.06 | 1.1 ± 0.08 | 9.5 ± 0.20 |
| 10 | −1 | 1 | 1 | −1 | 0 | −1 | 4.2 ± 0.10 | 1.4 ± 0.05 | 12.6 ± 0.20 |
| 11 | 1 | 1 | −1 | −1 | 1 | 0 | 4.2 ± 0.20 | 1.4 ± 0.05 | 12.8 ± 0.06 |
| 12 | −1 | −1 | 1 | 1 | −1 | 0 | 2.6 ± 0.20 | 0.9 ± 0.02 | 9.0 ± 0.20 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 3.9 ± 0.04 | 1.4 ± 0.04 | 11.9 ± 0.10 |
| 14 1 | −1 | 0 | −1 | 0 | −1 | 1 | 4.9 ± 0.10 | 1.6 ± 0.02 | 12.0 ± 0.05 |
| Conventional extractions 2 | - | - | - | - | - | - | 4.7 ± 0.20 | 2.1 ± 0.04 | 15.7 ± 0.30 |
| Pomace | Raspberry | Strawberry | Blackberry | |||
|---|---|---|---|---|---|---|
| Extract | Conventional Extractions 1 | UEAE | Conventional Extractions 1 | UEAE | Conventional Extractions 1 | UEAE |
| Sugars | 5.8 ± 0.8 a | 7.2 ± 0.9 a | 9 ± 1 b | 15.3 ± 0.5 a | 14 ± 1 b | 22 ± 3 a |
| Proteins | - | 7.0 ± 0.2 | - | 7.0 ± 0.6 a | - | 7.5 ± 0.9 a |
| Lipids | 16 ± 0.3 b | 12 ± 0.1 a | 6.3 ± 0.1 b | 3.5 ± 0.2 a | 18 ± 0.3 b | 11.2 ± 0.1 a |
| TPC | 4.7 ± 0.2 a | 4.9 ± 0.1 a | 3.8 ± 0.1 b | 4.40 ± 0.1 a | 5.1 ± 0.1 a | 5.3 ± 0.2 a |
| Ellagitannins 2 | 1.4 ± 0.05 b | 1.04 ± 0.03 a | 1.5 ± 0.2 b | 1.0 ± 0.2 a | 2.9 ± 0.3 b | 2.07 ± 0.09 a |
| Active polyphenol content 3 | 2.0 ± 0.07 b | 1.5 ± 0.04 a | 1.9 ± 0.04 b | 1.6 ± 0.06 a | 2.9 ± 0.2 b | 2.2 ± 0.03 a |
| Antioxidant activity 4 (%) | 42 ± 2 b | 36 ± 2 a | 50 ± 3 b | 37 ± 0.2 a | 57 ± 1 b | 47 ± 2 a |
| Composition (g/100 g) | UEAE Raspberry | UEAE Strawberry | UEAE Blackberry |
|---|---|---|---|
| Moisture | 10 ± 5 | 16 ± 1 | 18 ± 1 |
| Sugars | 19 ± 3 | 37 ± 1 | 42 ± 5 |
| Lipids | 24 ± 2 | 3.5 ± 0.4 | 14 ± 0.1 |
| Proteins | 18 ± 0.4 | 17 ± 2 | 15 ± 2 |
| Ashes | 13 ± 2 | 14 ± 0.6 | 8 ± 2 |
| TPC | 10.4 ± 0.9 | 9.1 ± 0.5 | 10.1 ± 0.7 |
| Ellagitannins 1 | 2.7 ± 0.1 | 2.3 ± 0.3 | 4.0 ± 0.3 |
| Active polyphenol content 2 | 3.9 ± 0.09 | 3.4 ± 0.2 | 4.8 ± 0.2 |
| Peak No. | RT (min) | Pic Proportion (%) 1 | m/z [M-H]− | Ions Fragments | Compounds Identification | |
|---|---|---|---|---|---|---|
| Raspberry Pomace | ||||||
| Conventional Extract 2 | UEAE Extract | |||||
| 1 | 4.70 | 0.4 | 1.7 | [633.07]− | [300.99]−, [275.02]− | HHDP-galloylglucose (GG) |
| 2 | 5.02 | 1.1 | 3.6 | [633.07]− | [300.99]−, [275.02]− | HHDP-GG |
| 3 | 5.63 | 0.7 | 1.8 | [289.07]− | [245.08]−, [179.03]− | Catechin |
| 4 | 6.35 | 1.4 | 1.6 | [858.07]2− | [783.07]−, [633.07]−, [469.00]−, [314.98]−, [300.99]−, [275.02]− | Degalloylated sanguiin H-6 (Roshenin B) |
| 5 | 6.49 | 3.5 | 3.5 | [783.07]2− | [897.04]−, [633.07]−, [469.00]−, [331.07]−, [314.98]−, [300.99]−, | Sanguiin H-10 or isomer |
| 6 | 6.52 | 4.8 | 1.5 | [577.13]− | [407.08]−, [289.07]−, [245.08]−, [125.02]− | Procyanidin dimer |
| 7 | 7.63 | 9.7 | 7.3 | [561.14]− | [407.08]−, [289.07]− | (epi)cat-epiafzelechin |
| 8 | 9.80 | 1.1 | 1.9 | [783.07]2− | [897.04]−, [633.07]−, [314.98]−, [300.99]−, [275.02]− | Sanguiin H-10 or isomer |
| 9 | 9.86 | 2.7 | 3.8 | [935.08]− | [783.07]−, [633.07]−, [463.05]−, [300.99]−, [275.02]−, | Casuarictin or isomer |
| 10 | 10.37 | 11.3 | 8.5 | [934.07]2− | [300.99]− | Sanguiin H-6 or isomer |
| 11 | 10.57 | 2.0 | 5.9 | [783.07]2− | [633.07]−, [314.98]−, [300.99]−, [275.02]− | Sanguiin H-10 or isomer |
| 12 | 11.43 | 11.9 | 4.5 | [1401.61]2− | [897.04]−, [633.07]−, [469.00]−, [300.99]− | Lambertianin C |
| 13 | 11.62 | 42.7 | 32.2 | [934.07]2− | [633.07]−, [300.99]− | Sanguiin H-6 or isomer |
| 14 | 11.84 | 0.6 | 0.5 | [433.04]− | [300.99]−, [201.07]− | Ellagic acid pentoside |
| 15 | 11.97 | 2.4 | 17.5 | [300.99]− | - | Ellagic acid (EA) |
| 16 | 13.61 | 0.9 | 0.2 | [469.05]2− [939.11]− | [617.08]−, [465.07]−, [295.05]−, [169.01]− | Pentagalloylglucose |
| 17 | 13.83 | 1.2 | 1.5 | [447.06]− | [315.01]−, [299.99]− | Methyl ellagic acid pentoside |
| 18 | 14.31 | 0.9 | 2.3 | [450.99]− | - | Ellagitannin derivative 3 |
| 19 | 14.64 | 0.8 | 0.2 | [542.03]2− [1085.08]− | [897.04]−, [745.03]−, [633.07]−, [450.99]−, [300.99]− | Ellagitannin derivative 4 |
| Strawberry pomace | ||||||
| Conventional extract 2 | UEAE extract | |||||
| 1 | 0.94 | 0.1 | 1.5 | [169.01]− | [125.02]− | Gallic acid |
| 2 | 1.18 | 1.4 | 3.1 | [331.07]− | [169.01]− | Galloylglucose (GG) |
| 3 | 2.10 | 2.5 | 4.2 | [783.07]− | [300.99]−, [275.01]− | di-HHDP-Glucose or pedunculagin |
| 4 | 6.65 | 1.5 | 22.4 | [291.01]− | - | Brevifolin carboxylic acid |
| 5 | 9.91 | 6.7 | 3.8 | [935.08]− [467.03]2− | [633.07]−, [463.05]−, [300.99]−, [275.01]− | di-HHDP-GG (casuarictin isomer) |
| 6 | 10.62 | 10.7 | 2.6 | [617.03]2− [1235.07]− | [631.06]−, [469.00]−, [314.98]−, [300.99]− | di-HHDP-GG derivative with EA group |
| 7 | 11.97 | 15.9 | 39.5 | [300.99]− | - | Ellagic acid (EA) |
| 8 | 12.34 | 5.2 | 1.3 | [1250.10]3− | [935.08]−, [897.04]−, [633.07]−, [300.99]− | Lambertianin C derivative (trimer of di-HHDP-GG with loss of a HHDP group) |
| 9 | 13.02 | 35.8 | 13.1 | [934.07]2− | [935.08]−, [897.04]−, [783.07]−, [633.07]−, [613.05]−, [300.99]− | Agrimoniin |
| 10 | 13.58 | 6.2 | 1 | [1401.61]2− | [897.04]−, [783.07]−, [633.07]−, [450.99]−, [300.99]− | Lambertianin C isomer |
| 11 | 13.73 | 2.2 | 4.4 | [447.09]− | [285.04]−, [284.03]− | Kaempferol glucoside |
| 12 | 14.00 | 3.0 | 0.8 | [1009.07]2− | [897.04]−, [633.07]−, [450.99]−, [300.99]− | Ellagitannin (non identified) |
| 13 | 15.86 | 8.8 | 2.3 | [593.13]− | [447.09]−, [285.04]−, [284.03]− | Kaempferol 3-O-coumaroyl-glucoside |
| Blackberry pomace | ||||||
| Conventional extract 2 | UEAE extract | |||||
| 1 | 2.07 | 5.5 | 2.7 | [783.07]− | [783.07]−, [481.07]−, [300.99]−, [275.02]− | di-HHDP-glucose (pedunculagin) |
| 2 | 4.70 | 3.8 | 1.5 | [783.07]− | [783.07]−, [481.07]−, [300.99]−, [275.02]−, | di-HHDP-glucose (pedunculagin isomer) |
| 3 | 4.97 | 2.0 | 11.5 | [633.07]− | [300.99]−, [275.02]− | HHDP-GG |
| 4 | 6.57 | 6.4 | 3.3 | [783.07]2− | [633.07]−, [469.01]−, [314.98]−, [331.07]−, [300.99]−, | Sanguiin H-10 or isomer |
| 5 | 10.56 | 3.8 | 9.4 | [783.07]2− | [897.04]−, [633.07]−, [469.01]−, [314.98]−, [300.99]− | Sanguiin H-10 or isomer |
| 6 | 11.10 | 1.5 | 4.8 | [1103.08]− | [897.04]−, [745.03]−, [633.07]−, [469.00]−, [314.98]−, [300.99]−, [275.02]− | Galloyl-di-HHDP galloylglucose (ex. sanguiin H-2) |
| 7 | 11.41 | 10.9 | 3.5 | [1401.06]2− | [897.04]−, [633.07]−, [469.01]−, [314.98]−, [300.99]− | Lambertianin C |
| 8 | 11.62 | 44.2 | 29.1 | [934.07]2− | [897.04]−, [633.07]−, [469.07]−, [314.98]−, [300.99]−, [275.02]− | Sanguiin H-6 or Isomer |
| 9 | 11.86 | 2.1 | 2.7 | [433.04]− | [300.99]− | Ellagic acid pentoside |
| 10 | 11.97 | 8.1 | 27.1 | [300.99]− | - | Ellagic acid |
| 11 | 12.34 | 5.7 | 0.9 | [935.08]− | [633.07]−, [463.05]−, [300.99]−, [275.02]− | di-HHDP-GG (casuarictin isomer) |
| 12 | 13.82 | 4.3 | 3.2 | [1103.08]− [551.04]2− | [935.08]−, [898.05]−, [633.07]−, [300.99]−, [275.02]− | Galloyl-di-HHDP galloylglucose (ex. sanguiin H-2) |
| 13 | 14.63 | 1.8 | 0.2 | [542.03]2− [1085.08]− | [897.04]−, [745.03]−, [633.07]−, [450.99]−, [300.99]−, [275.02]− | Ellagitannin derivative 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidson, M.; Louvet, F.; Meudec, E.; Landolt, C.; Grenier, K.; Périno, S.; Ouk, T.-S.; Saad, N. Optimized Single-Step Recovery of Lipophilic and Hydrophilic Compounds from Raspberry, Strawberry and Blackberry Pomaces Using a Simultaneous Ultrasound-Enzyme-Assisted Extraction (UEAE). Antioxidants 2023, 12, 1793. https://doi.org/10.3390/antiox12101793
Davidson M, Louvet F, Meudec E, Landolt C, Grenier K, Périno S, Ouk T-S, Saad N. Optimized Single-Step Recovery of Lipophilic and Hydrophilic Compounds from Raspberry, Strawberry and Blackberry Pomaces Using a Simultaneous Ultrasound-Enzyme-Assisted Extraction (UEAE). Antioxidants. 2023; 12(10):1793. https://doi.org/10.3390/antiox12101793
Chicago/Turabian StyleDavidson, Morag, François Louvet, Emmanuelle Meudec, Cornelia Landolt, Karine Grenier, Sandrine Périno, Tan-Sothéa Ouk, and Naïma Saad. 2023. "Optimized Single-Step Recovery of Lipophilic and Hydrophilic Compounds from Raspberry, Strawberry and Blackberry Pomaces Using a Simultaneous Ultrasound-Enzyme-Assisted Extraction (UEAE)" Antioxidants 12, no. 10: 1793. https://doi.org/10.3390/antiox12101793
APA StyleDavidson, M., Louvet, F., Meudec, E., Landolt, C., Grenier, K., Périno, S., Ouk, T.-S., & Saad, N. (2023). Optimized Single-Step Recovery of Lipophilic and Hydrophilic Compounds from Raspberry, Strawberry and Blackberry Pomaces Using a Simultaneous Ultrasound-Enzyme-Assisted Extraction (UEAE). Antioxidants, 12(10), 1793. https://doi.org/10.3390/antiox12101793






