Quinoline Derivatives: Promising Antioxidants with Neuroprotective Potential
Abstract
:1. Introduction
2. Materials and Methods
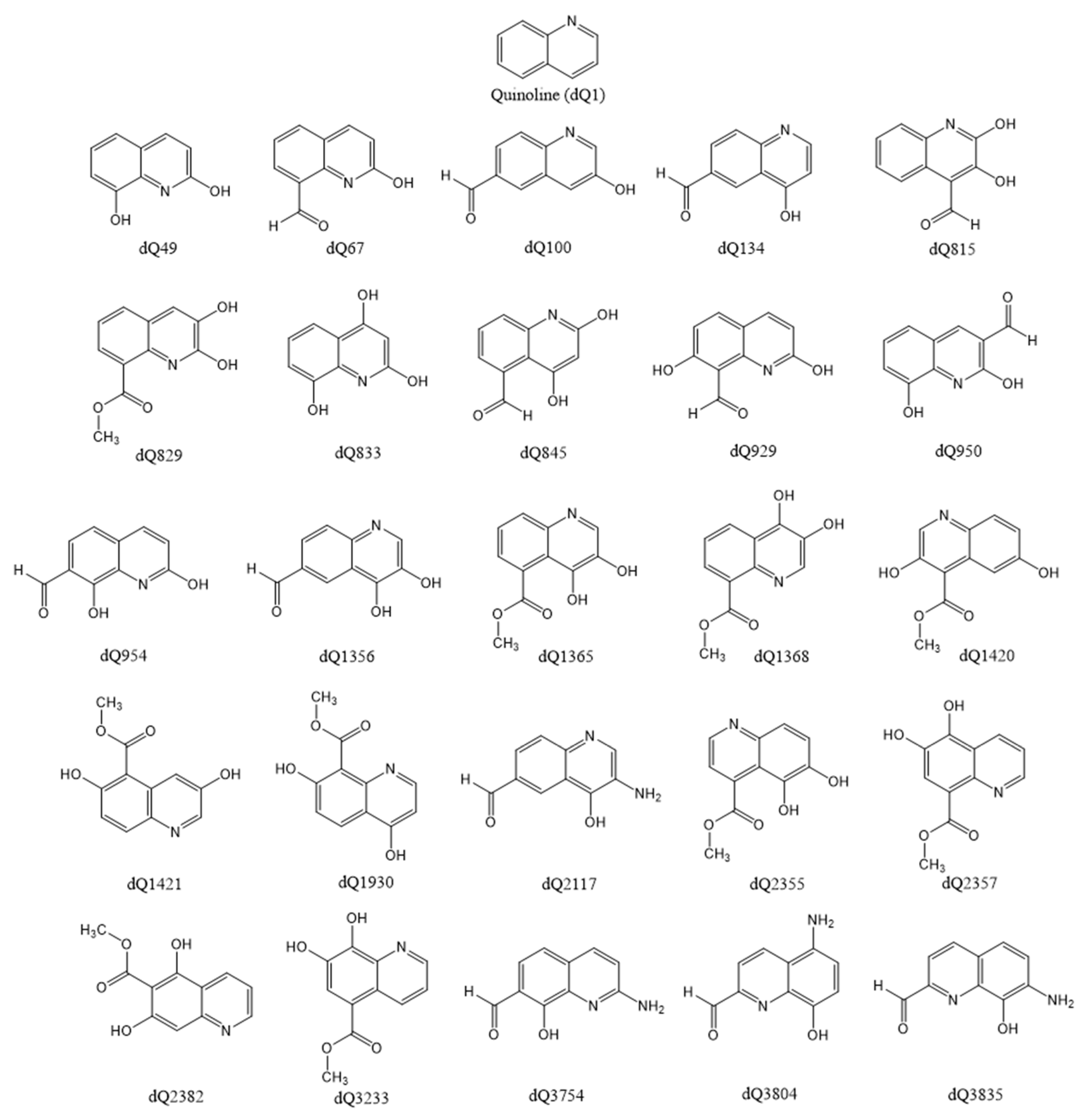
2.1. Construction of Derivatives and Estimation of Molecular Properties
2.2. DFT Calculations
2.3. Protein–Ligand Docking Details
3. Results and Discussion
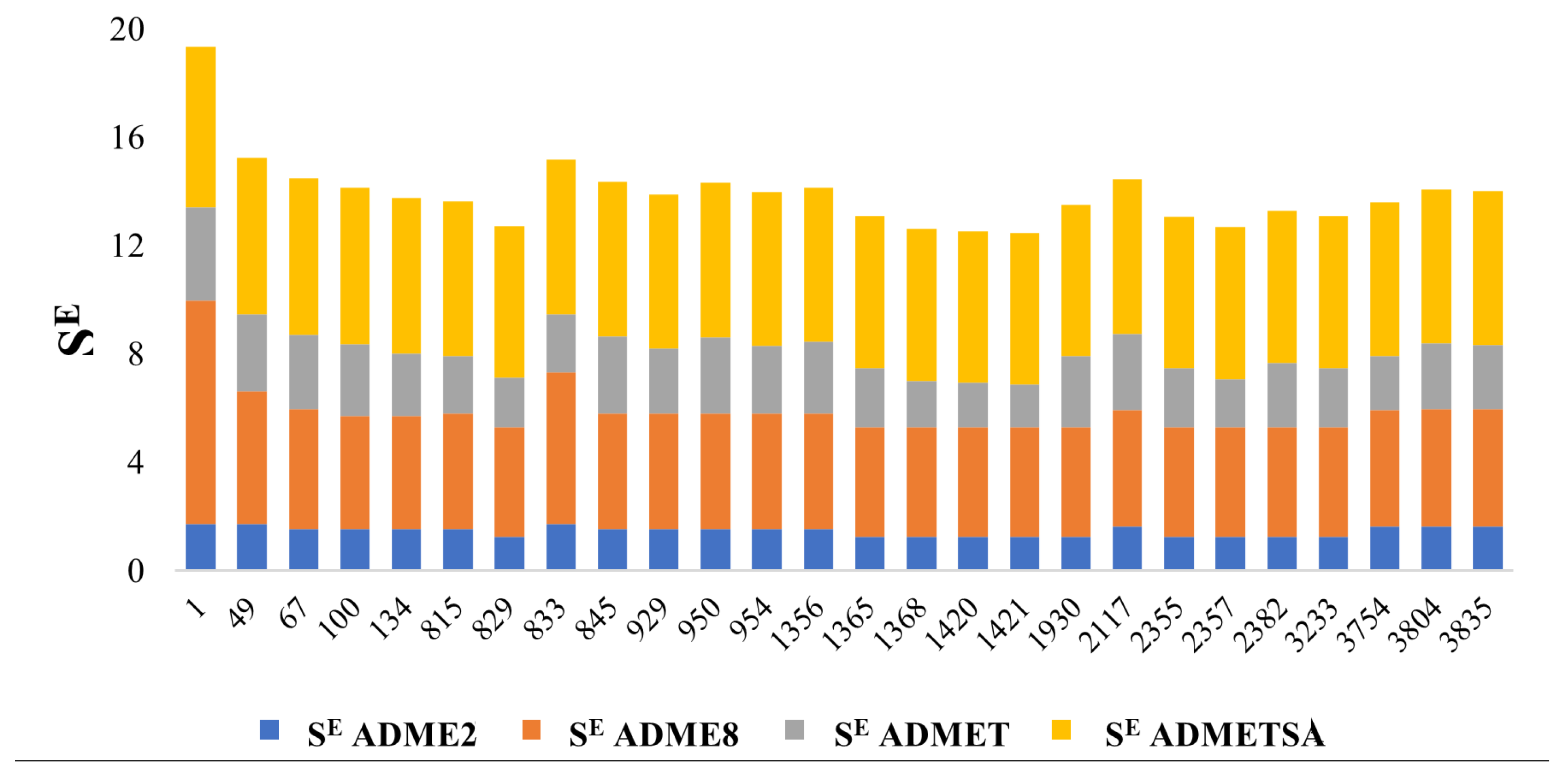
3.1. Screening the Chemical Space—Selection and Elimination Scores
3.2. Acid–Base Equilibria and Antioxidant Activity
3.3. Neuroprotection Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Natasha, C.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Phys. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, K.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell. Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Kuresh, A.Y.; Martin, A.; Joseph, J.A. Essential fatty acids and the brain: Possible health implications. Int. J. Dev. Neurosci. 2000, 18, 338–399. [Google Scholar]
- Lee, C.S.; Leong, K.W. Advances in microphysiological blood-brain barrier (BBB) models towards drug delivery. Curr. Opin. Biotechnol. 2020, 66, 78–87. [Google Scholar] [CrossRef]
- Bang, S.; Lee, S.R.; Ko, J.; Son, K.; Tahk, D.; Ahn, J.; Im, I.; Jeon, N.L. A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytes. Sci. Rep. 2017, 7, 8083. [Google Scholar] [CrossRef]
- Franke, H.; Galla, H.J.; Beuckmann, C.T. An improved low-permeability in vitro-model of the blood–brain barrier: Transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res. 1999, 818, 65–71. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and Oxidative Stress in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of reactive oxygen species in the progression of Alzheimer’s disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef]
- Zuo, L.; Motherwell, M.S. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 1568–1637. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic Stress and Oxidative Stress as Common Factors of the Pathogenesis of Depression and Alzheimer’s Disease: The Role of Antioxidants in Prevention and Treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Sayre, L.M.; Smith, M.A.; Perry, G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 2001, 8, 721–738. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.E.; Borrello, S.; Passantino, M.; Palazzotti, B.; Mordente, A.; Daniele, A. Oxidative stress and overexpression of manganeso superoxide dismutase in patients with Alzheimer’s disease. Neurosci. Lett. 1998, 250, 173–176. [Google Scholar] [CrossRef]
- Dhib-Jalbut, S.; Arnold, D.L.; Cleveland, D.W.; Fisher, M.; Friedlander, M.; Mouradian, M.M. Neurodegeneration and neuroprotection in multiple sclerosis and other neurodegenerative diseases. J. Neuroimmunol. 2006, 176, 198–215. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative Stress and β-Amyloid Protein in Alzheimer’s Disease. Neuromol. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Rad. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Chen, L.; Fan, Y.; Zhao, L.; Zhang, Q.; Wang, Z. The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorg. Chem. 2023, 131, 106301. [Google Scholar] [CrossRef]
- Everett, J.; Lermyte, F.; Brooks, J.; Tjendana-Tjhin, V.; Plascencia-Villa, G.; Hands-Portman, I.; Donnelly, J.M.; Billimoria, K.; Perry, G.; Zhu, X.; et al. Biogenic metallic elements in the human brain? Sci. Adv. 2021, 7, eabf6707. [Google Scholar] [CrossRef]
- Das, N.; Raymick, J.; Sarkar, S. Role of metals in Alzheimer’s disease. Metab. Brain. Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef]
- Brewer, G.J. Iron and Copper Toxicity in Diseases of Aging, Particularly Atherosclerosis and Alzheimer’s Disease. Exp. Biol. Med. 2007, 232, 323–335. [Google Scholar]
- Perry, G.; Taddeo, M.A.; Petersen, R.B.; Castellani, R.J.; Harris, P.L.R.; Siedlak, S.L.; Cash, A.D.; Liu, Q.; Nunomura, A.; Atwood, C.S.; et al. Adventiously-bound redox active iron and copper are at the center of oxidative damage in Alzheimer disease. Biometals 2003, 16, 77–81. [Google Scholar] [CrossRef]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease—Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef]
- Tatton, W.G.; Chalmers-Redman, R.; Brown, D.; Tatton, N. Apoptosis in Parkinson’s disease: Signals for neuronal degradation. Ann. Neurol. 2003, 53, S61–S72. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, A.I.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Nunes, C.; Laranjinha, J. Nitric oxide and dopamine metabolism converge via mitochondrial dysfunction in the mechanisms of neurodegeneration in Parkinson’s disease. Arch. Biochem. Biophys. 2021, 704, 108877. [Google Scholar] [CrossRef]
- Sun, Y.; Pham, A.N.; Waite, T.D. Elucidation of the interplay between Fe(II), Fe(III), and dopamine with relevance to iron solubilization and reactive oxygen species generation by catecholamines. J. Neurochem. 2016, 137, 955–968. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell. Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef]
- Forsberg, M.M.; Juvonen, R.O.; Helisalmi, P.; Leppänen, J.; Gogos, J.A.; Karayiorgou, M.; Männistö, P.T. Lack of increased oxidative stress in catechol-O-methyltransferase (COMT)-deficient mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 370, 279–289. [Google Scholar] [CrossRef]
- Delcambre, S.; Nonnenmacher, Y.; Hiller, K. Dopamine Metabolism and Reactive Oxygen Species Production. In Mitochondrial Mechanisms of Degeneration and Repair in Parkinson’s Disease; Buhlman, L., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Iuga, C.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. ROS initiated oxidation of dopamine under oxidative stress conditions in aqueous and lipidic environments. J. Phys. Chem. B 2011, 115, 12234–12246. [Google Scholar] [CrossRef]
- Chauhan, M.S.S.; Umar, T.; Aulakh, M.K. Quinolines: Privileged Scaffolds for Developing New Anti-neurodegenerative Agents. Chem. Sel. 2023, 8, e202204960. [Google Scholar] [CrossRef]
- Yadav, V.; Reang, J.; Sharma, V.; Majeed, J.; Sharma, P.C.; Sharma, K.; Giri, N.; Kumar, A.; Tonk, R.K. Quinoline-derivatives as privileged scaffolds for medicinal and pharmaceutical chemists: A comprehensive review. Chem. Biol. Drug Des. 2022, 100, 389–418. [Google Scholar] [CrossRef]
- Musiol, R. An overview of quinoline as a privileged scaffold in cancer drug discovery. Expert Opin. Drug Discov. 2017, 12, 583–597. [Google Scholar] [CrossRef]
- Bongarzone, S.; Bolognesi, M.L. The concept of privileged structures in rational drug design: Focus on acridine and quinoline scaffolds in neurodegenerative and protozoan diseases. Expert Opin. Drug Discov. 2011, 6, 251–268. [Google Scholar] [CrossRef]
- Yadav, P.; Shah, K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorg. Chem. 2021, 109, 104639. [Google Scholar] [CrossRef]
- Almansour, A.I.; Arumugam, N.; Prasad, S.; Kumar, R.S.; Alsalhi, M.S.; Alkaltham, M.F.; Al-Tamimi, H.b.A. Investigation of the Optical Properties of a Novel Class of Quinoline Derivatives and Their Random Laser Properties Using ZnO Nanoparticles. Molecules 2022, 27, 145. [Google Scholar] [CrossRef]
- Lewinska, G.; Sanetra, J.; Marszalek, K.W. Application of quinoline derivatives in third-generation photovoltaics. J. Mater. Sci. Mater. Electron 2021, 32, 18451–18465. [Google Scholar] [CrossRef]
- Aygün, B.; Alaylar, B.; Turhan, K.; Şakar, E.; Karadayı, M.; Abu Al-Sayyed, M.I.; Pelit, E.; Güllüce, M.; Karabulut, A.; Turgut, Z.; et al. Investigation of neutron and gamma radiation protective characteristics of synthesized quinoline derivatives. Int. J. Rad. Biol. 2020, 96, 1423–1434. [Google Scholar] [CrossRef]
- Gentile, D.; Fuochi, V.; Rescifina, A.; Furneri, P.M. New Anti SARS-Cov-2 Targets for Quinoline Derivatives Chloroquine and Hydroxychloroquine. Int. J. Mol. Sci. 2020, 21, 5856. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Yang, G.Z.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Zhang, J.; Lee, H.S. Biologically active quinoline and quinazoline alkaloids part II. Med. Res. Rev. 2018, 38, 1614–1660. [Google Scholar] [CrossRef]
- Summers, K.L.; Roseman, G.P.; Sopasis, G.J.; Millhauser, G.L.; Harris, H.H.; Pickering, I.J.; George, G.N. Copper(II) Binding to PBT2 Differs from That of Other 8-Hydroxyquinoline Chelators: Implications for the Treatment of Neurodegenerative Protein Misfolding Diseases. Inorg. Chem. 2020, 59, 17519–17534. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.K.; Singh, V.; Adhikari, D. Homogeneous nickel-catalyzed sustainable synthesis of quinoline and quinoxaline under aerobic conditions. J. Org. Chem. 2020, 85, 14971–14979. [Google Scholar] [CrossRef]
- El-Saghier, A.M.; El-Naggar, M.; Hussein Abdel, H.M.; El-Adasy, A.B.; Olish, M.A.; Abdelmonsef, A.H. Eco-Friendly Synthesis, Biological Evaluation, and In Silico Molecular Docking Approach of Some New Quinoline Derivatives as Potential Antioxidant and Antibacterial Agents. Front. Chem. 2020, 9, 679967. [Google Scholar] [CrossRef]
- Patel, A.; Patel, S.; Mehta, M.; Patel, Y.; Patel, R.; Shah, D.; Patel, D.; Shah, D.; Patel, M.; Patel, M.; et al. A review on synthetic investigation for quinoline- recent green approaches. Green Chem. Lett. Rev. 2022, 15, 337–372. [Google Scholar] [CrossRef]
- Jaiswal, A.; Sharma, A.K.; Jaiswal, S.; Mishra, A.; Singh, J.; Singh, J.; Siddiqui, I.R.J. Visible light induced eco sustainable synthesis of quinolines catalyzed by eosin Y. Heterocycl. Chem. 2023, 60, 1122. [Google Scholar] [CrossRef]
- Harikrishna, S.; Gangu, K.K.; Robert, A.R.; Ganja, H.; Kerru, M.; Maddila, S.; Jonnalagadda, S.B. An ecofriendly and reusable catalyst RuO2/MWCNT in the green synthesis of sulfonyl-quinolines. PSEP 2022, 159, 99–117. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef] [PubMed]
- Dib, M.; Ouchetto, H.; Ouchetto, K.; Hafid, A.; Khouili, M. Recent developments of quinoline derivatives and their potential biological activities. Curr. Org. Synth. 2022, 18, 248–269. [Google Scholar]
- Chu, X.M.; Wang, C.; Liu, W.; Liang, L.L.; Gong, K.K.; Zhao, C.Y.; Sun, K.L. Quinoline and quinolone dimers and their biological activities: An overview. Eur. J. Med. Chem. 2019, 161, 101–117. [Google Scholar] [CrossRef]
- Kumari, L.; Mazumder, A.; Pandey, D.; Yar, M.S.; Kumar, R.; Mazumder, R.; Gupta, S. Synthesis and biological potentials of quinoline analogues: A review of literature. Mini Rev. Org. Chem. 2019, 16, 653–688. [Google Scholar] [CrossRef]
- Loiseau, P.M.; Balaraman, K.; Barratt, G.; Pomel, S.; Durand, R.; Frézard, F.; Figadère, B. The Potential of 2-Substituted Quinolines as Antileishmanial Drug Candidates. Molecules 2022, 27, 2313. [Google Scholar] [CrossRef]
- Kucharski, D.J.; Jaszczak, M.K.; Boratyński, P.J. A Review of Modifications of Quinoline Antimalarials: Mefloquine and (hydroxy)Chloroquine. Molecules 2022, 27, 1003. [Google Scholar] [CrossRef]
- Zeleke, D.; Eswaramoorthy, R.; Belay, Z.; Melaku, Y. Synthesis and antibacterial, antioxidant, and molecular docking analysis of some novel quinoline derivatives. J. Chem. 2020, 2020, 1324096. [Google Scholar] [CrossRef]
- Moor, L.F.; Vasconcelos, T.R.; da R Reis, R.; Pinto, L.S.; da Costa, T.M. Quinoline: An attractive scaffold in drug design. Mini Rev. Med. Chem. 2021, 21, 2209–2226. [Google Scholar] [CrossRef]
- Tran, T.N.; Henary, M. Synthesis and Applications of Nitrogen-Containing Heterocycles as Antiviral Agents. Molecules 2022, 27, 2700. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Carvalho, D.T.; Sousa, E.; Pinto, E. New Antifungal Agents with Azole Moieties. Pharmaceuticals 2022, 15, 1427. [Google Scholar] [CrossRef] [PubMed]
- Katariya, K.D.; Shah, S.R.; Reddy, S.R. Anticancer, antimicrobial activities of quinoline based hydrazone analogues: Synthesis, characterization and molecular docking. Bioorg. Chem. 2020, 94, 103406. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.I.; Farrag, A.M.; Abbas, S.Y.; El Shehry, M.F.; Ragab, A.; Fayed, E.A. Novel structural hybrids of quinoline and thiazole moieties: Synthesis and evaluation of antibacterial and antifungal activities with molecular modeling studies. Bioorg. Chem. 2021, 110, 104803. [Google Scholar] [CrossRef]
- Douadi, K.; Chafaa, S.; Douadi, T.; Al-Noaimi, M.; Kaabi, I. Azoimine quinoline derivatives: Synthesis, classical and electrochemical evaluation of antioxidant, anti-inflammatory, antimicrobial activities and the DNA/BSA binding. J. Mol. Struct. 2020, 1217, 128305. [Google Scholar] [CrossRef]
- Amariucai-Mantu, D.; Mangalagiu, V.; Bejan, I.; Aricu, A.; Mangalagiu, I.I. Hybrid Azine Derivatives: A Useful Approach for Antimicrobial Therapy. Pharmaceutics 2022, 14, 2026. [Google Scholar] [CrossRef]
- Kalita, J.; Chetia, D.; Rudrapal, M. Design, synthesis, antimalarial activity and docking study of 7-chloro-4- (2-(substituted benzylidene)hydrazineyl)quinolines. J. Med. Chem. Drug Des. 2019, 2, 928–937. [Google Scholar] [CrossRef]
- Abdelbaset, M.S.; Abdel-Aziz, M.; Abuo-Rahma, G.E.D.A.; Abdelrahman, M.H.; Ramadan, M.; Youssif, B.G.M. Novel quinoline derivatives carrying nitrones/oximes nitric oxide donors: Design, synthesis, antiproliferative and caspase-3 activation activities. Arch. Pharm. 2018, 352, 1800270. [Google Scholar] [CrossRef]
- Rani, A.; Sharma, A.; Legac, J.; Rosenthal, P.J.; Singh, P.; Kumar, V. A trio of quinoline-isoniazid-phthalimide with promising antiplasmodial potential: Synthesis, invitro evaluation and heme-polymerization inhibition studies. Bioorg. Med. Chem. 2021, 39, 116159. [Google Scholar] [CrossRef]
- Ebenezer, O.; Jordaan, M.A.; Carena, G.; Bono, T.; Shapi, M.; Tuszynski, J.A. An Overview of the Biological Evaluation of Selected Nitrogen-Containing Heterocycle Medicinal Chemistry Compounds. Int. J. Mol. Sci. 2022, 23, 8117. [Google Scholar] [CrossRef]
- Senerovic, L.; Opsenica, D.; Moric, I.; Aleksic, I.; Spasić, M.; Vasiljevic, B. Quinolines and Quinolones as Antibacterial, Antifungal, Anti-virulence, Antiviral and Anti-parasitic Agents. Adv. Exp. Med. Biol. 2020, 1282, 37–69. [Google Scholar]
- Kaur, R.; Kumar, K. Synthetic and medicinal perspective of quinolines as antiviral agents. Eur. J. Med. Chem. 2021, 215, 113220. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, H.; Mishra, A.; Mishra, A.K. Aripiprazole: An FDA Approved Bioactive Compound to Treat Schizophrenia- A Mini Review. Curr. Drug. Discov. Technol. 2020, 17, 23–29. [Google Scholar] [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Lopez, E.G.; Reina, M.; Perez-Gonzalez, A.; Francisco-Marquez, M.; Hernandez-Ayala, L.F.; Castañeda-Arriaga, R.; Galano, A. CADMA-Chem: A Computational Protocol Based on Chemical Properties Aimed to Design Multifunctional Antioxidants. Int. J. Mol. Sci. 2022, 23, 13246. [Google Scholar] [CrossRef]
- RDKit: Open-Source Cheminformatics. Available online: https://www.rdkit.org (accessed on 1 June 2023).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Kochev, N.; Avramova, S.; Angelov, P.; Jeliazkova, N. Computational Prediction of Synthetic Accessibility of Organic Molecules with Ambit-Synthetic Accessibility Tool. Org. Chem. Ind. J. 2018, 14, 123. [Google Scholar]
- Martin, T. Toxicity Estimation Software Tool (TEST); U.S. Environmental Protection Agency: Washington, DC, USA, 2016. [Google Scholar]
- Reina, M.; Castañeda-Arriaga, R.; Pérez-González, A.; Guzman-Lopez, E.; Tan, D.-X.; Reiter, R.; Galano, A. A Computer-Assisted Systematic Search for Melatonin Derivatives with High Potential as Antioxidants. Melatonin Res. 2018, 27–58. [Google Scholar] [CrossRef]
- Zhong, H.A.; Mashinson, V.; Woolman, T.A.; Zha, M. Understanding the molecular properties and metabolism of top prescribed drugs. Curr. Top. Med. Chem. 2013, 13, 1290–1307. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 16, Revision C.01; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. (Eds.) Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of Density Functionals by Combining the Method of Constraint Satisfaction with Parametrization for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Castro-González, L.M.; Alvarez-Idaboy, J.R.; Galano, A. Computationally Designed Sesamol Derivatives Proposed as Potent Antioxidants. ACS Omega 2020, 5, 9566–9575. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Castañeda-Arriaga, R.; Guzmán-López, E.G.; Hernández-Ayala, L.F.; Galano, A. Chalcone Derivatives with a High Potential as Multifunctional Antioxidant Neuroprotectors. ACS Omega 2022, 7, 38254–38268. [Google Scholar] [CrossRef]
- Reina, M.; Guzmán-López, E.G.; Romeo, I.; Marino, T.; Russo, N.; Galano, A. Computationally designed: P -coumaric acid analogs: Searching for neuroprotective antioxidants. New J. Chem. 2021, 45, 14369–14380. [Google Scholar] [CrossRef]
- Galano, A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 2011, 13, 7178–7188. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R.; Francisco-Márquez, M. Physicochemical Insights on the Free Radical Scavenging Activity of Sesamol: Importance of the Acid/Base Equilibrium. J. Phys. Chem. B 2011, 115, 13101–13109. [Google Scholar] [CrossRef]
- Hirao, K.; Nakajima, T.; Chan, B.; Lee, H.J. The core ionization energies calculated by delta SCF and Slater’s transition state theory. J. Chem. Phys. 2023, 158, 064112. [Google Scholar] [CrossRef]
- Marvin 23.8.0, 2023, ChemAxon. Available online: http://www.chemaxon.com (accessed on 15 June 2023).
- Galano, A.; Pérez-González, A.; Castañeda-Arriaga, R.; Muñoz-Rugeles, L.; Mendoza-Sarmiento, G.; Romero-Silva, A.; Ibarra-Escutia, A.; Rebollar-Zepeda, A.M.; León-Carmona, J.R.; Hernández-Olivares, M.A.; et al. Empirically Fitted Parameters for Calculating pKaValues with Small Deviations from Experiments Using a Simple Computational Strategy. J. Chem. Inf. Model. 2016, 56, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-López, E.G.; Reina, M.; Hernández-Ayala, L.F.; Galano, A. Rational Design of Multifunctional Ferulic Acid Derivatives Aimed for Alzheimer’s and Parkinson’s Diseases. Antioxidants 2023, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, M.; Lerner, C.; Burgy, G.; Ehler, A.; Bissantz, C.; Jakob-Roetne, R.; Paulini, R.; Allemann, O.; Tissot, H.; Grünstein, D.; et al. Catechol-O-methyltransferase in complex with substituted 3′-deoxyribose bisubstrate inhibitors. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 253–260. [Google Scholar] [CrossRef]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: Safinamide and coumarin analogs. J. Med. Chem. 2007, 50, 5848–5852. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using Modeller. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 5.6.1–5.6.37. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- BIOVIA. Dassault Systèmes, Discovery Studio 2021 Client; Dassault Systèmes: San Diego, CA, USA, 2023. [Google Scholar]
- Learmonth, D.A.; Bonifácio, M.J.; Soares-da-Silva, P. Synthesis and Biological Evaluation of a Novel Series of “Ortho-Nitrated” Inhibitors of Catechol-O-methyltransferase. J. Med. Chem. 2005, 48, 8070–8078. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Hernández-Marin, E.; Galano, A. Xanthones as antioxidants: A theoretical study on the thermodynamics and kinetics of the single electron transfer mechanism. Food Funct. 2012, 3, 442–450. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Adv. Free Rad. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: How is structure related to function? Chem. Biol. Interact. 2008, 175, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Schalk, I.; Ehret-Sabatier, L.; Bouet, F.; Goeldner, M.; Hirth, C.; Axelsen, P.H.; Silman, I.; Sussman, J.L. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035. [Google Scholar] [CrossRef]
- Ma, J.; Ito, A. Tyrosine residues near the FAD binding site are critical for FAD binding and for the maintenance of the stable and active conformation of rat monoamine oxidase. J. Biochem. 2002, 131, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.E.; Binda, C.; Mattevi, A. The FAD binding sites of human monoamine oxidases A and B. Neurotoxicology 2004, 25, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.E.; Soares-Da-Silva, P. Medicinal chemistry of catechol O -methyltransferase (COMT) inhibitors and their therapeutic utility. J. Med. Chem. 2014, 57, 8692–8717. [Google Scholar] [CrossRef]






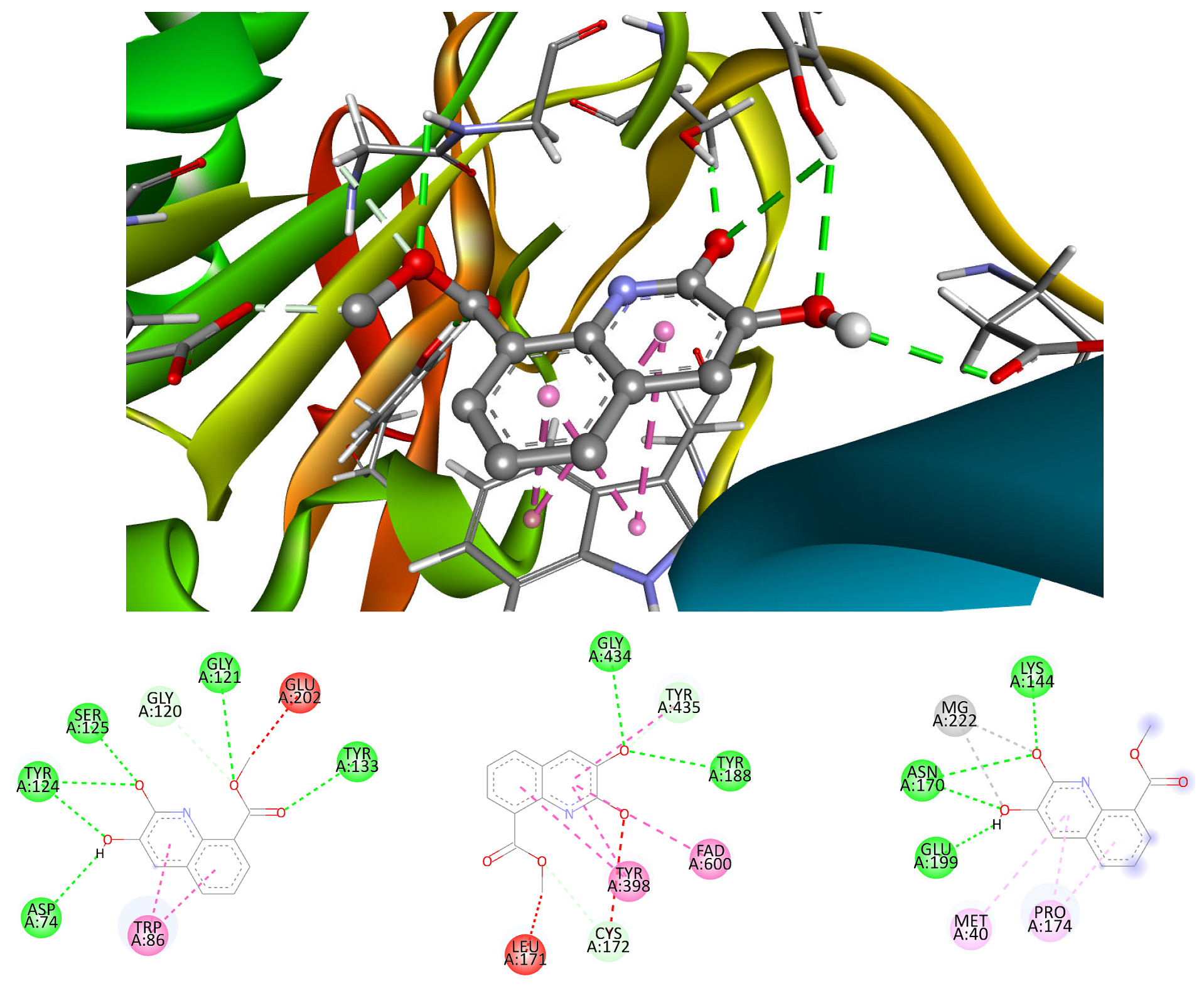
| Enzyme | Co-Crystallized Inhibitor | Substrate |
|---|---|---|
| COMT | Tolcapone [98] | Dopamine |
| MAOB | Safinamide [99] | Phenylethylamine |
| AChE | Donopezil [100] | Acetylcholine |
| dQ | X%H2dQ2+ | X%HdQ+ | X%dQ | X%H-1dQ− | X%H-2dQ2− | X% H-3dQ3− |
|---|---|---|---|---|---|---|
| 1 | --- | 0.0 | 100.0 | --- | --- | --- |
| 49 | --- | 0.0 | 79.6 | 29.4 | 0.0 | --- |
| 67 | --- | 0.0 | 98.7 | 1.3 | --- | --- |
| 100 | --- | 0.0 | 84.9 | 15.1 | --- | --- |
| 134 | --- | 0.0 | 100.0 | 0.0 | --- | --- |
| 815 | --- | 0.0 | 92.4 | 7.6 | 0.0 | --- |
| 829 | --- | 0.0 | 30.9 | 69.1 | 0.0 | --- |
| 833 | --- | 1.1 | 63.3 | 35.6 | 0.0 | 0.0 |
| 845 | --- | 0.0 | 98.7 | 1.3 | 0.0 | --- |
| 929 | --- | 0.0 | 88.6 | 11.4 | 0.0 | --- |
| 950 | --- | 0.0 | 98.8 | 1.2 | 0.0 | --- |
| 954 | --- | 0.0 | 59.7 | 40.3 | 0.0 | --- |
| 1356 | --- | 0.0 | 97.7 | 2.3 | 0.0 | --- |
| 1365 | --- | 0.0 | 68.1 | 31.2 | 0.0 | --- |
| 1368 | --- | 0.0 | 31.4 | 68.6 | 0.0 | --- |
| 1420 | --- | 0.3 | 28.4 | 71.3 | 0.0 | --- |
| 1421 | --- | 0.1 | 80.1 | 18.8 | 1.0 | --- |
| 1930 | --- | 0.0 | 56.8 | 43.2 | 0.0 | --- |
| 2117 | --- | 0.1 | 99.9 | 0.0 | --- | --- |
| 2355 | --- | 0.0 | 56.3 | 43.7 | 0.0 | --- |
| 2357 | --- | 0.0 | 68.6 | 31.4 | 0.0 | --- |
| 2382 | --- | 4.3 | 92.8 | 2.9 | --- | --- |
| 3233 | --- | 0.1 | 54.0 | 46.0 | --- | --- |
| 3754 | --- | 0.0 | 98.0 | 2.0 | --- | --- |
| 3804 | 0.0 | 0.0 | 98.0 | 2.0 | --- | --- |
| 3835 | 0.0 | 0.0 | 98.8 | 1.2 | --- | --- |
| dQ | ΔGBW (kcal/mol) | SP | ||
|---|---|---|---|---|
| COMT | MAO-B | AChE | ||
| Quinoline | −5.00 | −6.90 | −3.80 | 2.85 |
| 49 | −6.00 | −8.34 | −8.20 | 4.18 |
| 815 | −6.26 | −7.91 | −9.02 | 4.11 |
| 829 | −6.80 | −8.41 | −8.74 | 4.44 |
| 954 | −5.78 | −8.16 | −8.24 | 4.11 |
| 1368 | −5.96 | −8.07 | −8.24 | 4.13 |
| 2357 | −6.29 | −8.06 | −7.93 | 4.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ayala, L.F.; Guzmán-López, E.G.; Galano, A. Quinoline Derivatives: Promising Antioxidants with Neuroprotective Potential. Antioxidants 2023, 12, 1853. https://doi.org/10.3390/antiox12101853
Hernández-Ayala LF, Guzmán-López EG, Galano A. Quinoline Derivatives: Promising Antioxidants with Neuroprotective Potential. Antioxidants. 2023; 12(10):1853. https://doi.org/10.3390/antiox12101853
Chicago/Turabian StyleHernández-Ayala, Luis Felipe, Eduardo Gabriel Guzmán-López, and Annia Galano. 2023. "Quinoline Derivatives: Promising Antioxidants with Neuroprotective Potential" Antioxidants 12, no. 10: 1853. https://doi.org/10.3390/antiox12101853
APA StyleHernández-Ayala, L. F., Guzmán-López, E. G., & Galano, A. (2023). Quinoline Derivatives: Promising Antioxidants with Neuroprotective Potential. Antioxidants, 12(10), 1853. https://doi.org/10.3390/antiox12101853








