Korean Chestnut Honey Suppresses HSV-1 Infection by Regulating the ROS–NLRP3 Inflammasome Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Korean Chestnut Honey
2.3. Cells and Viruses
2.4. Plaque Assay
2.5. Cell Survival Assay
2.6. Evaluation of Antiviral Effects
2.7. Time-of-Addition Assay
2.8. Western Blot Analysis
2.9. Fluorescence Analysis
2.10. Antioxidant-Related Enzyme Activity
2.11. Immunofluorescence Staining
2.12. Cytokines
2.13. Statistical Analysis
3. Results
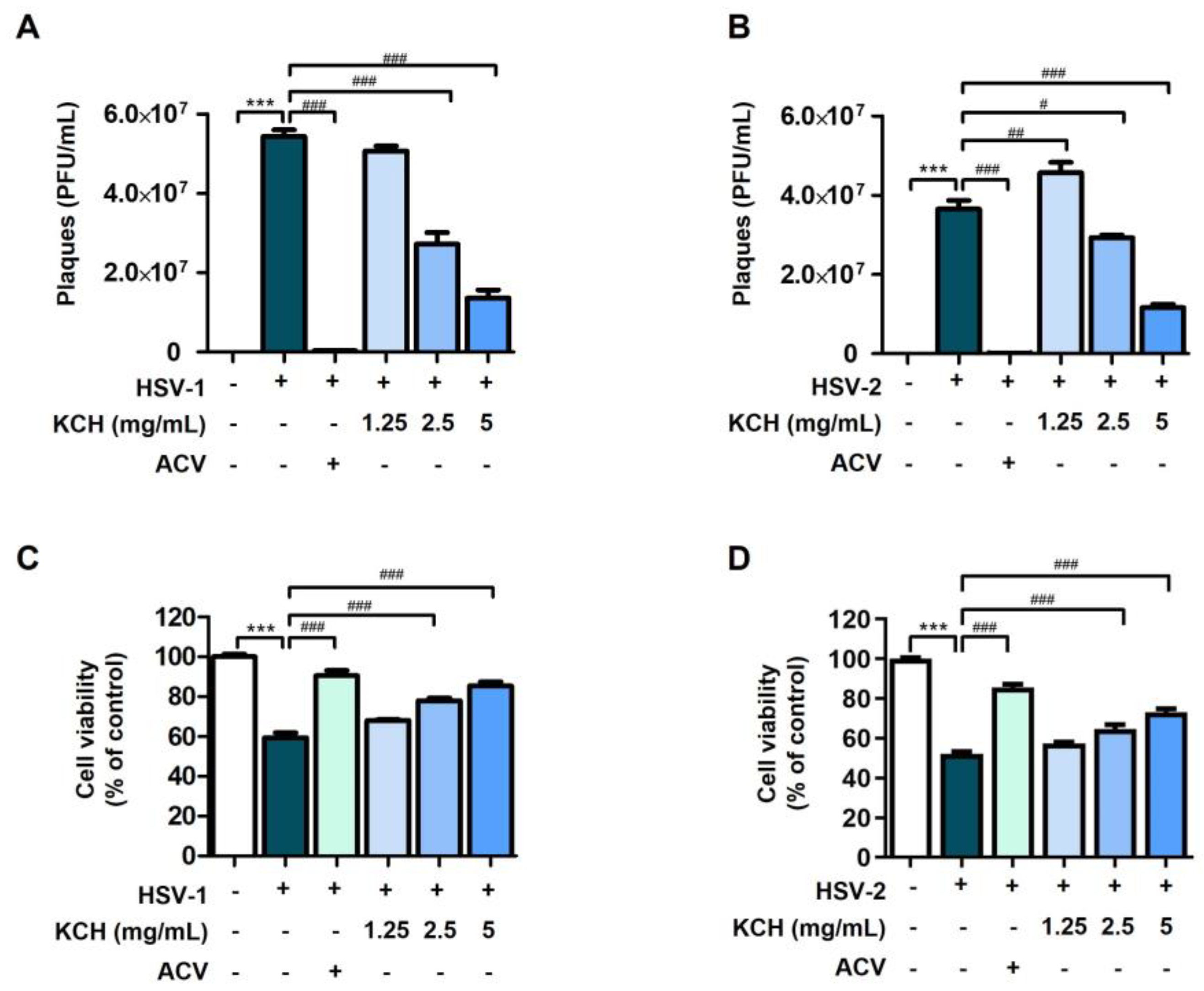
3.1. Antiviral Effects of KCH on HSV-1 and HSV-2 Infected in Vero Cells
3.2. KCH Inhibited HSV-1 Binding and Virucidal Activity
3.3. KCH Ameliorates Mitochondrial Dysfunction in Vero Cells Caused by HSV-1 Infection
3.4. KCH Reduced Inflammation Response in HSV-1-Infected Vero Cells
3.5. KCH Inhibits the Expression of the NLRP3 Inflammasome following HSV-1 Infection
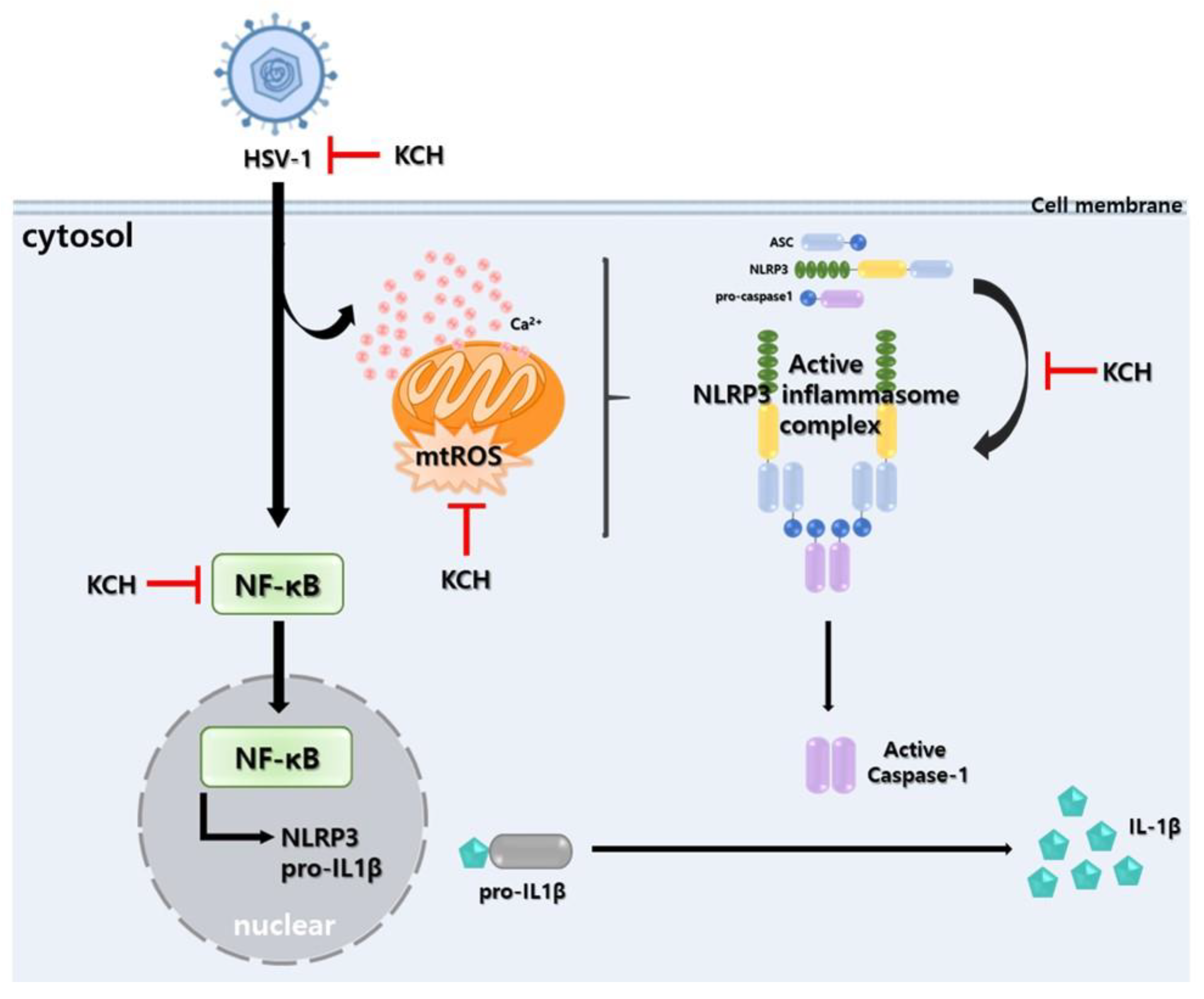
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, G.H.; Kim, J.; Kim, H.W.; Cho, J.W. Herpes simplex viruses (1 and 2) and varicella-zoster virus infections in an adult population with aseptic meningitis or encephalitis: A nine-year retrospective clinical study. Medicine 2021, 100, e27856. [Google Scholar] [CrossRef]
- Whitley, R.J. Herpes Simplex Virus Infections of the Central Nervous System. Continuum 2015, 21, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.P.; Mailles, A. Herpes simplex virus encephalitis update. Curr. Opin. Infect. Dis. 2019, 32, 239–243. [Google Scholar] [CrossRef]
- Xu, X.Q.; Xu, T.; Ji, W.; Wang, C.; Ren, Y.; Xiong, X.; Zhou, X.; Lin, S.H.; Xu, Y.; Qiu, Y. Herpes Simplex Virus 1-Induced Ferroptosis Contributes to Viral Encephalitis. mBio 2023, 14, e0237022. [Google Scholar] [CrossRef] [PubMed]
- Nash, A.; Ryan, E.J. The oncogenic gamma herpesviruses Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) hijack retinoic acid-inducible gene I (RIG-I) facilitating both viral and tumour immune evasion. Tumour Virus Res. 2022, 14, 200246. [Google Scholar] [CrossRef]
- Lee, J.; Song, C.H. Effect of Reactive Oxygen Species on the Endoplasmic Reticulum and Mitochondria during Intracellular Pathogen Infection of Mammalian Cells. Antioxidants 2021, 10, 872. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Panda, S.; Behera, S.; Alam, M.F.; Syed, G.H. Endoplasmic reticulum & mitochondrial calcium homeostasis: The interplay with viruses. Mitochondrion 2021, 58, 227–242. [Google Scholar] [CrossRef]
- Luo, Z.L.; Sun, H.Y.; Wu, X.B.; Cheng, L.; Ren, J.D. Epigallocatechin-3-gallate attenuates acute pancreatitis induced lung injury by targeting mitochondrial reactive oxygen species triggered NLRP3 inflammasome activation. Food Funct. 2021, 12, 5658–5667. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Zheng, M.; Kanneganti, T.D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 2020, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Aarreberg, L.D.; Esser-Nobis, K.; Driscoll, C.; Shuvarikov, A.; Roby, J.A.; Gale, M., Jr. Interleukin-1beta Induces mtDNA Release to Activate Innate Immune Signaling via cGAS-STING. Mol. Cell 2019, 74, 801–815.e6. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Place, D.E.; Kanneganti, T.D. DNA Sensing in the Innate Immune Response. Physiology 2020, 35, 112–124. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, W. NLRP3 Inflammasome-A Key Player in Antiviral Responses. Front. Immunol. 2020, 11, 211. [Google Scholar] [CrossRef]
- Zheng, Q.; Hua, C.; Liang, Q.; Cheng, H. The NLRP3 inflammasome in viral infection (Review). Mol. Med. Rep. 2023, 28, 160. [Google Scholar] [CrossRef] [PubMed]
- Piperi, E.; Papadopoulou, E.; Georgaki, M.; Dovrat, S.; Bar Illan, M.; Nikitakis, N.G.; Yarom, N. Management of oral herpes simplex virus infections: The problem of resistance. A narrative review. Oral. Dis. 2023. [Google Scholar] [CrossRef]
- Kim, B.; Kim, Y.S.; Hwang, Y.H.; Yang, H.J.; Li, W.; Kwon, E.B.; Kim, T.I.; Go, Y.; Choi, J.G. Quercus acuta Thunb. (Fagaceae) and Its Component, Isoquercitrin, Inhibit HSV-1 Replication by Suppressing Virus-Induced ROS Production and NF-kappaB Activation. Antioxidants 2021, 10, 1638. [Google Scholar] [CrossRef]
- Kim, B.; Kwon, E.B.; Yang, H.J.; Li, W.; Hwang, Y.H.; Kim, Y.S.; Pak, M.E.; Go, Y.; Choi, J.G. Vaccinium bracteatum Thunb Extract Inhibits HSV-1 Infection by Regulating ER Stress and Apoptosis. Antioxidants 2022, 11, 1773. [Google Scholar] [CrossRef]
- Kim, T.I.; Kwon, E.B.; Oh, Y.C.; Go, Y.; Choi, J.G. Mori ramulus and its Major Component Morusin Inhibit Herpes Simplex Virus Type 1 Replication and the Virus-Induced Reactive Oxygen Species. Am. J. Chin. Med. 2021, 49, 163–179. [Google Scholar] [CrossRef]
- Sanchez-Martin, V.; Morales, P.; Gonzalez-Porto, A.V.; Iriondo-DeHond, A.; Lopez-Parra, M.B.; Del Castillo, M.D.; Hospital, X.F.; Fernandez, M.; Hierro, E.; Haza, A.I. Enhancement of the Antioxidant Capacity of Thyme and Chestnut Honey by Addition of Bee Products. Foods 2022, 11, 3118. [Google Scholar] [CrossRef]
- Kwon, E.B.; Kim, S.G.; Kim, Y.S.; Kim, B.; Han, S.M.; Lee, H.J.; Choi, H.M.; Choi, J.G. Castanea crenata honey reduces influenza infection by activating the innate immune response. Front. Immunol. 2023, 14, 1157506. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.B.; Kim, Y.S.; Hwang, Y.H.; Kim, B.; Lee, S.B.; Park, S.K.; Choi, M.S.; Ha, H.; Choi, J.G. Antiviral activity of soybean GL 2626/96 (Glycine max) ethanolic extract against influenza A virus in vitro and in vivo. Biomed. Pharmacother. 2022, 156, 113780. [Google Scholar] [CrossRef]
- Ravi, B.; Foyer, C.H.; Pandey, G.K. The integration of reactive oxygen species (ROS) and calcium signalling in abiotic stress responses. Plant Cell Environ. 2023, 46, 1985–2006. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, R.; Zhong, W. Host Calcium Channels and Pumps in Viral Infections. Cells 2019, 9, 94. [Google Scholar] [CrossRef]
- Torres, S.; Segales, P.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondria and the NLRP3 Inflammasome in Alcoholic and Nonalcoholic Steatohepatitis. Cells 2022, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Ramanujan, V.K.; Becker, C.; Fett, S.; Underhill, D.M.; Wolf, A.J. Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation. Sci. Immunol. 2023, 8, eade7652. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52. [Google Scholar] [CrossRef]
- Carty, M.; Guy, C.; Bowie, A.G. Detection of Viral Infections by Innate Immunity. Biochem. Pharmacol. 2021, 183, 114316. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Karaba, A.H.; Figueroa, A.; Massaccesi, G.; Botto, S.; DeFilippis, V.R.; Cox, A.L. Herpes simplex virus type 1 inflammasome activation in proinflammatory human macrophages is dependent on NLRP3, ASC, and caspase-1. PLoS ONE 2020, 15, e0229570. [Google Scholar] [CrossRef] [PubMed]
- Dominic, A.; Le, N.T.; Takahashi, M. Loop Between NLRP3 Inflammasome and Reactive Oxygen Species. Antioxid. Redox Signal. 2022, 36, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.S.; Hossain, M.G.; Moni, A.; Rahman, M.M.; Rahman, U.H.; Alam, M.; Kundu, S.; Rahman, M.M.; Hannan, M.A.; Uddin, M.J. Prospects of honey in fighting against COVID-19: Pharmacological insights and therapeutic promises. Heliyon 2020, 6, e05798. [Google Scholar] [CrossRef]
- Kwon, E.B.; Kim, Y.S.; Han, S.M.; Kim, S.G.; Choi, J.G. The protective effect of Tilia amurensis honey on influenza A virus infection through stimulation of interferon-mediated IFITM3 signaling. Biomed. Pharmacother. 2022, 153, 113259. [Google Scholar] [CrossRef]
- Piacentini, R.; Civitelli, L.; Ripoli, C.; Marcocci, M.E.; De Chiara, G.; Garaci, E.; Azzena, G.B.; Palamara, A.T.; Grassi, C. HSV-1 promotes Ca2+ -mediated APP phosphorylation and Abeta accumulation in rat cortical neurons. Neurobiol. Aging 2011, 32, 2323.e13–2323.e26. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef]
- Abe, T.; Marutani, Y.; Shoji, I. Cytosolic DNA-sensing immune response and viral infection. Microbiol. Immunol. 2019, 63, 51–64. [Google Scholar] [CrossRef]
- Liu, N.; Pang, X.; Zhang, H.; Ji, P. The cGAS-STING Pathway in Bacterial Infection and Bacterial Immunity. Front. Immunol. 2021, 12, 814709. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Lemoff, A.; Wang, G.; Zarek, C.; Lowe, A.; Yan, N.; Reese, T.A. Reactive oxygen species oxidize STING and suppress interferon production. Elife 2020, 9, e57837. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, E.-B.; Kim, Y.S.; Kim, B.; Kim, S.-G.; Na, S.-J.; Go, Y.; Choi, H.M.; Lee, H.J.; Han, S.M.; Choi, J.-G. Korean Chestnut Honey Suppresses HSV-1 Infection by Regulating the ROS–NLRP3 Inflammasome Pathway. Antioxidants 2023, 12, 1935. https://doi.org/10.3390/antiox12111935
Kwon E-B, Kim YS, Kim B, Kim S-G, Na S-J, Go Y, Choi HM, Lee HJ, Han SM, Choi J-G. Korean Chestnut Honey Suppresses HSV-1 Infection by Regulating the ROS–NLRP3 Inflammasome Pathway. Antioxidants. 2023; 12(11):1935. https://doi.org/10.3390/antiox12111935
Chicago/Turabian StyleKwon, Eun-Bin, Young Soo Kim, Buyun Kim, Se-Gun Kim, Sung-Joon Na, Younghoon Go, Hong Min Choi, Hye Jin Lee, Sang Mi Han, and Jang-Gi Choi. 2023. "Korean Chestnut Honey Suppresses HSV-1 Infection by Regulating the ROS–NLRP3 Inflammasome Pathway" Antioxidants 12, no. 11: 1935. https://doi.org/10.3390/antiox12111935
APA StyleKwon, E.-B., Kim, Y. S., Kim, B., Kim, S.-G., Na, S.-J., Go, Y., Choi, H. M., Lee, H. J., Han, S. M., & Choi, J.-G. (2023). Korean Chestnut Honey Suppresses HSV-1 Infection by Regulating the ROS–NLRP3 Inflammasome Pathway. Antioxidants, 12(11), 1935. https://doi.org/10.3390/antiox12111935






