Resveratrol and Its Derivatives in Inflammatory Skin Disorders—Atopic Dermatitis and Psoriasis: A Review
Abstract
:1. Introduction
2. Resveratrol and Its Derivatives
3. Resveratrol and Its Derivatives in Inflammatory Diseases
4. Resveratrol and Its Derivatives in Atopic Dermatitis and Psoriasis
Overview of Selected Studies
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guttman-Yassky, E.; Krueger, J.G.; Lebwohl, M.G. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp. Dermatol. 2018, 27, 409–417. [Google Scholar] [CrossRef]
- Yaghmaie, P.; Koudelka, C.W.; Simpson, E.L. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Silverberg, J.I. Epidemiology of childhood atopic dermatitis. Clin. Dermatol. 2015, 33, 281–288. [Google Scholar] [CrossRef]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Vyas, J.; Finlay, A.Y. Counting the burden: Atopic dermatitis and health-related quality of life. Acta Derm. Venereol. 2020, 100, 330–340. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on atopic dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef]
- Torres, T. Atopic dermatitis: The new therapeutic revolution in dermatology. Acta Med. Port. 2017, 30, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. How to Define Atopic Dermatitis? Dermatol. Clin. 2017, 35, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I. Public health burden and epidemiology of atopic dermatitis. Dermatol. Clin. 2017, 35, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, G.; de Bruin-Weller, M.; Aoki, V.; Kabashima, K.; Deleuran, M.; Puig, L.; Bansal, A.; Rossi, A.B. Nomenclature and clinical phenotypes of atopic dermatitis. Ther. Adv. Chronic Dis. 2018, 9, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Rakkhit, T.; Panko, J.M.; Christensen, T.E.; Wong, B.; Nelson, T.; Papenfuss, J.; Hansen, C.B.; Callis-Duffin, K.; Krueger, G.G. Plaque thickness and morphology in psoriasis vulgaris associated with therapeutic response. Br. J. Dermatol. 2009, 160, 1083–1089. [Google Scholar] [CrossRef]
- Schäbitz, A.; Eyerich, K.; Garzorz-Stark, N. So close, and yet so far away: The dichotomy of the specific immune response and inflammation in psoriasis and atopic dermatitis. J. Intern. Med. 2021, 290, 27–39. [Google Scholar] [CrossRef]
- Noda, S.; Krueger, J.G.; Guttman-Yassky, E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J. Allergy Clin. Immunol. 2015, 135, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: Latest evidence and clinical considerations. Drugs Context 2018, 17, 212530. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Christensen, R.; Lavrijsen, A.; Arents, B.W.M. Emollients and moisturisers for eczema. Cochrane Database Syst. Rev. 2017, 2, CD012119. [Google Scholar] [PubMed]
- Hon, K.L.E.; Ching, G.K.; Leung, T.F.; Choi, C.Y.; Lee, K.K.C.; Ng, P.C. Estimating emollient usage in patients with eczema. Clin. Exp. Dermatol. 2010, 35, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.H.I.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef]
- Eichenfield, L.E.; Wynnis, L.T.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.; Gooderham, M.; Torres, T. New Topical Therapies in Development for Atopic Dermatitis. Drugs 2022, 82, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Von Kobyletzki, L.; Svensson, Å.; Apfelbacher, C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: Systematic review and meta-analysis of randomized controlled trials. Br. J. Dermatol. 2011, 164, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Yan, J.; Wang, F.S. Two topical calcineurin inhibitors for the treatment of atopic dermatitis in pediatric patients: A meta-analysis of randomized clinical trials. J. Dermatol. Treat. 2010, 21, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.M.; Lebwohl, M.; Lim, H.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J. Am. Acad. Dermatol. 2009, 60, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2019, 80, 1029–1072. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Leonardi, C.L.; Davis, D.M.R.; Gelfand, J.M.; Lichten, J.; Mehta, N.N.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Elewski, B.E.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol. 2019, 80, 1073–1113. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA-J. Am. Med. Assoc. 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Lim, H.W.; Stoff, B.; Connor, C.; Cordoro, K.M.; Lebwohl, M.; Armstrong, A.W.; Davis, D.M.R.; Elewski, B.E.; Gelfand, J.M.; et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J. Am. Acad. Dermatol. 2019, 81, 775–804. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Kabashima, K.; Eyerich, K. Mechanisms of skin autoimmunity: Cellular and soluble immune components of the skin. J. Allergy Clin. Immunol. 2020, 146, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, S.C.; Hsu, S.Y.; Lin, Y.A.; Shih, C.M.; Huang, C.Y. Annoying Psoriasis and Atopic Dermatitis: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 4898. [Google Scholar] [CrossRef] [PubMed]
- de Brito Oliveira, A.L.; Monteiro, V.V.S.; Navegantes-Lima, K.C.; Reis, J.F.; de Souza Gomes, R.; Rodrigues, D.V.S.; de França Gaspar, S.L.; Monteiro, M.C. Resveratrol role in autoimmune disease—A mini-review. Nutrients 2017, 9, 1306. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The use of micro-and nanocarriers for resveratrol delivery into and across the skin in different skin diseases—A literature review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Hung, C.F.; Sung, H.C.; Yang, S.C.; Yu, H.P.; Fang, J.Y. The bioactivities of resveratrol and its naturally occurring derivatives on skin. J. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Siemann, E.H.; Creasy, L.L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; Lorgeril, M.D. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Wine phenolics. Ann. N. Y Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Biasutto, L.; Mattarei, A.; Azzolini, M.; La Spina, M.; Sassi, N.; Romio, M.; Paradisi, C.; Zoratti, M. Resveratrol derivatives as a pharmacological tool. Ann. N. Y Acad. Sci. 2017, 1403, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Grau, L.; Soucek, R.; Pujol, M.D. Resveratrol derivatives: Synthesis and their biological activities. Eur. J. Med. Chem. 2023, 246, 114962. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Wang, K.T.; Chen, L.G.; Tseng, S.H.; Huang, J.S.; Hsieh, M.S.; Wang, C.C. Anti-inflammatory effects of resveratrol and oligostilbenes from vitis thunbergii var. taiwaniana against lipopolysaccharide-induced arthritis. J. Agric. Food Chem. 2011, 59, 3649–3656. [Google Scholar] [CrossRef]
- Ishihata, A.; Maruki-Uchida, H.; Gotoh, N.; Kanno, S.; Aso, Y.; Togashi, S.; Sai, M.; Ito, T.; Katano, Y. Vascular- and hepato-protective effects of passion fruit seed extract containing piceatannol in chronic high-fat diet-fed rats. Food Funct. 2016, 7, 4075–4081. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Hung, C.F.; Lin, Y.K.; Huang, Z.R.; Fang, J.Y. Delivery of resveratrol, a red wine polyphenol, from solutions and hydrogels via the skin. Biol. Pharm. Bull. 2008, 31, 955–962. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-loaded nanoparticles based on poly(epsiloncaprolactone) and poly(D,L-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef] [PubMed]
- Badri, W.; Miladi, K.; Nazari, Q.A.; Greige-Gerges, H.; Fessi, H.; Elaissari, A. Encapsulation of NSAIDs for inflammation management: Overview, progress, challenges and prospects. Int. J. Pharm. 2016, 515, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Luo, L.J.; Yang, C.J.; Lai, J.Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.A.; Lucio, M.L.C.; Lima, J.; Reis, S. Resveratrol in Medicinal Chemistry: A Critical Review of its Pharmacokinetics, Drug-Delivery, and Membrane Interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, R.; Zhao, X.; Zheng, G.; Wang, Z.; Wang, P. Resveratrol raises in vitro anticancer effects of paclitaxel in NSCLC cell line A549 through CO X-2 expression. Korean J. Physiol. Pharmacol. 2017, 21, 465–474. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Mou, S.F.; Chen, X.Q.; Gong, L.L.; Ge, W.S. Anti-inflammatory activity of resveratrol prevents inflammation by inhibiting NF-κB in animal models of acute pharyngitis. Mol. Med. Rep. 2018, 17, 1269–1274. [Google Scholar] [CrossRef]
- Wang, G.; Hu, Z.; Song, X.; Cui, Q.; Fu, Q.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Analgesic and Anti-Inflammatory Activities of Resveratrol through Classic Models in Mice and Rats. Evid. Based Complement. Alternat Med. 2017, 2017, 5197567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, J.; Shi, J.S. Anti-inflammatory activities of resveratrol in the brain: Role of resveratrol in microglial activation. Eur. J. Pharmacol. 2010, 636, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Park, E.J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- Rahal, K.; Schmiedlin-Ren, P.; Adler, J.; Dhanani, M.; Sultani, V.; Rittershaus, A.C.; Reingold, L.; Zhu, J.; McKenna, B.J.; Christman, G.M.; et al. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 613–623. [Google Scholar] [CrossRef]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of Resveratrol, Curcumin and Simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de la Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010, 633, 78–84. [Google Scholar] [CrossRef]
- Jha, R.K.; Ma, Q.; Lei, Z.; Sha, H. Resveratrol Ameliorates the Deleterious Effect of Severe Acute Pancreatitis. Cell Biochem. Biophys. 2012, 62, 397–402. [Google Scholar] [CrossRef]
- Sha, H.; Ma, Q.; Jha, R.K.; Wu, Z.; Qingyuan, Z.; Wang, Z.; Ma, Z.; Luo, X.; Liu, C. Resveratrol Suppresses Microcirculatory Disturbance in a Rat Model of Severe Acute Pancreatitis. Cell Biochem. Biophys. 2013, 67, 1059–1065. [Google Scholar] [CrossRef]
- Djoko, B.; Chiou, R.Y.Y.; Shee, J.J.; Liu, Y.W. Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-induced inflammation of RAW 264.7 macrophages. J. Agric. Food Chem. 2007, 55, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.; Kim, B.H.; Hong, J.T.; Lee, C.K.; Ahn, B.; Nam, S.Y.; Han, S.B.; Kim, Y. Resveratrol down-regulates interferon-γ-inducible inflammatory genes in macrophages: Molecular mechanism via decreased STAT-1 activation. J. Nutr. Biochem. 2011, 22, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.L.; Yang, J.Y.; Dong, Y.X.; Wang, J.M.; Cui, Y.H.; Ikeshima, T.; Zhao, Y.Q.; Wu, C.F. Resveratrol inhibits nitric oxide and TNF-α production by lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2005, 5, 185–193. [Google Scholar] [CrossRef]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.C.; Kang, O.H.; Choi, J.G.; Chae, H.S.; Lee, Y.S.; Brice, O.O.; Jung, H.J.; Hong, S.H.; Lee, Y.M.; Kwon, S.Y. Anti-inflammatory effect of resveratrol by inhibition of IL-8 production in LPS-induced THP-1 cells. Am. J. Chin. Med. 2009, 37, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Jang, H.J.; Chae, H.S.; Oh, Y.C.; Choi, J.G.; Lee, Y.S.; Kim, J.H.; Kim, Y.C.; Sohn, D.H.; Park, H.; et al. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: Pivotal roles of NF-κB and MAPK. Pharmacol. Res. 2009, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef]
- Ko, Y.J.; Kim, H.H.; Kim, E.J.; Katakura, Y.; Lee, W.S.; Kim, G.S.; Ryu, C.H. Piceatannol inhibits mast cell-mediated allergic inflammation. Int. J. Mol. Med. 2013, 31, 951–958. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol. Oxid. Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Serial Review: Oxidative DNA Damage and Repair. Science 2002, 32, 1102–1115. [Google Scholar]
- Dobrică, E.C.; Cozma, M.A.; Găman, M.A.; Voiculescu, V.M.; Găman, A.M. The Involvement of Oxidative Stress in Psoriasis: A Systematic Review. Antioxidants 2022, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Bang, J.S.; Lee, W.J. Preventative Effects of Antioxidants against PM 10 on Serum IgE Concentration, Mast Cell Counts, Inflammatory Cytokines, and Keratinocyte Differentiation Markers in DNCB-Induced Atopic Dermatitis Mouse Model. Antioxidants 2022, 11, 1334. [Google Scholar] [CrossRef]
- Park, S.; Seok, J.K.; Kwak, J.Y.; Suh, H.J.; Kim, Y.M.; Boo, Y.C. Anti-Inflammatory Effects of Pomegranate Peel Extract in THP-1 Cells Exposed to Particulate Matter PM10. Evid. Based Complement. Alternat Med. 2016, 2016, 6836080. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super. Sanita 2007, 43, 394–405. [Google Scholar]
- Ilves, L.; Ottas, A.; Kaldvee, B.; Abram, K.; Soomets, U.; Zilmer, M.; Jaks, V.; Kingo, K. Metabolomic analysis of skin biopsies from patients with atopic dermatitis reveals hallmarks of inflammation, disrupted barrier function and oxidative stress. Acta Derm. Venereol. 2021, 101, 1–8. [Google Scholar] [CrossRef]
- Tsukahara, H.; Shibata, R.; Ohshima, Y.; Todoroki, Y.; Sato, S.; Ohta, N.; Hiraoka, M.; Yoshida, A.; Nishima, S.; Mayumi, M. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003, 72, 2509–2516. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Blagov, A.; Sukhorukov, V.; Guo, S.; Zhang, D.; Eremin, I.; Orekhov, A. The Role of Oxidative Stress in the Induction and Development of Psoriasis. Front. Biosci. 2023, 28, 118. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free Radic. Res. 2016, 50, 585–595. [Google Scholar] [CrossRef]
- Kirmit, A.; Kader, S.; Aksoy, M.; Bal, C.; Nural, C.; Aslan, O. Trace elements and oxidative stress status in patients with psoriasis. Postep. Dermatol. Alergol. 2020, 37, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Dainichi, T.; Hanakawa, S.; Kabashima, K. Classification of inflammatory skin diseases: A proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity. J. Dermatol. Sci. 2014, 76, 81–89. [Google Scholar] [CrossRef]
- Wu, Z. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J. Dermatol. Sci. 2014, 75, 16–23. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Guy, R.H.; Anissimov, Y.G. Modelling drug flux through microporated skin. J. Control. Release 2016, 241, 194–199. [Google Scholar] [CrossRef]
- Tsai, M.J.; Lu, I.J.; Fu, Y.S.; Fang, Y.P.; Huang, Y.B.; Wu, P.C. Nanocarriers enhance the transdermal bioavailability of resveratrol: In-vitro and in-vivo study. Colloids Surf. B Biointerfaces 2016, 148, 650–656. [Google Scholar] [CrossRef]
- Sarama, R.; Matharu, P.K.; Abduldaiem, Y.; Corrêa, M.P.; Gil, C.D.; Greco, K.V. In Vitro Disease Models for Understanding Psoriasis and Atopic Dermatitis. Front. Bioeng. Biotechnol. 2022, 10, 251. [Google Scholar] [CrossRef]
- Kabashima, K.; Nomura, T. Revisiting murine models for atopic dermatitis and psoriasis with multipolar cytokine axes. Curr. Opin. Immunol. 2017, 48, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Manzke, V.; Erpenbeck, L. Animal models of psoriasis—Highlights and drawbacks. J. Allergy Clin. Immunol. 2021, 147, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Reich, K.; Keren, A.; Kabashima, K.; Steinhoff, M.; Paus, R. Mouse models of atopic dermatitis: A critical reappraisal. Exp. Dermatol. 2021, 30, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in mice. Int. Immunopharmacol. 2014, 23, 617–623. [Google Scholar] [CrossRef]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W.; et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef]
- Katoh, N.; Hirano, S.; Suehiro, M.; Masuda, K.; Kishimoto, S. The characteristics of patients with atopic dermatitis demonstrating a positive reaction in a scratch test after 48 hours against house dust mite antigen. J. Dermatol. 2004, 31, 720–726. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with Dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, J.; Kim, J.; Kim, H.; Kang, H.; Kim, E.H.; Chang, J.H.; Kim, Y.E.; Choi, Y.J.; Lee, K.W.; et al. 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J. Ethnopharmacol. 2014, 151, 365–371. [Google Scholar] [CrossRef]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Ayyildiz, Z.A.; Bagriyanik, A.; Uzuner, N.; Karaman, O. Resveratrol ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis-like lesions through effects on the epithelium. PeerJ 2016, 4, e1889. [Google Scholar] [CrossRef] [PubMed]
- Bangash, Y.; Saleem, A.; Akhtar, M.F.; Anwar, F.; Akhtar, B.; Sharif, A.; Khan, M.I.; Khan, A. Pterostilbene reduces the progression of atopic dermatitis via modulating inflammatory and oxidative stress biomarkers in mice. Inflammopharmacology 2023, 31, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Dagouassat, M.; Lanone, S.; Boczkowski, J. Interaction of matrix metalloproteinases with pulmonary pollutants. Eur. Respir. J. 2012, 39, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem.-Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef]
- Kjær, T.N.; Thorsen, K.; Jessen, N.; Stenderup, K.; Pedersen, S.B. Resveratrol ameliorates imiquimod-induced psoriasis-like skin inflammation in mice. PLoS ONE 2015, 10, e0126599. [Google Scholar] [CrossRef]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of resveratrol-enriched rice on skin inflammation and pruritus in the NC/Nga mouse model of atopic dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lin, Y.K.; Yang, S.C.; Alalaiwe, A.; Lin, C.J.; Fang, J.Y.; Lin, C.F. Percutaneous absorption of resveratrol and its oligomers to relieve psoriasiform lesions: In silico, in vitro and in vivo evaluations. Int. J. Pharm. 2020, 585, 119507. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, Y.K.; Lin, C.F.; Wang, P.W.; Chen, E.L.; Fang, J.Y. Elucidating the skin delivery of aglycone and glycoside flavonoids: How the structures affect cutaneous absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef]
- Shin, J.W.; Lee, H.S.; Na, J.I.; Huh, C.H.; Park, K.C.; Choi, H.R. Resveratrol inhibits particulate matter-induced inflammatory responses in human keratinocytes. Int. J. Mol. Sci. 2020, 21, 3446. [Google Scholar] [CrossRef]
- Moon, P.D.; Han, N.R.; Lee, J.S.; Jee, H.W.; Kim, J.H.; Kim, H.M.; Jeong, H.J. Effects of resveratrol on thymic stromal lymphopoietin expression in mast cells. Medicina 2021, 57, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.S.; Park, S.Y.; Lee, Y.J. Resveratrol induces human keratinocyte damage via the activation of class III histone deacetylase, Sirt1. Oncol. Rep. 2016, 35, 524–529. [Google Scholar] [CrossRef] [PubMed]

| Resveratrol and Its Derivatives—Stages of Action | |
|---|---|
| Atopic dermatitis | ↑ Skin barrier function ↓ Scratching ↓ Edema ↓ Hyperplasia ↓ Immune cell infiltration |
| Psoriasis | ↓ Keratinocyte proliferation ↓ Hyperplasia ↓ Plaque ↓ Neutrophil infiltration |
| Authors [Reference] | Year | Cell/Animal/ Model Type | Induction Methods | Resveratrol Dosage | Molecular Target and Effect |
|---|---|---|---|---|---|
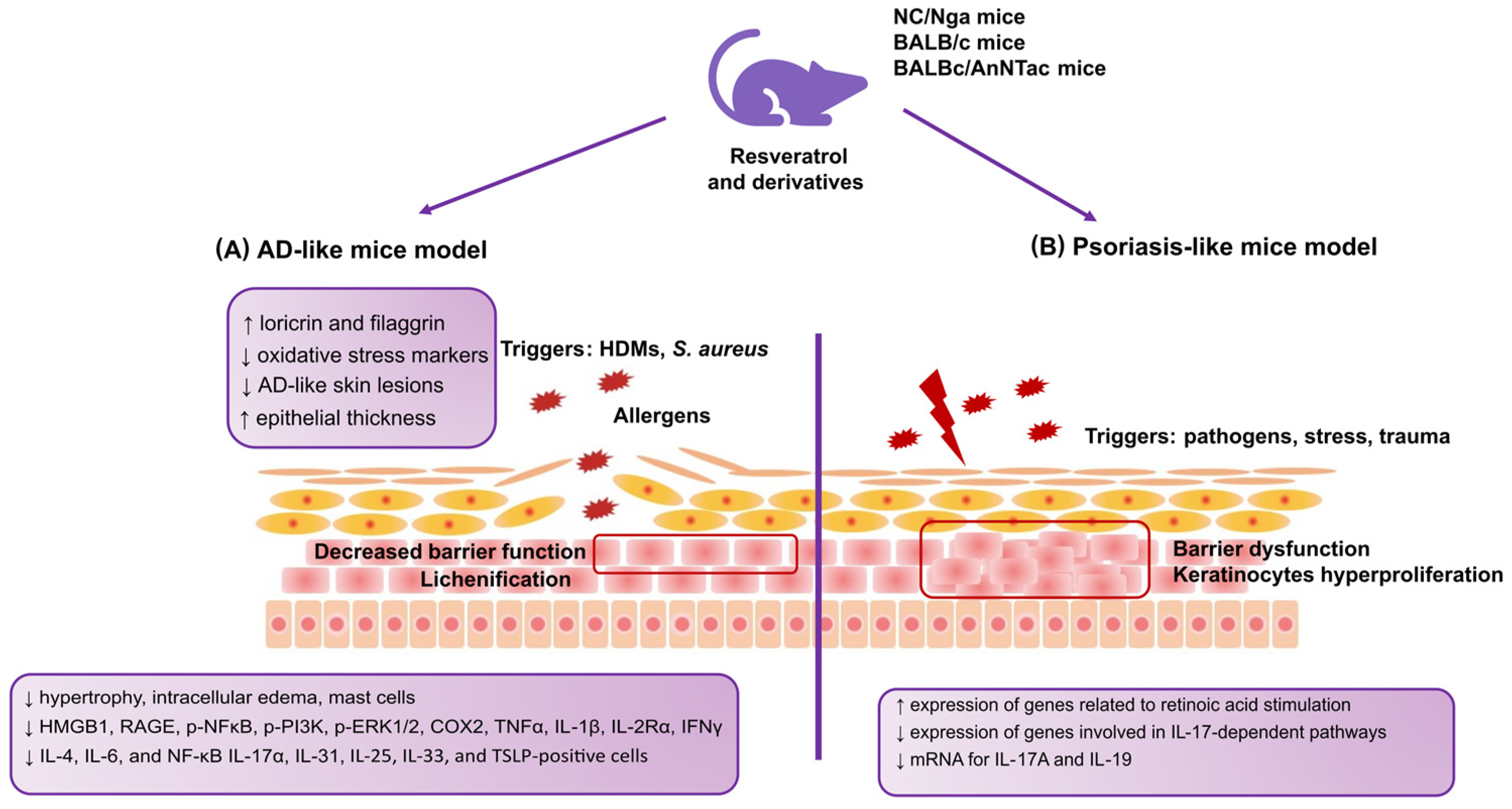
| Karuppagounder et al. [105] | 2014 | NC/Nga mice; AD-like model | HDM extract (100 mg of DfE cream) | 20 mg/kg for 2 weeks | ↓ Hypertrophy ↓ Intracellular edema ↓ Mast cells ↓ Infiltration of inflammatory cells ↓ HMGB1 ↓ RAGE ↓ p-NFκB ↓ p-PI3K ↓ p-ERK1/2 ↓ COX2 ↓ TNFα ↓ IL-1β ↓ IL-2Rα ↓ IFNγ ↓ IL-4 |
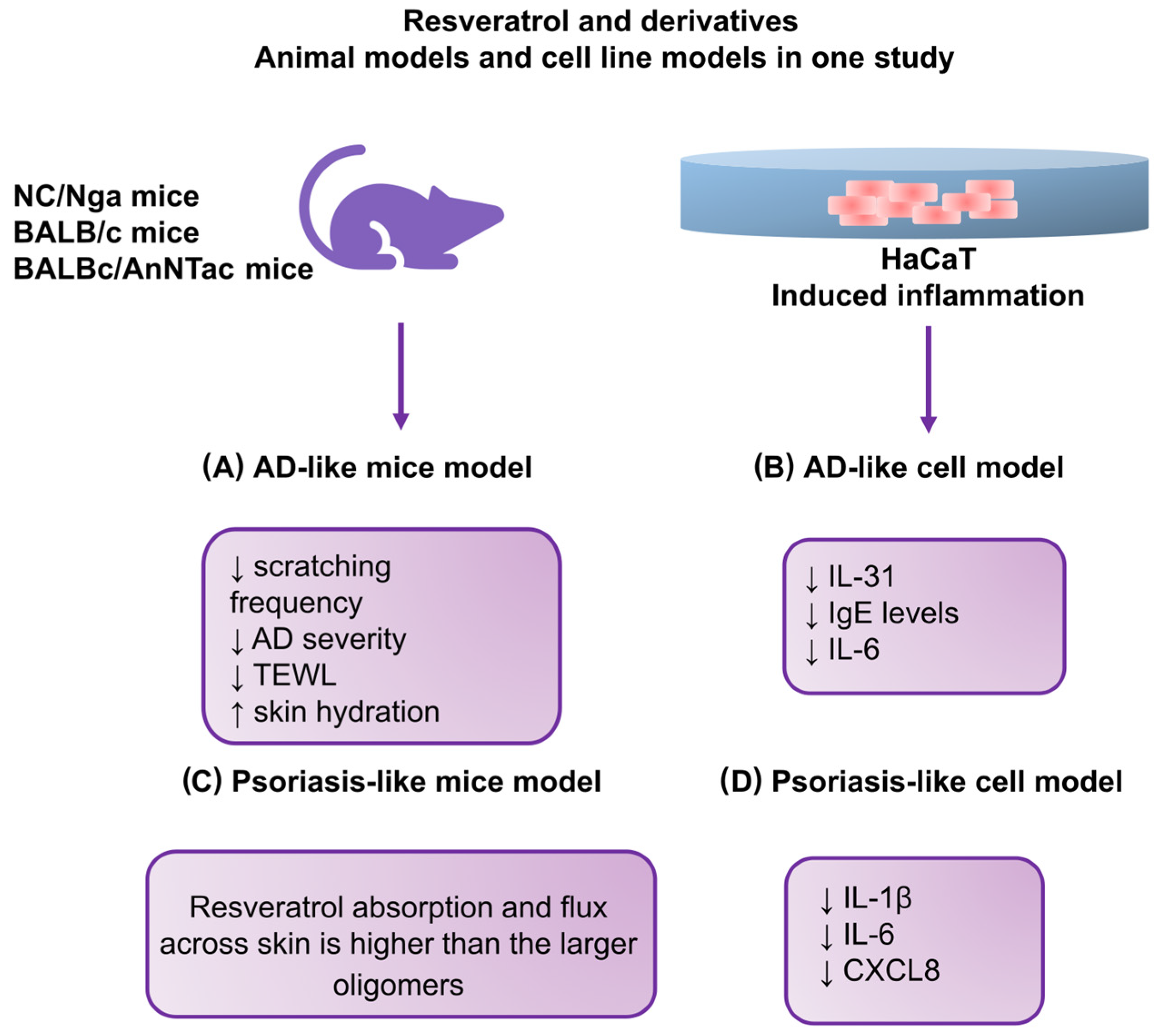
| Kang et al. [117] | 2019 | NC/Nga mice; AD-like model | 200 μL of 0.4% DNCB solution | RR; 2.5%, 5 mg/200 μL; and DNCB + resveratrol 2.5%, 5 mg/200 μL | ↓ Scratching frequency ↓ Dermatitis severity ↓ TEWL ↑ Skin hydration |
| Kang et al. [117] | 2019 | HaCaT and a 3D skin model; AD-like model | TNF-α and IFN-γ (each 10 ng/mL) | RR 10 ng/mL | ↓ IL-31 and IgE levels ↓ IL-6 |
| Sozmen et al. [111] | 2016 | BALB/c mice; AD-like model | 100 μL of 0.5% DNFB | Resveratrol (30 mg/kg/day), administered repeatedly during the 6th week | ↑ Epithelial thickness ↓ IL-25-, IL-33-, and TSLP-positive cells in the epithelium ↓ Caspase-3-positive cells in the epithelium |
| Bangash et al. [112] | 2023 | AD-like mouse model | 200 μL of 1% DNCB dissolved in acetone–olive oil mixture (3:1) on the dorsal skin; 10 μL of 1% DNCB on the right ear; on the seventh day, the animals were resubjected to 0.5% DNCB on the dorsal skin (200 μL) and right ear (10 μL) | Daily topical pterostilbene at 0.2, 0.6, and 1% for 28 days | ↓ IgE ↓ Epidermal thickness ↓ Oxidative stress markers in the skin ↓ IL-4 ↓ IL-6 ↓ TNF-α ↓ NF-κB |
| Kwack et al. [86] | 2022 | BALBc/AnNTac mice; AD-like model | 100 μL of 2% DNCB | DNCB+PM10 +5 μM dieckol; DNCB+PM10 +5 μM punicalagin; DNCB + PM10 + 1 μM EGCG; DNCB + PM10 + 1 μM resveratrol; DNCB + PM10 + 10 μg/mL SHE | ↓ IgE ↓ Spleen weight ↓ Epidermal thickness ↓ Mast cell counts ↓ IL-1β, IL-4, IL-6, IL-17α, IL-25, IL-31 ↓ TSLP Antioxidants prevented the downregulation of keratinocyte differentiation markers, including loricrin and fillagrin |
| Kjaer et al. [116] | 2015 | BALBc/AnNTac mice; Psoriasis-like model | Daily dose of 62.5 mg of 5% IMQ cream | 400 mg/kg animal/day of resveratrol based on average food intake | ↓ mRNA levels of IL-17A and IL-19 |
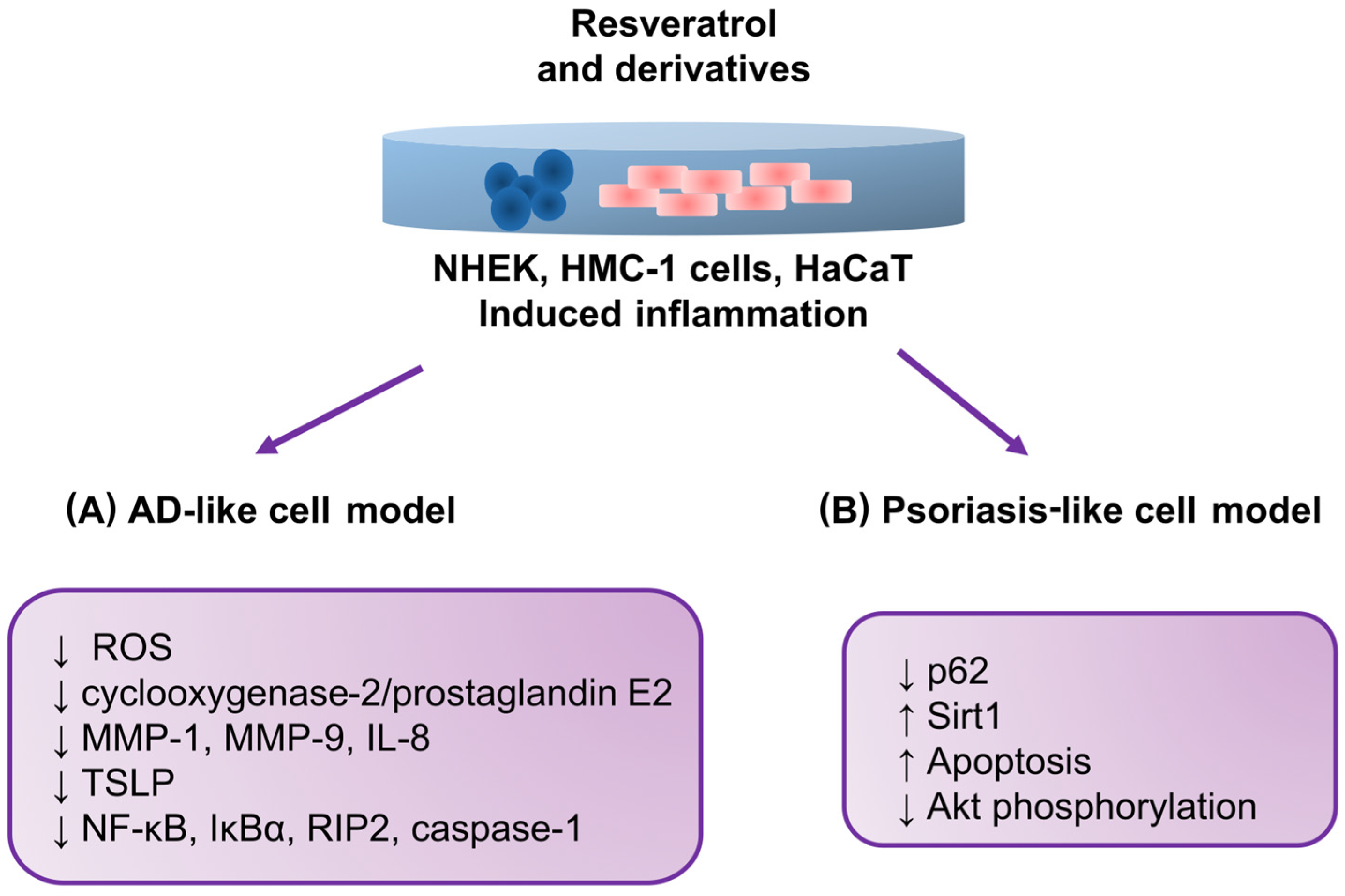
| Shin et al. [120] | 2020 | NHEKs; Skin inflammation model | PM 0, 1.25 μg/mL, 3 μg/mL, 6 μg/mL, 12 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL | Resveratrol 0, 0.01 μg/mL, 0.1 μg/mL, 1 μg/mL, 10 μg/mL, 50 μg/mL, 100 μM | ↓ PM-induced aryl hydrocarbon receptor activation ↓ ROS formation in keratinocytes ↓ Subsequent cellular inflammatory response by inhibiting mitogen-activated protein kinase activation ↓ PM-induced cyclooxygenase-2/prostaglandin E2 and proinflammatory cytokine expression, including that of matrix metalloproteinase (MMP)-1, MMP-9, and interleukin-8 |
| Moon et al. [121] | 2020 | HMC-1 cells; AD-like model | PMACI (0.128 ± 0.008 ng/mL) | Resveratrol (0.03, 0.3, and 3 μM) | ↓ TSLP production and mRNA expression ↓ NF-κB activation ↓ IκBα phosphorylation ↓ Activation of receptor-interacting protein 2 and caspase-1 ↓ Upregulation of intracellular calcium |
| Lee et al. [122] | 2016 | HaCaT psoriasis-like model | Basal expression | Resveratrol (5, 10, 20, and 50 μM) | ↑ apoptosis ↑ Sirt1 expression ↓ the p62 level ↓ Sirt1 Resveratrol-induced Sirt1 blocked Akt phosphorylation. ↓ Akt pathways by inducing Sirt1, thus leading to cell death |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marko, M.; Pawliczak, R. Resveratrol and Its Derivatives in Inflammatory Skin Disorders—Atopic Dermatitis and Psoriasis: A Review. Antioxidants 2023, 12, 1954. https://doi.org/10.3390/antiox12111954
Marko M, Pawliczak R. Resveratrol and Its Derivatives in Inflammatory Skin Disorders—Atopic Dermatitis and Psoriasis: A Review. Antioxidants. 2023; 12(11):1954. https://doi.org/10.3390/antiox12111954
Chicago/Turabian StyleMarko, Monika, and Rafał Pawliczak. 2023. "Resveratrol and Its Derivatives in Inflammatory Skin Disorders—Atopic Dermatitis and Psoriasis: A Review" Antioxidants 12, no. 11: 1954. https://doi.org/10.3390/antiox12111954
APA StyleMarko, M., & Pawliczak, R. (2023). Resveratrol and Its Derivatives in Inflammatory Skin Disorders—Atopic Dermatitis and Psoriasis: A Review. Antioxidants, 12(11), 1954. https://doi.org/10.3390/antiox12111954








