Geranylgeranylacetone Ameliorates Skin Inflammation by Regulating and Inducing Thioredoxin via the Thioredoxin Redox System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. ICD Model
2.3. Animal Treatment
2.4. Histologic Analysis of ICD
2.5. Immunohistochemistry
2.6. Cell Culture and Cell Processing
2.7. RNA Interference of Nrf2
2.8. Protein Extraction and Western Blot Analysis
2.9. Flow Cytometry Assays for ROS Production
2.10. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
2.11. Statistical Analyses
3. Results
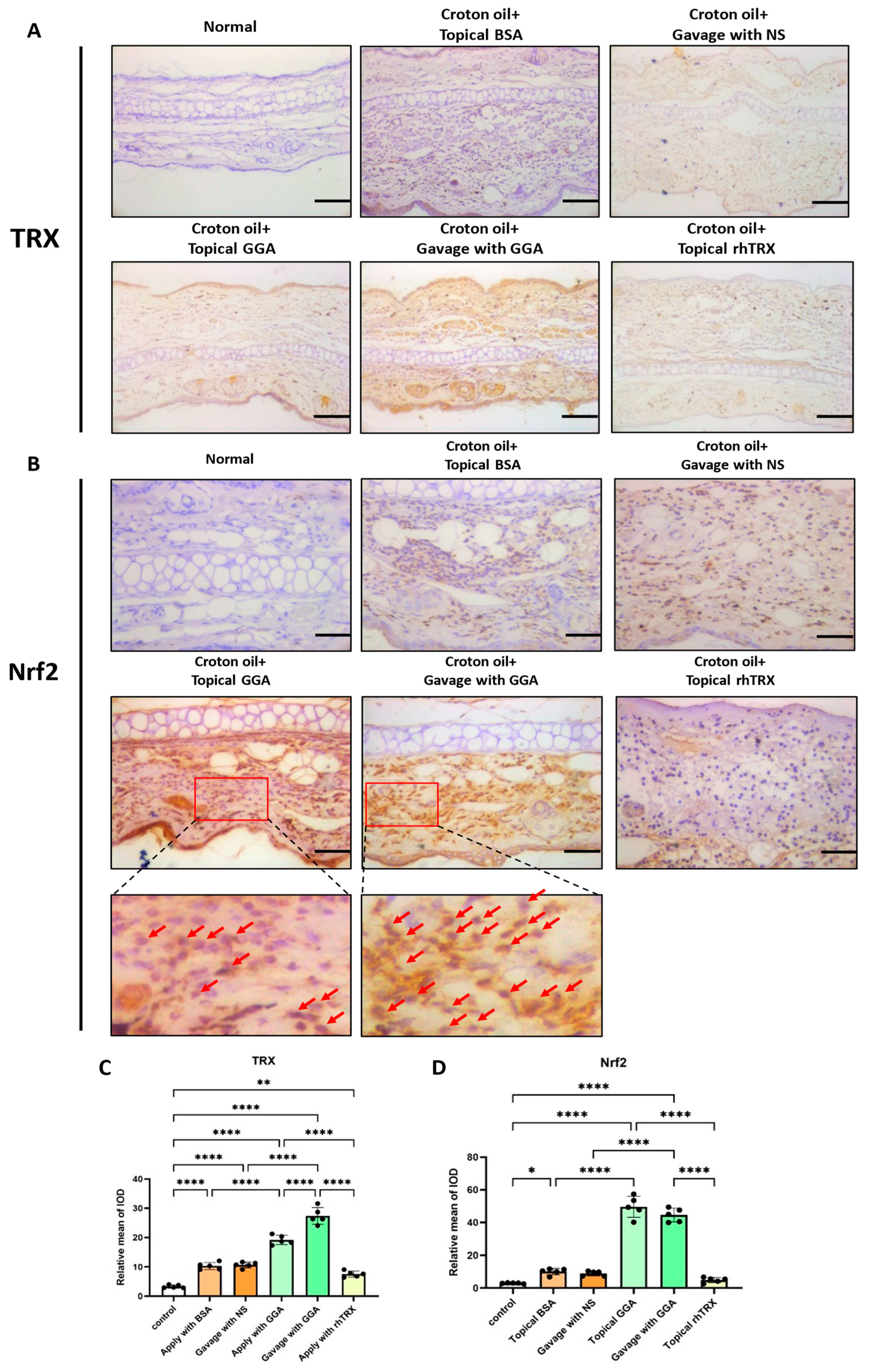
3.1. Induction of TRX and Nrf2 Expression by GGA in ICD Induced by Croton Oil
3.2. GGA Suppressed ICD Induced by Croton Oil
3.3. GGA Inhibited TNF-α, IL-1β, GM-CSF, and 8-OHdG Expression in ICD Induced by Croton Oil
3.4. GGA Inhibited PMA-Induced TNF-α, IL-1β, GM-CSF, and NLRP3 Expression in Murine Keratinocytes
3.5. GGA Scavenged ROS Production in PAM212 Cells Stimulated by PMA
3.6. Induction of TRX by GGA in PAM212 Cells Was Associated with ROS/TXNIP and PI3K/Akt/Nrf2 Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmgren, A. Thioredoxin. Annu. Rev. Biochem. 1985, 54, 237–271. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Matsuo, Y.; Fukunaga, A.; Ono, R.; Nishigori, C.; Yodoi, J. Thioredoxin ameliorates cutaneous inflammation by regulating the epithelial production and release of pro-inflammatory cytokines. Front. Immunol. 2013, 4, 269. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Hu, G.; Shi, D.; Gan, L.; Zhang, H.; Yao, X.; Fang, J. Fluorophore-Dependent Cleavage of Disulfide Bond Leading to a Highly Selective Fluorescent Probe of Thioredoxin. Anal. Chem. 2019, 91, 8524–8531. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Tamura, S.; Watanabe, I.; Iwasaki, T.; Yodoi, J. Enhanced resistancy of thioredoxin-transgenic mice against influenza virus-induced pneumonia. Immunol. Lett. 2002, 82, 165–170. [Google Scholar] [CrossRef]
- Kawasaki, K.; Nishio, A.; Nakamura, H.; Uchida, K.; Fukui, T.; Ohana, M.; Yoshizawa, H.; Ohashi, S.; Tamaki, H.; Matsuura, M.; et al. Helicobacter felis-induced gastritis was suppressed in mice overexpressing thioredoxin-1. Lab. Investig. 2005, 85, 1104–1117. [Google Scholar] [CrossRef]
- Lu, T.; Zong, M.; Fan, S.; Lu, Y.; Yu, S.; Fan, L. Thioredoxin 1 is associated with the proliferation and apoptosis of rheumatoid arthritis fibroblast-like synoviocytes. Clin. Rheumatol. 2018, 37, 117–125. [Google Scholar] [CrossRef]
- Sun, X.; Chen, L.; He, Z. PI3K/Akt-Nrf2 and Anti-Inflammation Effect of Macrolides in Chronic Obstructive Pulmonary Disease. Curr. Drug Metab. 2019, 20, 301–304. [Google Scholar] [CrossRef]
- Go, Y.-M.; Jones, D.P. Redox control systems in the nucleus: Mechanisms and functions. Antioxid. Redox Signal. 2010, 13, 489–509. [Google Scholar] [CrossRef]
- Harris, C.; Hansen, J.M. Nrf2-mediated resistance to oxidant-induced redox disruption in embryos. Birth Defects Res. B Dev. Reprod. Toxicol. 2012, 95, 213–218. [Google Scholar] [CrossRef]
- Yoshihara, E.; Masaki, S.; Matsuo, Y.; Chen, Z.; Tian, H.; Yodoi, J. Thioredoxin/Txnip: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014, 4, 514. [Google Scholar] [CrossRef]
- Shirakabe, H.; Takemoto, T.; Kobayashi, K.; Ogoshi, K.; Kimura, K.; Nakamura, K.; Watanabe, H. Clinical evaluation of teprenone, a mucosal protective agent, in the treatment of patients with gastric ulcers: A nationwide, multicenter clinical study. Clin. Ther. 1995, 17, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Chitapanarux, T.; Lertprasertsuke, N.; Kongnak, A. Teprenone for the prevention of low-dose aspirin-induced gastric mucosal injury in Helicobacter pylori-negative patients. Scand. J. Gastroenterol. 2019, 54, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Nakamura, H.; Arai, T.; Ishii, H.; Bai, J.; Itoh, T.; Fukuda, K.; Yodoi, J. Geranylgeranylacetone enhances expression of thioredoxin and suppresses ethanol-induced cytotoxicity in cultured hepatocytes. Biochem. Biophys. Res. Commun. 2000, 275, 825–830. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.Y.; Kang, S.W.; Chun, G.S.; Ban, J.Y. Protective effect of geranylgeranylacetone against hydrogen peroxide-induced oxidative stress in human neuroblastoma cells. Life Sci. 2015, 131, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kokura, S.; Yoshida, N.; Okuda, T.; Nakabe, N.; Sakamoto, N.; Isozaki, Y.; Hattori, T.; Handa, O.; Takagi, T.; Naito, Y.; et al. Hyperthermia ameliorates 2,4,6-trinitrobenzene sulphonic acid-induced colitis in rats: The role of heat shock proteins. Int. J. Hyperth. 2007, 23, 17–28. [Google Scholar] [CrossRef]
- Nakabe, N.; Kokura, S.; Shimozawa, M.; Katada, K.; Sakamoto, N.; Ishikawa, T.; Handa, O.; Takagi, T.; Naito, Y.; Yoshida, N.; et al. Hyperthermia attenuates TNF-alpha-induced up regulation of endothelial cell adhesion molecules in human arterial endothelial cells. Int. J. Hyperth. 2007, 23, 217–224. [Google Scholar] [CrossRef]
- Guo, S.; Zhen, Y.; Wang, A. Geranylgeranylacetone exerts neuroprotective roles through medicating the phosphatidylinositol-3 kinase/Akt signaling pathway in an intracerebral hemorrhage rat model. Int. J. Neurosci. 2018, 128, 893–898. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, K.; Fan, P.; Chen, C.; Hu, J.; Huang, J.; Lu, W.; Xu, J.; Xu, S.; Ran, J.; et al. Geranylgeranylacetone-induced heat shock protein70 expression reduces retinal ischemia-reperfusion injury through PI3K/AKT/mTOR signaling. Exp. Eye Res. 2023, 229, 109416. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Fujiki, M.; Uchida, S.; Morishige, M.; Momii, Y.; Ishii, K. A Single Oral Dose of Geranylgeranylacetone Upregulates Vascular Endothelial Growth Factor and Protects against Kainic Acid-Induced Neuronal Cell Death: Involvement of the Phosphatidylinositol-3 Kinase/Akt Pathway. Pathobiology 2017, 84, 184–191. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, G.; Zhang, H.; Zhu, L.; Cheng, G.; Wang, Y.; Li, Y.; Wu, W. Dexmedetomidine alleviates hepatic ischaemia-reperfusion injury via the PI3K/AKT/Nrf2-NLRP3 pathway. J. Cell. Mol. Med. 2021, 25, 9983–9994. [Google Scholar] [CrossRef]
- Pu, Z.; Shen, C.; Zhang, W.; Xie, H.; Wang, W. Avenanthramide C from Oats Protects Pyroptosis through Dependent ROS-Induced Mitochondrial Damage by PI3K Ubiquitination and Phosphorylation in Pediatric Pneumonia. J. Agric. Food Chem. 2022, 70, 2339–2353. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lv, H.; Li, H.; Ci, X.; Peng, L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun. Signal. 2019, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Törmä, H.; Geijer, S.; Gester, T.; Alpholm, K.; Berne, B.; Lindberg, M. Variations in the mRNA expression of inflammatory mediators, markers of differentiation and lipid-metabolizing enzymes caused by sodium lauryl sulphate in cultured human keratinocytes. Toxicol. Vitr. 2006, 20, 472–479. [Google Scholar] [CrossRef]
- Smith, H.R.; Basketter, D.A.; McFadden, J.P. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin. Exp. Dermatol. 2002, 27, 138–146. [Google Scholar] [CrossRef]
- Gittler, J.K.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: Implications for contact dermatitis. J. Allergy Clin. Immunol. 2013, 131, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Yodoi, J.; Tian, H.; Masutani, H.; Nakamura, H. Thiol redox barrier; local and systemic surveillance against stress and inflammatory diseases. Arch. Biochem. Biophys. 2016, 595, 88–93. [Google Scholar] [CrossRef]
- Huang, J.; Feng, X.; Zeng, J.; Zhang, S.; Zhang, J.; Guo, P.; Yu, H.; Sun, M.; Wu, J.; Li, M.; et al. Aberrant HO-1/NQO1-Reactive Oxygen Species-ERK Signaling Pathway Contributes to Aggravation of TPA-Induced Irritant Contact Dermatitis in Nrf2-Deficient Mice. J. Immunol. 2022, 208, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Dekigai, H.; Nakamura, H.; Bai, J.; Tanito, M.; Masutani, H.; Hirota, K.; Matsui, H.; Murakami, M.; Yodoi, J. Geranylgeranylacetone promotes induction and secretion of thioredoxin in gastric mucosal cells and peripheral blood lymphocytes. Free Radic. Res. 2001, 35, 23–30. [Google Scholar] [CrossRef]
- Tanito, M.; Kwon, Y.-W.; Kondo, N.; Bai, J.; Masutani, H.; Nakamura, H.; Fujii, J.; Ohira, A.; Yodoi, J. Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage. J. Neurosci. 2005, 25, 2396–2404. [Google Scholar] [CrossRef]
- Luo, F.-C.; Qi, L.; Lv, T.; Wang, S.-D.; Liu, H.; Nakamura, H.; Yodoi, J.; Bai, J. Geranylgeranylacetone protects mice against morphine-induced hyperlocomotion, rewarding effect, and withdrawal syndrome. Free Radic. Biol. Med. 2012, 52, 1218–1227. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, X.; Huang, M.; Li, X.; Li, Y.; Zhou, X.; Bai, J. Geranylgeranylacetone blocks the reinstatement of morphine-conditioned place preference. Neuropharmacology 2018, 143, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.-C.; Zhao, L.; Deng, J.; Liang, M.; Zeng, X.-S.; Liu, H.; Bai, J. Geranylgeranylacetone protects against morphine-induced hepatic and renal damage in mice. Mol. Med. Rep. 2013, 7, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Li, Y.; Jia, J.; Shi, Z.; Bai, J. Protective effect of geranylgeranylacetone against methamphetamine-induced neurotoxicity in rat pheochromocytoma cells. Pharmacology 2013, 92, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, M.; Li, J.; Li, C.; Xu, X.; Gu, W. Geranylgeranylacetone ameliorates lung ischemia/reperfusion injury by HSP70 and thioredoxin redox system: NF-kB pathway involved. Pulm. Pharmacol. Ther. 2015, 32, 109–115. [Google Scholar] [CrossRef]
- Nakai, K.; Yoneda, K.; Kubota, Y. Oxidative stress in allergic and irritant dermatitis: From basic research to clinical management. Recent. Pat. Inflamm. Allergy Drug Discov. 2012, 6, 202–209. [Google Scholar] [CrossRef]
- Sun, Q.; Kim, O.-S.; He, Y.; Lim, W.; Ma, G.; Kim, B.; Kim, Y.; Kim, O. Role of E2F1/SPHK1 and HSP27 During Irradiation in a PMA-Induced Inflammatory Model. Photobiomodul. Photomed. Laser Surg. 2020, 38, 512–520. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef]
- Xu, C.; Li, L.; Wang, C.; Jiang, J.; Li, L.; Zhu, L.; Jin, S.; Jin, Z.; Lee, J.J.; Li, G.; et al. Effects of G-Rh2 on mast cell-mediated anaphylaxis via AKT-Nrf2/NF-κB and MAPK-Nrf2/NF-κB pathways. J. Ginseng Res. 2022, 46, 550–560. [Google Scholar] [CrossRef]
- Fu, C.; Wu, Y.; Liu, S.; Luo, C.; Lu, Y.; Liu, M.; Wang, L.; Zhang, Y.; Liu, X. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 2022, 289, 115021. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Yamaguchi, Y.; Kondo, N.; Masutani, H.; Yodoi, J. Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene 2003, 22, 1860–1865. [Google Scholar] [CrossRef]
- Chorley, B.N.; Campbell, M.R.; Wang, X.; Karaca, M.; Sambandan, D.; Bangura, F.; Xue, P.; Pi, J.; Kleeberger, S.R.; Bell, D.A. Identification of novel NRF2-regulated genes by ChIP-Seq: Influence on retinoid X receptor alpha. Nucleic Acids Res. 2012, 40, 7416–7429. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Masutani, H.; Kim, Y.-C.; Nishikawa, M.; Ohira, A.; Yodoi, J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Kishimoto, C.; Nakamura, H.; Makita, T.; Ishii, K.; Okuda, N.; Yodoi, J.; Sasayama, S. Serum thioredoxin and alpha-tocopherol concentrations in patients with major risk factors. Circ. J. 2005, 69, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef]
- Patwari, P.; Higgins, L.J.; Chutkow, W.A.; Yoshioka, J.; Lee, R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006, 281, 21884–21891. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Dalmas, É.; Sauter, N.S.; Böni-Schnetzler, M. Inflammation in obesity and diabetes: Islet dysfunction and therapeutic opportunity. Cell Metab. 2013, 17, 860–872. [Google Scholar] [CrossRef]
- Waldhart, A.N.; Dykstra, H.; Peck, A.S.; Boguslawski, E.A.; Madaj, Z.B.; Wen, J.; Veldkamp, K.; Hollowell, M.; Zheng, B.; Cantley, L.C.; et al. Phosphorylation of TXNIP by AKT Mediates Acute Influx of Glucose in Response to Insulin. Cell Rep. 2017, 19, 2005–2013. [Google Scholar] [CrossRef]
- Huy, H.; Song, H.Y.; Kim, M.J.; Kim, W.S.; Kim, D.O.; Byun, J.-E.; Lee, J.; Park, Y.-J.; Kim, T.-D.; Yoon, S.R.; et al. TXNIP regulates AKT-mediated cellular senescence by direct interaction under glucose-mediated metabolic stress. Aging Cell 2018, 17, e12836. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Yuan, Q.; Zhu, L.; Li, F.; Wang, H.; Kong, D.; Hao, J. TXNIP, a novel key factor to cause Schwann cell dysfunction in diabetic peripheral neuropathy, under the regulation of PI3K/Akt pathway inhibition-induced DNMT1 and DNMT3a overexpression. Cell Death Dis. 2021, 12, 642. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, L.; Zhu, T.; Jiao, K. LncRNA FOXD3-AS1 aggravates myocardial ischemia/reperfusion injury by inactivating the Redd1/AKT/GSK3β/Nrf2 signaling pathway via the miR-128/TXNIP axis. J. Biochem. Mol. Toxicol. 2022, 36, e23218. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, R.; Zhang, S.; Deng, J.; Jia, J.J.; Sun, B.; Zhou, X.; Bai, J. Thioredoxin-1 regulates calcium homeostasis in MPP+/MPTP-induced Parkinson’s disease models. Eur. J. Neurosci. 2021, 54, 4827–4837. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Z.; Sukumar, P.; Zeng, F.; Li, J.; Jairaman, A.; English, A.; Naylor, J.; Ciurtin, C.; Majeed, Y.; Milligan, C.J.; et al. TRPC channel activation by extracellular thioredoxin. Nature 2008, 451, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S.; Matsura, T.; Yamashita, A.; Horie, S.; Ohata, S.; Kusumoto, C.; Nishida, T.; Minami, Y.; Inagaki, Y.; Ishibe, Y.; et al. Geranylgeranylacetone ameliorates inflammatory response to lipopolysaccharide (LPS) in murine macrophages: Inhibition of LPS binding to the cell surface. J. Clin. Biochem. Nutr. 2007, 41, 115–123. [Google Scholar] [CrossRef] [PubMed]








| Name | Sequence | |
|---|---|---|
| Forward | Reverse | |
| siNRF2(1) | 5′-GAGGAUGGAAGCCUUACUTT-3′ | 5′-AGUAAGGCUUUCCAUCCUCTC-3′ |
| siNRF2(2) | 5′-GGAGGCAAGACAUAGAUCUTT-3′ | 5′-AGAUCUAUGUCUUGCCUCCTT-3′ |
| siNRF2(3) | 5′-CCGAAUUACAGUGUCUUAATT-3′ | 5′-UUAAGACACUGUAAUUCGGTT-3′ |
| Nrf2 | 5′-CAGCATGACTGATTTAAGCAG-3′ | 5′-CAGCTGCTTTTTCGTGTGTGTATTA-3′ |
| TRX1 | 5′-TTCCCTCTGTGACAAGTTCC-3′ | 5′-TCAAGCTTTCTCTTGTTAGCAC-3′ |
| TXNIP | 5′-GTCTTGAGGTGGTCTTCAAC-3′ | 5′-TCACACACTTCCACTATTACCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, T.; You, Y.; Fan, W.; Wang, J.; Chen, Y.; Li, S.; Hong, S.; Wang, Y.; Cao, R.; Yodoi, J.; et al. Geranylgeranylacetone Ameliorates Skin Inflammation by Regulating and Inducing Thioredoxin via the Thioredoxin Redox System. Antioxidants 2023, 12, 1701. https://doi.org/10.3390/antiox12091701
Jin T, You Y, Fan W, Wang J, Chen Y, Li S, Hong S, Wang Y, Cao R, Yodoi J, et al. Geranylgeranylacetone Ameliorates Skin Inflammation by Regulating and Inducing Thioredoxin via the Thioredoxin Redox System. Antioxidants. 2023; 12(9):1701. https://doi.org/10.3390/antiox12091701
Chicago/Turabian StyleJin, Tiancheng, Yitong You, Wenjie Fan, Junyang Wang, Yuhao Chen, Shujing Li, Siyuan Hong, Yaxuan Wang, Ruijie Cao, Junji Yodoi, and et al. 2023. "Geranylgeranylacetone Ameliorates Skin Inflammation by Regulating and Inducing Thioredoxin via the Thioredoxin Redox System" Antioxidants 12, no. 9: 1701. https://doi.org/10.3390/antiox12091701
APA StyleJin, T., You, Y., Fan, W., Wang, J., Chen, Y., Li, S., Hong, S., Wang, Y., Cao, R., Yodoi, J., & Tian, H. (2023). Geranylgeranylacetone Ameliorates Skin Inflammation by Regulating and Inducing Thioredoxin via the Thioredoxin Redox System. Antioxidants, 12(9), 1701. https://doi.org/10.3390/antiox12091701







