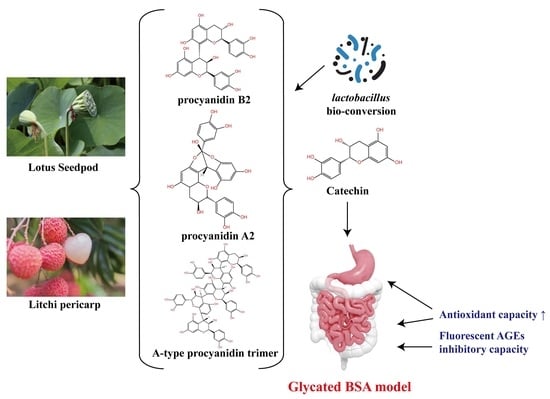
Phenolic Profile and Bioactivity Changes of Lotus Seedpod and Litchi Pericarp Procyanidins: Effect of Probiotic Bacteria Biotransformation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Extraction of LSPC and LPPC
2.3. Growth Experiments
2.4. Procyanidin Metabolite Analysis
2.4.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4.2. UPLC–HRMS Analysis
2.5. AGE Inhibition Capacity after GI Digestion In Vitro
2.6. Antioxidant Capacity of Digestive Metabolites
2.6.1. ABTS Assay
2.6.2. ORAC Assay
2.6.3. FRAP Assay
2.7. Statistical Analysis
3. Results
3.1. LAB Growth Analysis
3.2. Procyanidin Metabolite Analysis
3.2.1. Identification of LSPC and LPPC
3.2.2. Characterization of LSPC Metabolite Composition
3.2.3. Characterization of LPPC Metabolite Composition
3.3. Inhibition Effect of LSPC and LPPC Metabolites on AGEs Fluorescent Formation
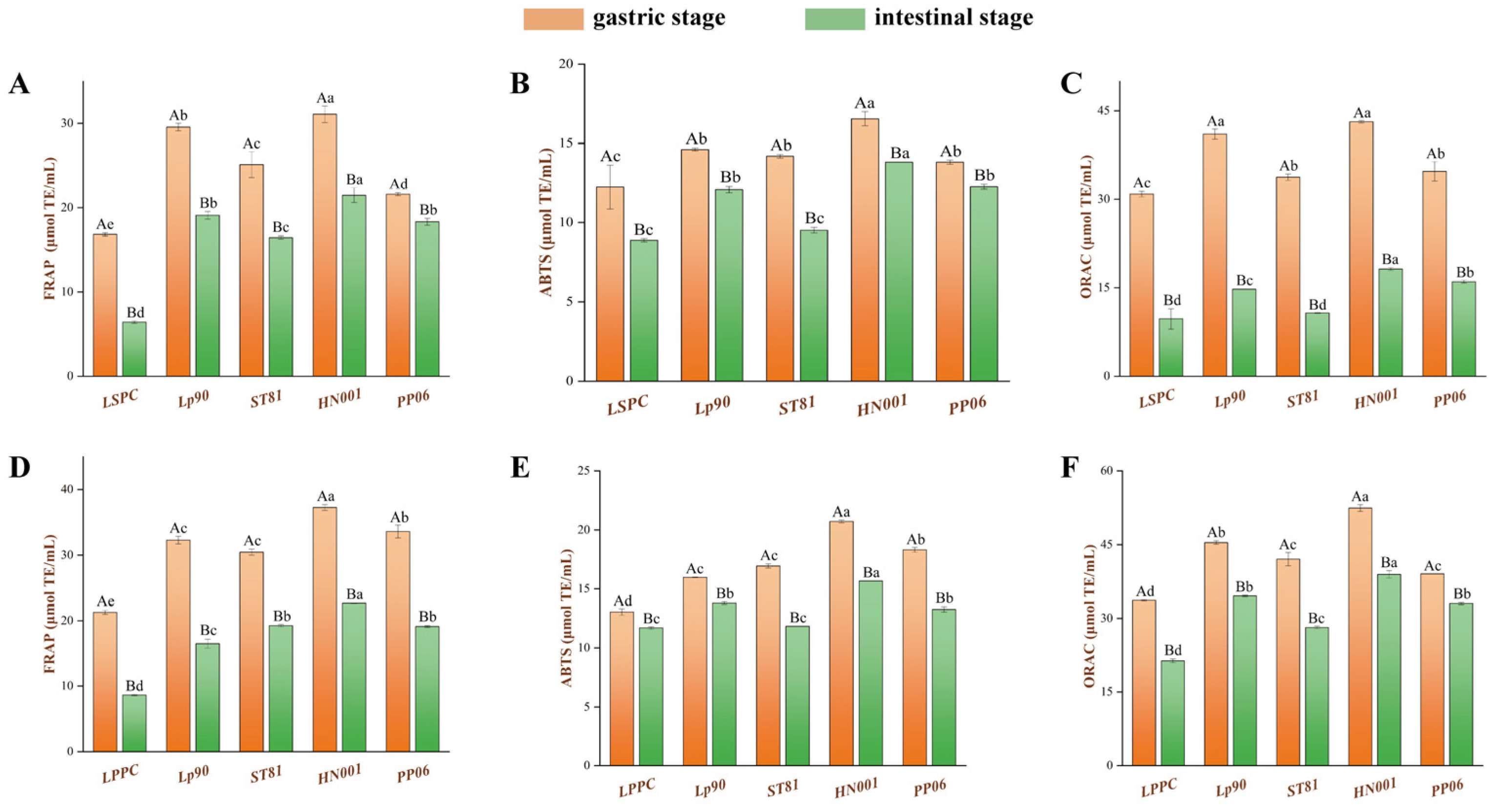
3.4. Antioxidant Capacity Effect of LSPC and LPPC Metabolites
3.4.1. Antioxidant Capacity Analysis
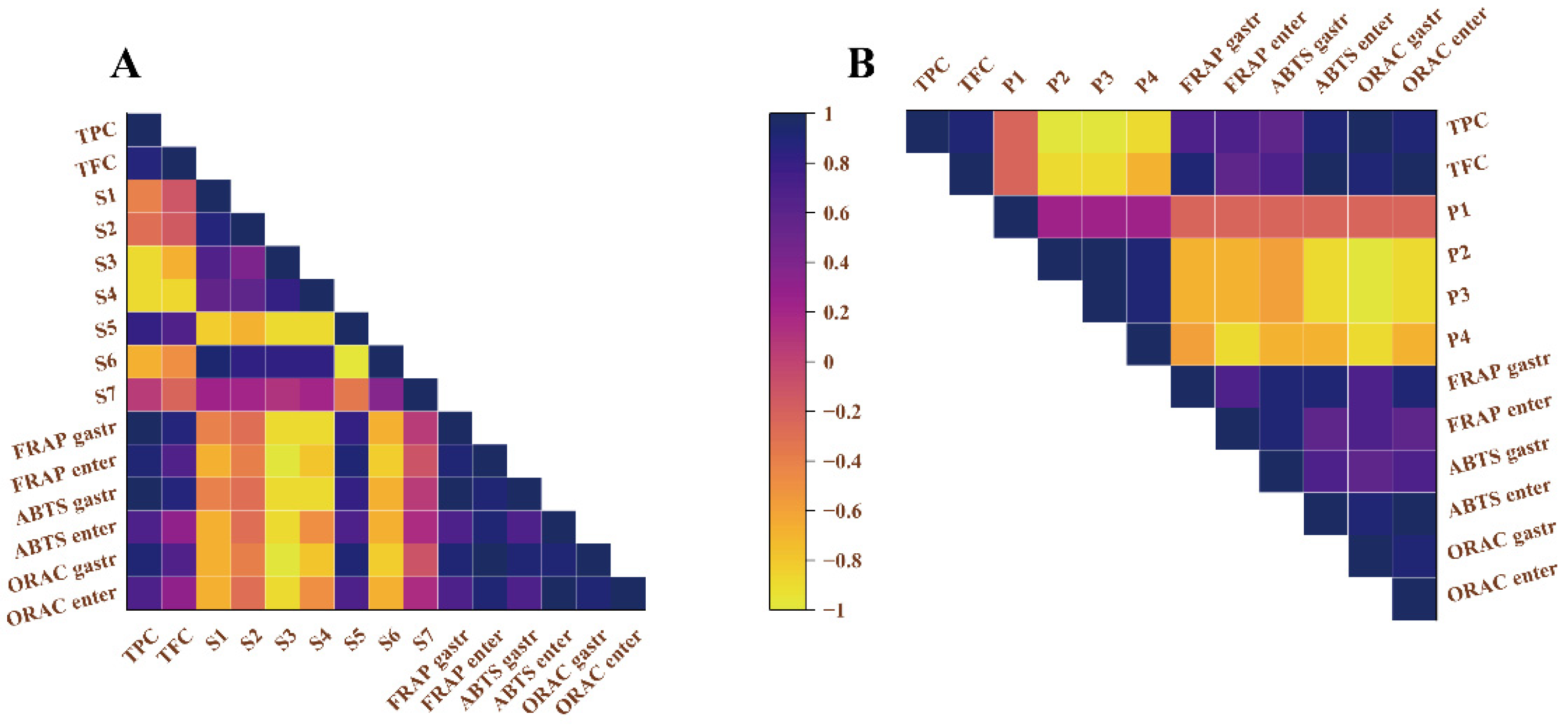
3.4.2. Correlation Analysis between Antioxidant Activity and Metabolite Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.F.; Shen, Z.C.; Li, J.; Liang, T.; Lin, X.F.; Li, Y.P.; Zeng, W.; Zou, Q.; Shen, J.L.; Wang, X.Y. Phytochemicals, biological activity, and industrial application of lotus seedpod (Receptaculum Nelumbinis): A review. Front. Nutr. 2022, 9, 1022794. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Hernández, A.M.; Muñiz-Márquez, D.B.; Wong-Paz, J.E.; Aguilar-Zárate, P.; de la Rosa-Hernández, M.; Larios-Cruz, R.; Aguilar, C.N. Characterization by HPLC–ESI–MS2 of native and oxidized procyanidins from litchi (Litchi chinensis) pericarp. Food Chem. 2019, 291, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Tang, F.; Hu, C.; Cheng, L.; Lv, Z.; Shen, Y.; Li, W.; Xiao, G.; Dong, H.; Wu, Q. Debate: Could the litchi pericarp oligomeric procyanidins bioconverted by Lactobacillus plantarum increase the inhibitory capacity on advanced glycation end products? Front. Nutr. 2022, 9, 961078. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, K.; Chen, Y.; Ouyang, Y.; Feng, Y.; Li, S.; Zhang, L.; Feng, N. Effect of lotus seedpod oligomeric procyanidins on AGEs formation in simulated gastrointestinal tract and cytotoxicity in Caco-2 cells. Food Funct. 2021, 12, 3527–3538. [Google Scholar] [CrossRef]
- Fruet, A.P.B.; Giotto, F.M.; Fonseca, M.A.; Nörnberg, J.L.; de Mello, A.S. Effects of the incorporation of tannin extract from quebracho colorado wood on color parameters, lipid oxidation, and sensory attributes of beef patties. Foods 2020, 9, 667. [Google Scholar] [CrossRef]
- Sui, Y.; Shi, J.; Cai, S.; Xiong, T.; Xie, B.; Sun, Z.; Mei, X. Metabolites of procyanidins from litchi chinensis pericarp with xanthine oxidase inhibitory effect and antioxidant activity. Front. Nutr. 2021, 8, 676346. [Google Scholar] [CrossRef]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The efficacy of administering fruit-derived polyphenols to improve health biomarkers, exercise performance and related physiological responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef]
- Xu, X.; Xie, H.; Wang, Y.; Wei, X. A-type proanthocyanidins from lychee seeds and their antioxidant and antiviral activities. J. Agric. Food Chem. 2010, 58, 11667–11672. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, H.; Lv, Z.; Li, S.; Hu, B.; Guan, Y.; Xie, B.; Sun, Z. Oligomeric procyanidins of lotus seedpod inhibits the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Chem. 2013, 138, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.Z.; Dong, X.Q.; Zhu, W.; Zhang, Y.; Li, C.M. Metabolites and changes in antioxidant activity of A-Type and B-Type Proanthocyanidin dimers after incubation with rat intestinal microbiota. J. Agric. Food Chem. 2015, 63, 8991–8998. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mirghafourvand, M.; Homayouni Rad, A.; Mohammad Alizadeh Charandabi, S.; Fardiazar, Z.; Shokri, K. The effect of probiotic yogurt on constipation in pregnant women: A randomized controlled clinical trial. Iran. Red Crescent Med. J. 2016, 18, e39870. [Google Scholar] [CrossRef] [PubMed]
- Yamanushi, M.; Shimura, M.; Nagai, H.; Hamada-Sato, N. Antihypertensive effects of abalone viscera fermented with Lactiplantibacillus pentosus SN001 via angiotensin-converting enzyme inhibition. Food Chem. X 2022, 13, 100239. [Google Scholar] [CrossRef] [PubMed]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Victoria Moreno-Arribas, M.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, B.; Sun, S.; Liu, Q.; Zhu, J.; Zhou, X.; Zhang, H.; Wu, Q.; Wang, L. Analysis of proanthocyanidins and flavonols in the seedpods of Chinese Antique Lotus: A rich source of antioxidants. Food Chem. 2023, 415, 135756. [Google Scholar] [CrossRef]
- Dos Santos, K.M.; de Oliveira, I.C.; Lopes, M.A.; Cruz, A.P.; Buriti, F.C.; Cabral, L.M. Addition of grape pomace extract to probiotic fermented goat milk: The effect on phenolic content, probiotic viability and sensory acceptability. J. Sci. Food Agric. 2017, 97, 1108–1115. [Google Scholar] [CrossRef]
- Ye, Z.; Liang, L.; Xu, Y.; Yang, J.; Li, Y. Probiotics Influence Gut Microbiota and Tumor Immune Microenvironment to Enhance Anti-Tumor Efficacy of Doxorubicin. Probiotics Antimicrob. 2023. [Google Scholar] [CrossRef]
- Ivanova, I.; Miteva, V.; Stefanova, T.; Pantev, A.; Budakov, I.; Danova, S.; Moncheva, P.; Nikolova, I.; Dousset, X.; Boyaval, P. Characterization of a bacteriocin produced by Streptococcus thermophilus 81. Int. J. Food Microbiol. 1998, 42, 147–158. [Google Scholar] [CrossRef]
- Li, M.; Zeng, M.; He, Z.; Zheng, Z.; Qin, F.; Tao, G.; Zhang, S.; Chen, J. Effects of long-term exposure to free Nε-(Carboxymethyl)lysine on rats fed a high-fat diet. J. Agric. Food Chem. 2015, 63, 10995–11001. [Google Scholar] [CrossRef]
- Wu, H.; Shu, L.; Liang, T.; Li, Y.; Liu, Y.; Zhong, X.; Xing, L.; Zeng, W.; Zhao, R.; Wang, X. Extraction optimization, physicochemical property, antioxidant activity, and α-glucosidase inhibitory effect of polysaccharides from lotus seedpods. J. Sci. Food Agric. 2022, 102, 4065–4078. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, T.; Yin, L.; Man, S.; Ye, S.; Ma, L. Polyphenol-rich extract from litchi chinensis seeds alleviates hypertension-induced renal damage in rats. J. Agric. Food Chem. 2021, 69, 2138–2148. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Trossolo, E.; Tonini, S.; Filannino, P.; Gobbetti, M.; Di Cagno, R. Fermented whey ewe’s milk-based fruit smoothies: Bio-recycling and enrichment of phenolic compounds and improvement of protein digestibility and antioxidant activity. Antioxidants 2023, 12, 1091. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Akther, S.; Jothi, J.S.; Badsha, M.R.; Rahman, M.M.; Das, G.B.; Alim, M.A. Drying methods effect on bioactive compounds, phenolic profile, and antioxidant capacity of mango powder. J. King Saud Univ. Sci. 2023, 35, 102370. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Le, T.T.; Larsen, L.B.; Su, G.; Liang, Y.; Li, B. Digestibility of glyoxal-glycated β-casein and β-lactoglobulin and distribution of peptide-bound advanced glycation end products in gastrointestinal digests. J. Agric. Food Chem. 2017, 65, 5778–5788. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.S.; Léonil, J.; Henry, G.; Cauty, C.; Carvalho, A.F.; Bouhallab, S. Heating and glycation of β-lactoglobulin and β-casein: Aggregation and in vitro digestion. Food Res. Int. 2014, 55, 70–76. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, Q.; Xiao, J.; Tang, S.; Chen, Y.; Shen, Y.; Xu, N.; Zhou, M.; Hu, Y.; Wang, C.; et al. Catechin-iron as a new inhibitor to control advanced glycation end-products formation during vinegar storage. LWT 2019, 112, 108245. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Datta, S.; Mallick, B.; Dhar, P.; Ghosh, S. Lutein content and in vitro antioxidant activity of different cultivars of Indian marigold flower (Tagetes patula L.) extracts. J. Agric. Food Chem. 2010, 58, 8259–8264. [Google Scholar] [CrossRef]
- Wang, M.; Lu, Y.; Wu, Q.; Chen, G.; Zhao, H.; Ho, C.T.; Li, S. Biotransformation and gut microbiota-mediated bioactivity of flavonols. J. Agric. Food Chem. 2023, 71, 8317–8331. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Gobbetti, M.; De Angelis, M.; Di Cagno, R. Hydroxycinnamic acids used as external acceptors of electrons: An energetic advantage for strictly heterofermentative lactic acid bacteria. Appl. Environ. Microbiol. 2014, 80, 7574–7582. [Google Scholar] [CrossRef]
- Laosee, W.; Kantachote, D.; Chansuwan, W.; Sirinupong, N. Effects of probiotic fermented fruit juice-based biotransformation by lactic acid bacteria and Saccharomyces boulardii CNCM I-745 on anti-salmonella and antioxidative properties. J. Microbiol. Biotechnol. 2022, 32, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pan, Q.H.; Shi, Y.; Duan, C.Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Gänzle, M.G. Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 2010, 58, 9214–9220. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Tabasco, R.; Monagas, M.; Requena, T.; Peláez, C.; Moreno-Arribas, M.V.; Bartolomé, B. Capability of Lactobacillus plantarum IFPL935 to catabolize flavan-3-ol compounds and complex phenolic extracts. J. Agric. Food Chem. 2012, 60, 7142–7151. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Paz de Peña, M.; Concepción, C.; Alan, C. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013, 39, 623–632. [Google Scholar] [CrossRef]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Zi, J.; Valiente, J.; Zeng, J.; Zhan, J. Metabolism of quercetin by Cunninghamella elegans ATCC 9245. J. Biosci. Bioeng. 2011, 112, 360–362. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Plaza-Vinuesa, L.; de las Rivas, B.; Villamiel, M.; Muñoz, R.; Moreno, F.J. Production of α-rhamnosidases from Lactobacillus plantarum WCFS1 and their role in deglycosylation of dietary flavonoids naringin and rutin. J. Biol. Macromol. 2021, 193, 1093–1102. [Google Scholar] [CrossRef]
- te Poele, E.M.; Grijpstra, P.; van Leeuwen, S.S.; Dijkhuizen, L. Glucosylation of catechol with the GTFA glucansucrase enzyme from Lactobacillus reuteri and sucrose as donor substrate. Bioconjug. Chem. 2016, 27, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Illiano, A.; Carpentieri, A.; Spinelli, M.; Melchiorre, C.; Fontanarosa, C.; di Serio, M.; Amoresano, A. Quantification of polyphenols and metals in Chinese tea infusions by mass spectrometry. Foods 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced antioxidant activity for apple juice fermented with Lactobacillus plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.R.; Wang, Z.X.; Li, Y.J.; Kan, H.; Kong, K.W.; Zhang, X.C. The influence of probiotic fermentation on the active compounds and bioactivities of walnut flowers. J. Food Biochem. 2022, 46, e13887. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; González-Manzano, S.; González-Paramás, A.; Santos-Buelga, C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharm. Biomed. Anal. 2010, 51, 443–449. [Google Scholar] [CrossRef]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef]
- Yoo, N.H.; Jang, D.S.; Yoo, J.L.; Lee, Y.M.; Kim, Y.S.; Cho, J.H.; Kim, J.S. Erigeroflavanone, a flavanone derivative from the flowers of Erigeron annuus with protein glycation and aldose reductase inhibitory activity. J. Nat. Prod. 2008, 71, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Kim, J.S. Constituents of the flowers of platycodon grandiflorum with inhibitory activity on advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharm. Res. 2010, 33, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; McIntyre, K.; Saleem, A.; Haddad, P.S.; Arnason, J.T. The relationship between antiglycation activity and procyanidin and phenolic content in commercial grape seed products. Can. J. Physiol. Pharmacol. 2012, 90, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Tea polyphenol (−)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef]
- Moscovici, A.M.; Joubran, Y.; Briard-Bion, V.; Mackie, A.; Dupont, D.; Lesmes, U. The impact of the Maillard reaction on the in vitro proteolytic breakdown of bovine lactoferrin in adults and infants. Food Funct. 2014, 5, 1898–1908. [Google Scholar] [CrossRef]
- de Morais, F.P.R.; Pessato, T.B.; Rodrigues, E.; Peixoto Mallmann, L.; Mariutti, L.R.B.; Netto, F.M. Whey protein and phenolic compound complexation: Effects on antioxidant capacity before and after in vitro digestion. Food Res. Int. 2020, 133, 109104. [Google Scholar] [CrossRef]
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V.; Tang, N.; Andersen, M.L.; Chatterton, D.E.W. Modulation of gastrointestinal digestion of β-lactoglobulin and micellar casein following binding by (−)-epigallocatechin-3-gallate (EGCG) and green tea flavanols. Food Funct. 2020, 11, 6038–6053. [Google Scholar] [CrossRef]
- Kaur, P.; Robin; Mehta, R.G.; Singh, B.; Arora, S. Development of aqueous-based multi-herbal combination using principal component analysis and its functional significance in HepG2 cells. BMC Complement. Altern. Med. 2019, 19, 18. [Google Scholar] [CrossRef]
- Lv, Q.; Luo, F.; Zhao, X.; Liu, Y.; Hu, G.; Sun, C.; Li, X.; Chen, K. Identification of proanthocyanidins from litchi (Litchi chinensis Sonn.) pulp by LC-ESI-Q-TOF-MS and their antioxidant activity. PLoS ONE 2015, 10, e0120480. [Google Scholar] [CrossRef]
- Lyu, Q.; Kuo, T.H.; Sun, C.; Chen, K.; Hsu, C.C.; Li, X. Comprehensive structural characterization of phenolics in litchi pulp using tandem mass spectral molecular networking. Food Chem. 2019, 282, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.S.; Xie, B.J.; Cao, Y.P.; Wu, H.; Sun, Z.D.; Xiao, D. Characterization of oligomeric procyanidins and identification of quercetin glucuronide from lotus (Nelumbo nucifera Gaertn.) Seedpod. J. Agric. Food Chem. 2012, 60, 2825–2829. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Tan, H.Y.; Chen, J.; Wang, M. Characterization of tyrosinase inhibitors in the twigs of Cudrania tricuspidata and their structure-activity relationship study. Fitoterapia 2013, 84, 242–247. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Sui, Y.; Li, S.; Shi, J.; Cai, S.; Xiong, T.; Cai, F.; Zhou, L.; Zhao, S.; Mei, X. Phenolic Profile and Bioactivity Changes of Lotus Seedpod and Litchi Pericarp Procyanidins: Effect of Probiotic Bacteria Biotransformation. Antioxidants 2023, 12, 1974. https://doi.org/10.3390/antiox12111974
Wen J, Sui Y, Li S, Shi J, Cai S, Xiong T, Cai F, Zhou L, Zhao S, Mei X. Phenolic Profile and Bioactivity Changes of Lotus Seedpod and Litchi Pericarp Procyanidins: Effect of Probiotic Bacteria Biotransformation. Antioxidants. 2023; 12(11):1974. https://doi.org/10.3390/antiox12111974
Chicago/Turabian StyleWen, Junren, Yong Sui, Shuyi Li, Jianbin Shi, Sha Cai, Tian Xiong, Fang Cai, Lei Zhou, Shengnan Zhao, and Xin Mei. 2023. "Phenolic Profile and Bioactivity Changes of Lotus Seedpod and Litchi Pericarp Procyanidins: Effect of Probiotic Bacteria Biotransformation" Antioxidants 12, no. 11: 1974. https://doi.org/10.3390/antiox12111974
APA StyleWen, J., Sui, Y., Li, S., Shi, J., Cai, S., Xiong, T., Cai, F., Zhou, L., Zhao, S., & Mei, X. (2023). Phenolic Profile and Bioactivity Changes of Lotus Seedpod and Litchi Pericarp Procyanidins: Effect of Probiotic Bacteria Biotransformation. Antioxidants, 12(11), 1974. https://doi.org/10.3390/antiox12111974