The Effects of Dietary Silybin Supplementation on the Growth Performance and Regulation of Intestinal Oxidative Injury and Microflora Dysbiosis in Weaned Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
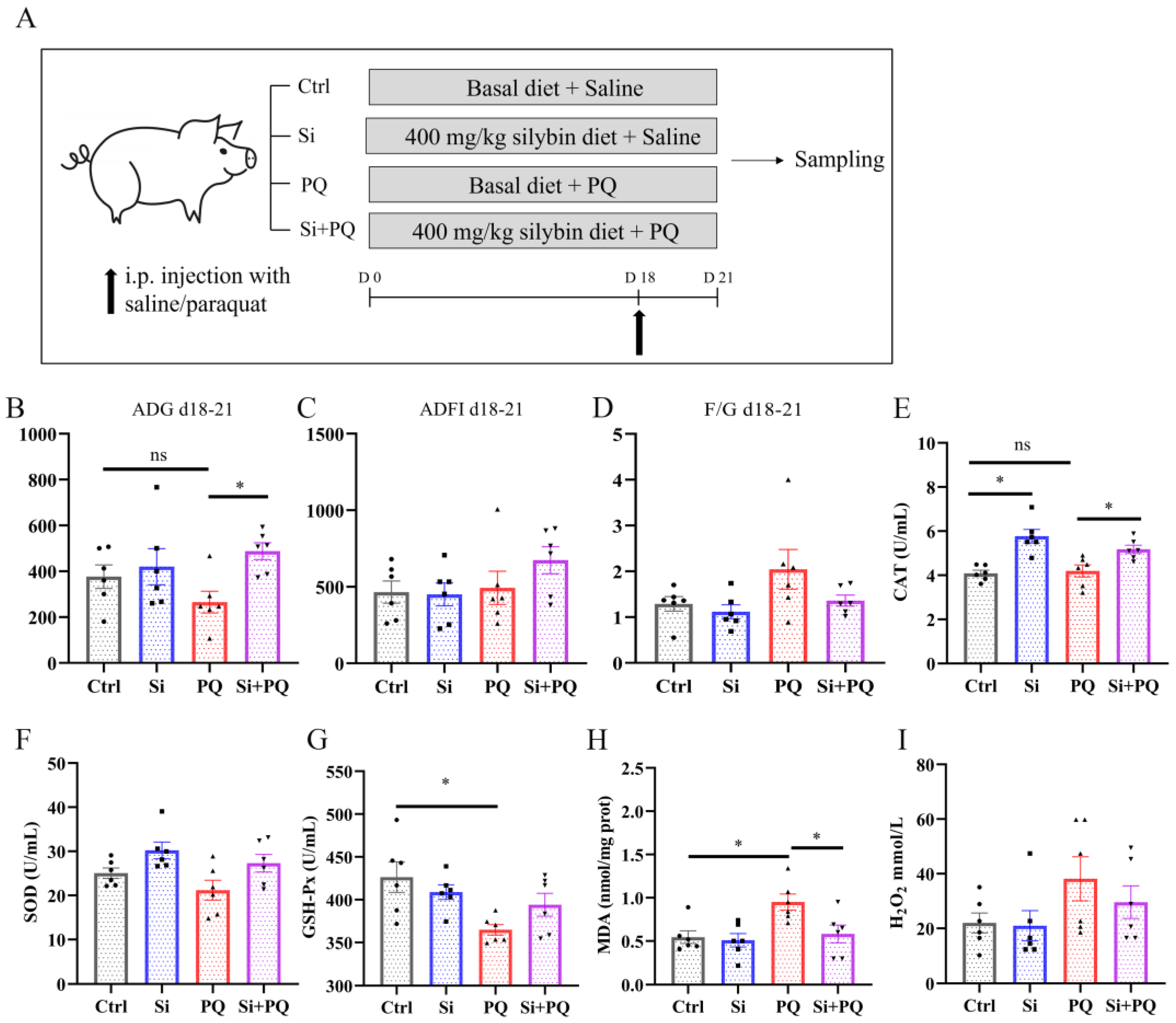
2.2. Animals and Experimental Design
2.3. Growth Performance and Diarrhea Incidence
2.4. Sample Collection
2.5. Assay of Antioxidant Indices in Plasma and Intestinal Mucosa
2.6. The Activities of Mitochondrial Complex and ATP Content Assay
2.7. Caspase 3 and Caspase 9 Activity
2.8. Detection of Biomarkers of Intestinal Barrier Dysfunction
2.9. Mucin 2 Content Assay
2.10. Histopathological Staining
2.11. Real-Time Quantitative PCR Analysis (qPCR)
2.12. Western Blotting Analysis
2.13. Gut Microbiome Analysis
2.14. Determinations of SCFAs in the Cecal Digesta
2.15. Statistical Analysis
3. Results
3.1. Effects of Dietary Silybin Supplementation on Growth Performance, Diarrhea Incidence, and Antioxidant Capacity in Weaned Piglets (Trial 1)
3.2. Dietary Silybin Supplementation Alleviated the Redox Imbalance and Eliminated the Growth Retardation Induced by Paraquat in Piglets (Trial 2)
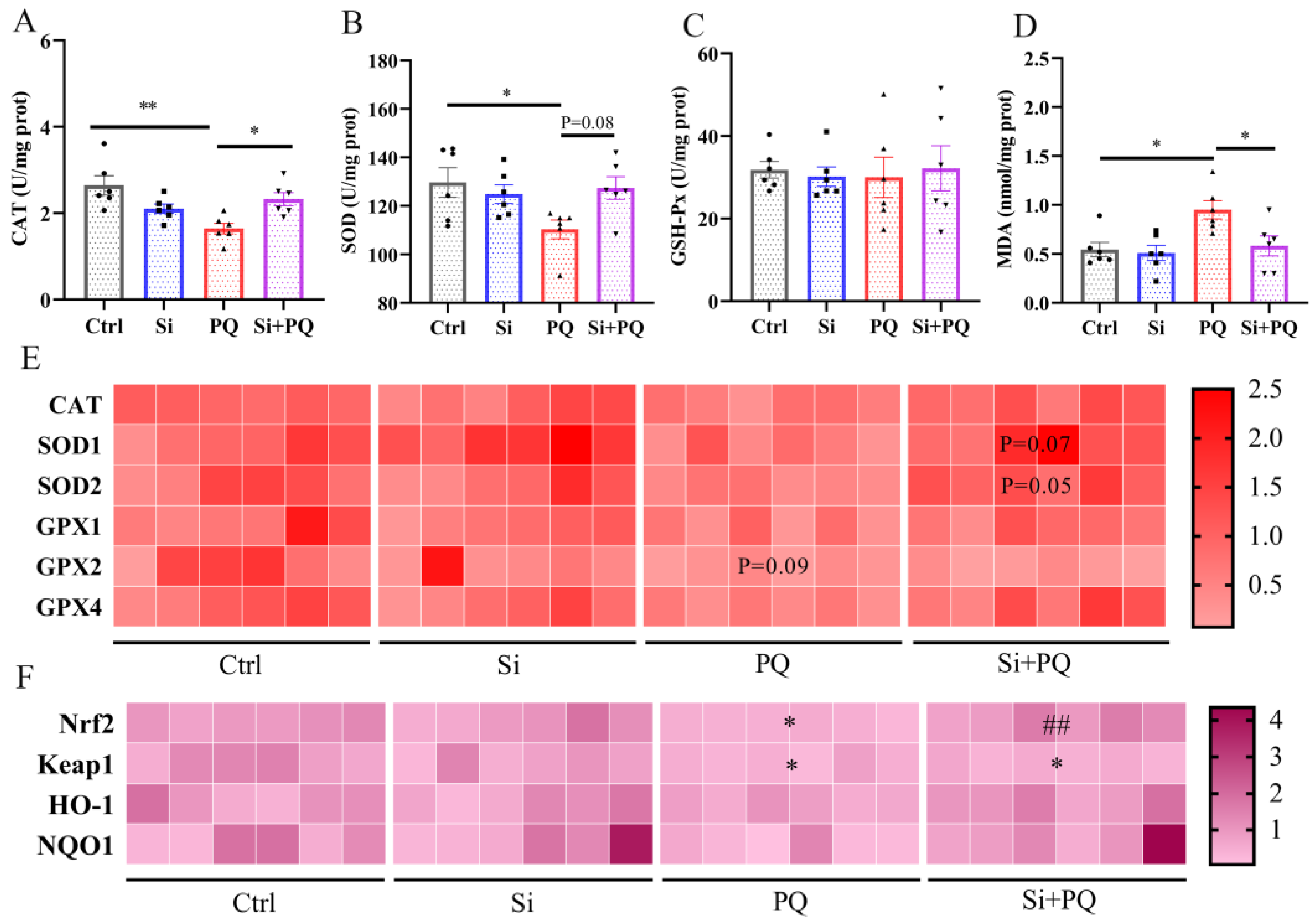
3.3. Dietary Silybin Administration Alleviated Paraquat-Induced Intestinal Redox Imbalance in Piglets (Trial 2)
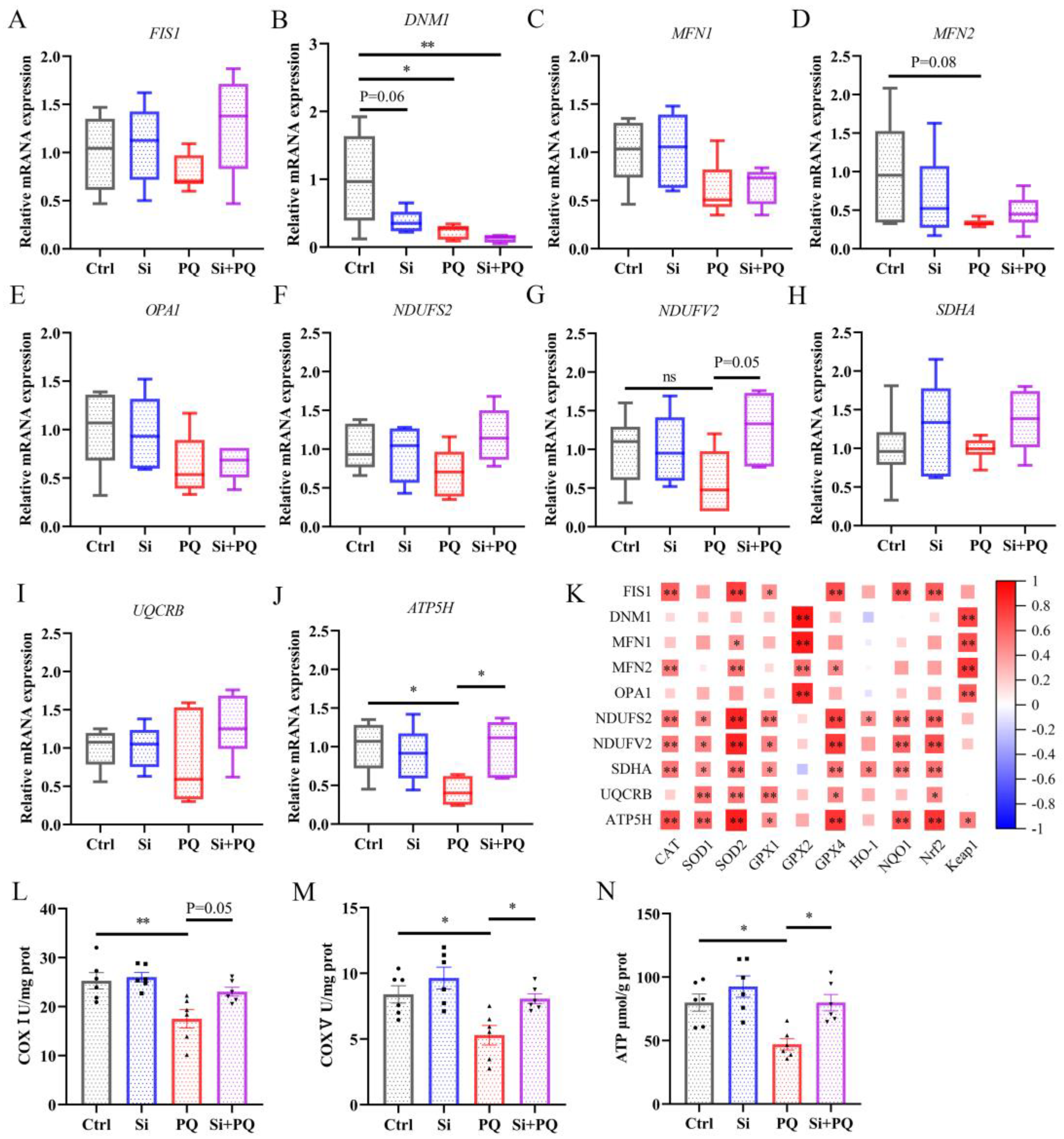
3.4. Dietary Silybin Supplementation Protected against PQ-Induced Mitochondrial Injury (Trial 2)
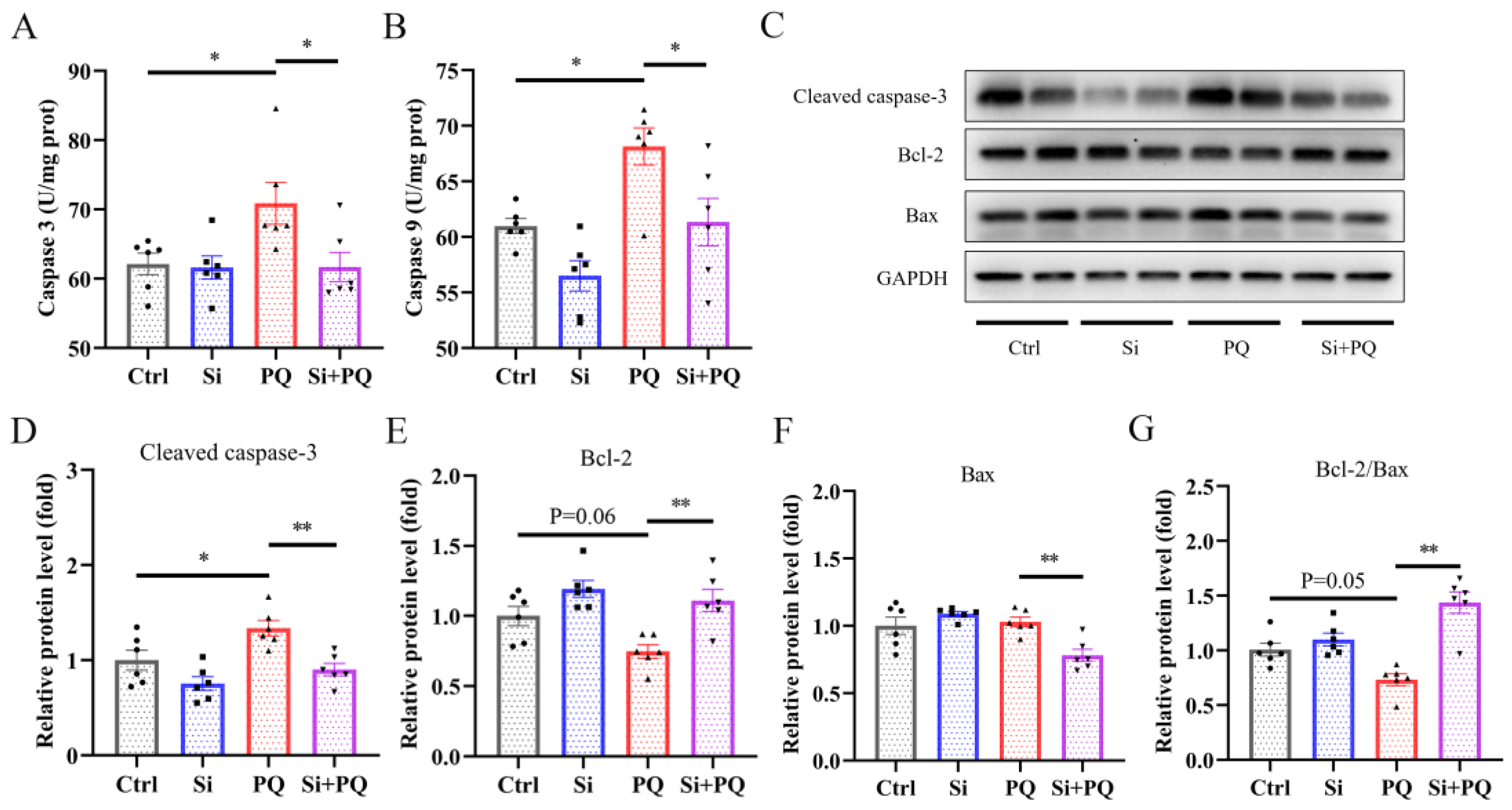
3.5. Dietary Silybin Addition Inhibited PQ-Induced Intestinal Apoptotic (Trial 2)
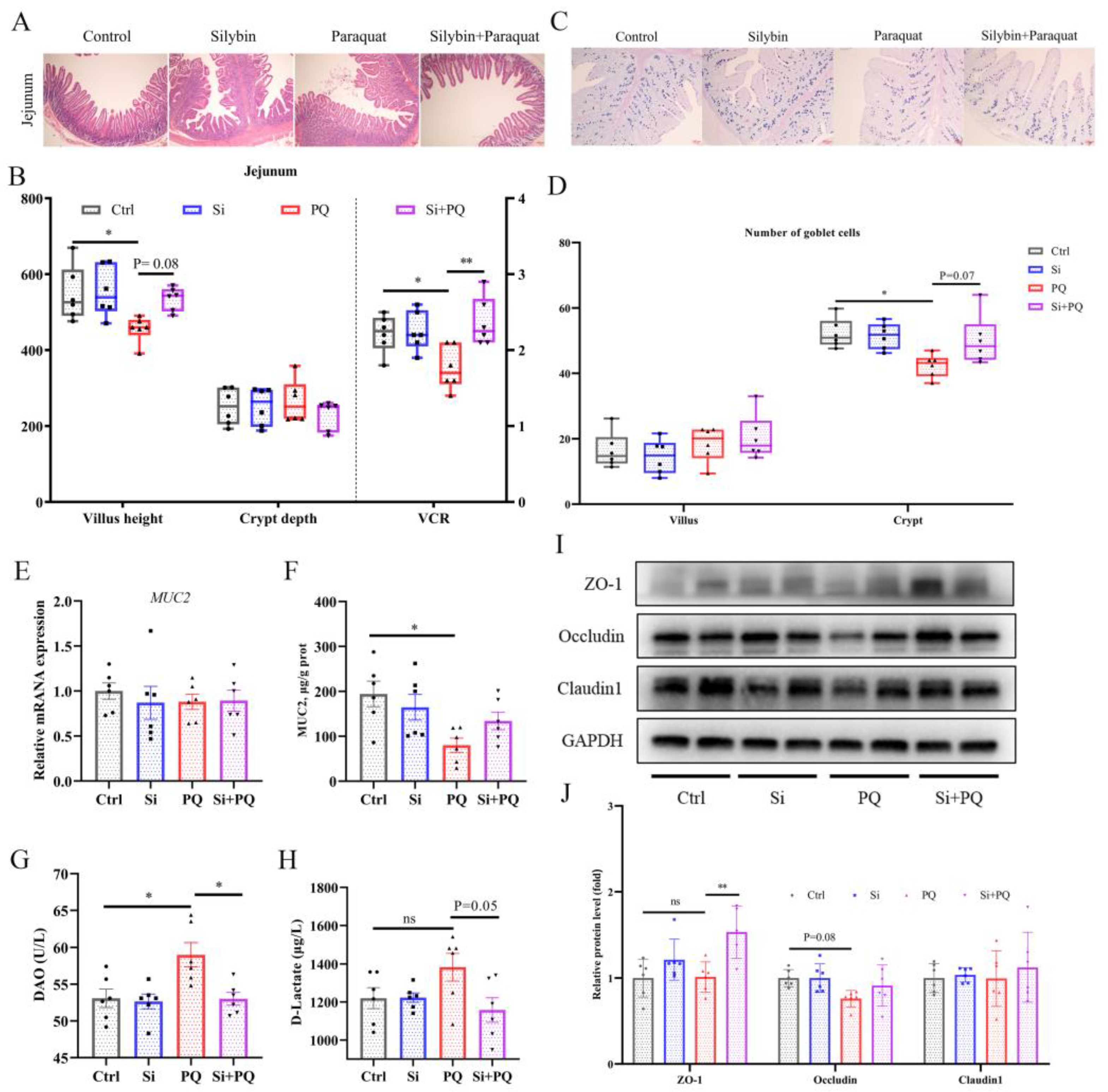
3.6. Dietary Silybin Addition Ameliorated PQ-Induced Intestinal Barrier Injury (Trial 2)
3.7. Dietary Silybin Addition Improved PQ-Induced Intestinal Microbiota Disorder (Trial 2)
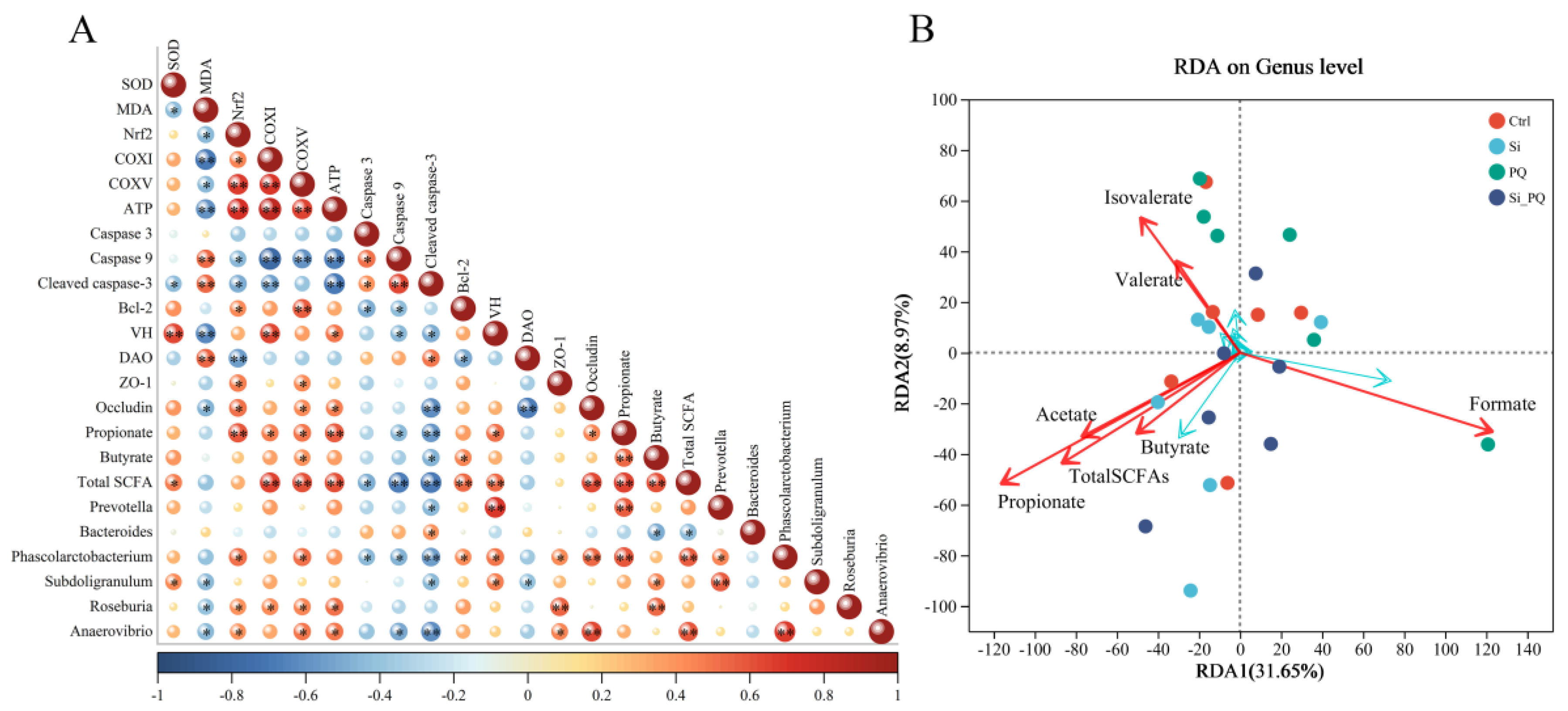
3.8. The Intestinal Microbiota and SCFAs Were Associated with Intestinal Homeostasis (Trial 2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yu, B.; Yu, J.; Zheng, P.; Huang, Z.; Luo, J.; Mao, X.; He, J.; Yan, H.; Wu, J.; et al. Lower abundance of Bacteroides and metabolic dysfunction are highly associated with the post-weaning diarrhea in piglets. Sci. China Life Sci. 2022, 65, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xu, B.; Chen, Y.; Yang, W.; Xu, Y.; Huang, J.; Duo, T.; Mao, Y.; Zhou, G.; Yan, X.; et al. Dietary ellagic acid supplementation attenuates intestinal damage and oxidative stress by regulating gut microbiota in weanling piglets. Anim. Nutr. 2022, 11, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, D.; Zhang, J.; Yuan, J. Metabolism, Transport and Drug–Drug Interactions of Silymarin. Molecules 2019, 24, 3693. [Google Scholar] [CrossRef]
- Fraschini, F.; Demartini, G.; Esposti, D. Pharmacology of Silymarin. Clin. Drug Investig. 2002, 22, 51–65. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Faryadi, S.; Sheikhahmadi, A.; Farhadi, A.; Nourbakhsh, H. Effects of silymarin and nano-silymarin on performance, egg quality, nutrient digestibility, and intestinal morphology of laying hens during storage. Ital. J. Anim. Sci. 2021, 20, 1633–1644. [Google Scholar] [CrossRef]
- Shanmugam, S.; Park, J.H.; Cho, S.; Kim, I.H. Silymarin seed extract supplementation enhances the growth performance, meat quality, and nutrients digestibility, and reduces gas emission in broilers. Anim. Biosci. 2022, 35, 1215–1222. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, S.; Lin, Y.; Fang, Z.; Xu, S.; Feng, B.; Zhuo, Y.; Li, J.; Che, L.; Jiang, X.; et al. Effects of silymarin supplementation during transition and lactation on reproductive performance, milk composition and haematological parameters in sows. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.H.; Zhang, Q.; Sampath, V.; Kim, I. PSIV-10 Effect of Micelle Silymarin Supplementation on the Growth Performance, Nutrient Digestibility, Fecal Microbiome, Gas Emissions, Blood Profile, Meat Quality, and Antioxidant Property in Finishing Pigs. J. Anim. Sci. 2022, 100, 150–151. [Google Scholar] [CrossRef]
- Jahanian, E.; Mahdavi, A.H.; Jahanian, R. Silymarin improved the growth performance via modulating the microbiota and mucosal immunity in Escherichia coli-challenged broiler chicks. Livest. Sci. 2021, 249, 104529. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, X.; Jia, X.; Jiang, X.; Che, L.; Lin, Y.; Zhuo, Y.; Feng, B.; Fang, Z.; Li, J.; et al. Silymarin Modulates Microbiota in the Gut to Improve the Health of Sow from Late Gestation to Lactation. Animals 2022, 12, 2202. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Liu, Z. Signaling pathways involved in paraquat-induced pulmonary toxicity: Molecular mechanisms and potential therapeutic drugs. Int. Immunopharmacol. 2022, 113, 109301. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, R.; Wang, N.; Deng, Y.; Tan, B.; Yin, Y.; Qi, M.; Wang, J. Ellagic Acid Alleviates Oxidative Stress by Mediating Nrf2 Signaling Pathways and Protects against Paraquat-Induced Intestinal Injury in Piglets. Antioxidants 2022, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef]
- Qi, M.; Wang, N.; Xiao, Y.; Deng, Y.; Zha, A.; Tan, B.; Wang, J.; Yin, Y.; Liao, P. Ellagic acid ameliorates paraquat-induced liver injury associated with improved gut microbial profile. Environ. Pollut. 2022, 293, 118572. [Google Scholar] [CrossRef]
- Wei, Z.X.; Cai, L.; Zhao, X.M.; Jiang, X.R.; Li, X.L. Effects of Spermidine on Cell Proliferation, Migration, and Inflammatory Response in Porcine Enterocytes. Front. Biosci.-Landmark 2022, 27, 194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, X.; Cai, L.; Zhang, Y.; Ding, H.; Yin, J.; Li, X. Effects of daidzein on antioxidant capacity in weaned pigs and IPEC-J2 cells. Anim. Nutr. 2022, 11, 48–59. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, W.; Zhai, T.; You, J.; Chen, Y. Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Pharm. Sin. 2019, 9, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Dobiasova, S.; Rehorova, K.; Kucerova, D.; Biedermann, D.; Kanova, K.; Petraskova, L.; Koucka, K.; Vaclavikova, R.; Valentova, K.; Ruml, T.; et al. Multidrug Resistance Modulation Activity of Silybin Derivatives and Their Anti-Inflammatory Potential. Antioxidants 2020, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Świątkiewicz, M.; Florek, M.; Wojtaszewska, I. Impact of milk thistle (Silybum marianum L.) seeds in fattener diets on pig performance and carcass traits and fatty acid profile and cholesterol of meat, backfat and liver. Livest. Sci. 2020, 239, 104180. [Google Scholar] [CrossRef]
- Janocha, A.; Milczarek, A.; Pietrusiak, D. Impact of Milk Thistle (Silybum marianum [L.] Gaertn.) Seeds in Broiler Chicken Diets on Rearing Results, Carcass Composition, and Meat Quality. Animals 2021, 11, 1550. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Yin, R.X.; Gao, H.; Huang, F.; Wu, J.Z.; Pan, S.L.; Lin, W.X.; Yang, D.Z. Association of the SPTLC3 rs364585 polymorphism and serum lipid profiles in two Chinese ethnic groups. Lipids Health Dis. 2017, 16, 1. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, M.; Wang, Y.; Zhang, L.; Zhao, H.; Zhao, M. Silymarin attenuated paraquat-induced cytotoxicity in macrophage by regulating Trx/TXNIP complex, inhibiting NLRP3 inflammasome activation and apoptosis. Toxicol. In Vitro 2018, 46, 265–272. [Google Scholar] [CrossRef]
- Zhang, Q.; Ahn, J.M.; Kim, I.H. Micelle silymarin supplementation to sows’ diet from day 109 of gestation to entire lactation period enhances reproductive performance and affects serum hormones and metabolites. J. Anim. Sci. 2021, 99, skab354. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Liu, D. Silibinin ameliorats H2O2-induced cell apoptosis and oxidative stress response by activating Nrf2 signaling in trophoblast cells. Acta Histochem. 2020, 122, 151620. [Google Scholar] [CrossRef]
- Guerbette, T.; Boudry, G.; Lan, A. Mitochondrial function in intestinal epithelium homeostasis and modulation in diet-induced obesity. Mol. Metab. 2022, 63, 101546. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Liu, P.C.; Liu, W.W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Protective effects of silibinin against ethanol- or acetaldehyde-caused damage in liver cell lines involve the repression of mitochondrial fission. Toxicol. In Vitro 2022, 80, 105330. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Stanca, E.; Lunetti, P.; Blonda, M.; Tamborra, R.; Siculella, L.; Vendemiale, G.; Capobianco, L.; Giudetti, A.M. Silybin exerts antioxidant effects and induces mitochondrial biogenesis in liver of rat with secondary biliary cirrhosis. Free Radic. Biol. Med. 2014, 73, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bellance, N.; Lestienne, P.; Rossignol, R. Mitochondria: From bioenergetics to the metabolic regulation of carcinogenesis. Front. Biosci.-Landmark 2009, 14, 4015–4034. [Google Scholar] [CrossRef]
- Chithra, Y.; Dey, G.; Ghose, V.; Chandramohan, V.; Gowthami, N.; Vasudev, V.; Srinivas Bharath, M.M. Mitochondrial Complex I Inhibition in Dopaminergic Neurons Causes Altered Protein Profile and Protein Oxidation: Implications for Parkinson’s disease. Neurochem. Res. 2023, 48, 2360–2389. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Chen, J.; Su, Y.; Lin, F.; Iqbal, M.; Mehmood, K.; Zhang, H.; Shi, D. Effect of paraquat on cytotoxicity involved in oxidative stress and inflammatory reaction: A review of mechanisms and ecological implications. Ecotoxicol. Environ. Saf. 2021, 224, 112711. [Google Scholar] [CrossRef]
- Imam, O.S.; Mohammed, M.H.A.G. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur. Cytokine Netw. 2013, 24, 114–121. [Google Scholar] [CrossRef]
- Song, Z.; Song, M.; Lee, D.Y.W.; Liu, Y.; Deaciuc, I.V.; McClain, C.J. Silymarin Prevents Palmitate-Induced Lipotoxicity in HepG2 Cells: Involvement of Maintenance of Akt Kinase Activation. Basic Clin. Pharmacol. 2007, 101, 262–268. [Google Scholar] [CrossRef]
- Martinou, J.C.; Youle, R.J. Mitochondria in Apoptosis: Bcl-2 Family Members and Mitochondrial Dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef]
- El-Boghdady, N.A.; Abdeltawab, N.F.; Nooh, M.M. Resveratrol and Montelukast Alleviate Paraquat-Induced Hepatic Injury in Mice: Modulation of Oxidative Stress, Inflammation, and Apoptosis. Oxid. Med. Cell. Longev. 2017, 2017, 9396425. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Chen, Y.; Deng, F.; Yan, X.; Zhong, R.; Meng, Q.; Liu, L.; Zhao, Y.; Zhang, S.; Chen, L.; et al. Xylooligosaccharide-mediated gut microbiota enhances gut barrier and modulates gut immunity associated with alterations of biological processes in a pig model. Carbohyd. Polym. 2022, 294, 119776. [Google Scholar] [CrossRef] [PubMed]
- Jahanian, E.; Mahdavi, A.H.; Asgary, S.; Jahanian, R. Effects of dietary inclusion of silymarin on performance, intestinal morphology and ileal bacterial count in aflatoxin-challenged broiler chicks. J. Anim. Physiol. Anim. Nutr. 2017, 101, e43–e54. [Google Scholar] [CrossRef]
- Trachsel, J.; Briggs, C.; Gabler, N.K.; Allen, H.K.; Loving, C.L. Dietary Resistant Potato Starch Alters Intestinal Microbial Communities and Their Metabolites, and Markers of Immune Regulation and Barrier Function in Swine. Front. Immunol. 2019, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, Y.; Wang, Y.; Fan, R.; Hu, X.; Zhang, F.; Yang, J.; Chen, J. The Role of Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Front. Immunol. 2022, 13, 871713. [Google Scholar] [CrossRef]
- Sasu, A.; Herman, H.; Mariasiu, T.; Rosu, M.; Balta, C.; Anghel, N.; Miutescu, E.; Cotoraci, C.; Hermenean, A. Protective effects of silymarin on epirubicin-induced mucosal barrier injury of the gastrointestinal tract. Drug Chem. Toxicol. 2015, 38, 442–451. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial Tight Junctions in Intestinal Inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Xia, X.; Li, G.; Ding, Y.; Ren, T.; Zheng, J.; Kan, J. Effect of Whole Grain Qingke (Tibetan Hordeum vulgare L. Zangqing 320) on the Serum Lipid Levels and Intestinal Microbiota of Rats under High-Fat Diet. J. Agric. Food Chem. 2017, 65, 2686–2693. [Google Scholar] [CrossRef]
- Kumari, R.; Ahuja, V.; Paul, J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013, 19, 3404–3414. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, L.; Fang, X.; Guo, Z.; Wang, X.; Shi, B.; Meng, Q. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nagai, F.; Morotomi, M. Characterization of Phascolarctobacterium succinatutens sp. nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl. Environ. Microbiol. 2012, 78, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Nagao-Kitamoto, H.; Leslie, J.L.; Kitamoto, S.; Jin, C.; Thomsson, K.A.; Gillilland, M.G.; Kuffa, P.; Goto, Y.; Jenq, R.R.; Ishii, C.; et al. Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 2020, 26, 608–617. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Li, X.Y.; Ji, H.F. Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl. Microbiol. Biotechnol. 2019, 103, 7141–7149. [Google Scholar] [CrossRef]
- Le Roy, T.; Moens de Hase, E.; Van Hul, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Depommier, C.; Druart, C.; Everard, A.; Maiter, D.; et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut 2022, 71, 534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bao, L.; Qiu, M.; Wu, K.; Zhao, Y.; Feng, L.; Xiang, K.; Zhang, N.; Hu, X.; Fu, Y. Commensal cow Roseburia reduces gut-dysbiosis-induced mastitis through inhibiting bacterial translocation by producing butyrate in mice. Cell Rep. 2022, 41, 111681. [Google Scholar] [CrossRef]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]

| Item | Silybin Level, mg/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | ANOVA | Linear | Quadratic | ||
| BW, kg | |||||||||
| Day 0 | 8.28 | 8.28 | 8.28 | 8.28 | 8.28 | 0.49 | 1.00 | 1.00 | 1.00 |
| Day 14 | 10.39 | 10.60 | 10.57 | 10.23 | 11.00 | 0.53 | 0.88 | 0.51 | 0.59 |
| Day 28 | 16.05 | 16.05 | 15.85 | 16.18 | 16.51 | 0.81 | 0.99 | 0.62 | 0.84 |
| Day 42 | 23.33 | 23.42 | 23.63 | 24.41 | 24.80 | 1.12 | 0.86 | 0.28 | 0.88 |
| ADG 2, g | |||||||||
| Day 0–14 | 151 | 166 | 164 | 140 | 194 | 16.76 | 0.25 | 0.15 | 0.23 |
| Day 14–28 | 404 | 389 | 377 | 422 | 394 | 26.05 | 0.82 | 0.92 | 0.86 |
| Day 28–42 | 520 | 526 | 556 | 588 | 592 | 25.53 | 0.20 | 0.03 | 0.34 |
| Day 0–42 | 358 | 360 | 366 | 388 | 393 | 17.56 | 0.54 | 0.11 | 0.73 |
| ADFI 3, g | |||||||||
| Day 0–14 | 270 | 297 | 280 | 262 | 316 | 22.26 | 0.47 | 0.29 | 0.36 |
| Day 14–28 | 659 | 674 | 671 | 700 | 692 | 29.66 | 0.90 | 0.43 | 0.62 |
| Day 28–42 | 996 | 927 | 963 | 1038 | 977 | 38.63 | 0.46 | 0.68 | 0.61 |
| Day 0–42 | 642 | 633 | 638 | 672 | 662 | 26.63 | 0.86 | 0.42 | 0.75 |
| FCR 4 | |||||||||
| Day 0–14 | 1.81 | 1.86 | 1.72 | 1.96 | 1.65 | 0.11 | 0.30 | 0.34 | 0.23 |
| Day 14–28 | 1.67 | 1.74 | 1.79 | 1.68 | 1.78 | 0.08 | 0.77 | 0.53 | 0.89 |
| Day 28–42 | 1.92 a,x | 1.77 ab | 1.74 ab,y | 1.77 ab | 1.65 b | 0.05 | 0.02 | 0.00 | 0.35 |
| Day 0–42 | 1.80 | 1.76 | 1.75 | 1.74 | 1.68 | 0.03 | 0.24 | 0.03 | 0.91 |
| DI 5, % | |||||||||
| Day 0–14 | 8.93 a | 5.36 ab | 6.55 a | 7.32 a | 2.98 b | - | 0.02 | - | - |
| Item | Silybin Level, mg/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | ANOVA | Linear | Quadratic | ||
| Day 14 | |||||||||
| CAT, U/mL | 2.14 | 2.47 | 1.93 | 2.08 | 2.05 | 0.23 | 0.55 | 0.51 | 0.71 |
| SOD, U/mL | 29.23 b | 35.77 a | 34.53 a | 34.11 a | 35.19 a | 0.78 | 0.00 | 0.01 | 0.01 |
| GSH-Px, U/mL | 380 | 411 | 373 | 374 | 394 | 15.3 | 0.39 | 0.93 | 0.41 |
| MDA, nmol/mL | 2.82 x | 2.82 | 2.29 | 2.23 y | 2.39 | 0.14 | 0.03 | 0.03 | 0.02 |
| Day 42 | |||||||||
| CAT, U/mL | 3.88 b | 4.08 ab | 4.23 ab | 4.39 ab | 4.7 a | 0.18 | 0.05 | 0.00 | 0.56 |
| SOD, U/mL | 32.56 | 30.84 | 32.68 | 31.13 | 35.04 | 1.08 | 0.16 | 0.08 | 0.15 |
| GSH-Px, U/mL | 374 | 366 | 352 | 401 | 399 | 13.3 | 0.08 | 0.04 | 0.95 |
| MDA, nmol/mL | 2.36 a,x | 2.26 ab | 2.21 ab | 1.83 ab,y | 1.73 b | 0.14 | 0.01 | 0.00 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Gao, G.; Yin, C.; Bai, R.; Li, Y.; Sun, W.; Pi, Y.; Jiang, X.; Li, X. The Effects of Dietary Silybin Supplementation on the Growth Performance and Regulation of Intestinal Oxidative Injury and Microflora Dysbiosis in Weaned Piglets. Antioxidants 2023, 12, 1975. https://doi.org/10.3390/antiox12111975
Cai L, Gao G, Yin C, Bai R, Li Y, Sun W, Pi Y, Jiang X, Li X. The Effects of Dietary Silybin Supplementation on the Growth Performance and Regulation of Intestinal Oxidative Injury and Microflora Dysbiosis in Weaned Piglets. Antioxidants. 2023; 12(11):1975. https://doi.org/10.3390/antiox12111975
Chicago/Turabian StyleCai, Long, Ge Gao, Chenggang Yin, Rong Bai, Yanpin Li, Wenjuan Sun, Yu Pi, Xianren Jiang, and Xilong Li. 2023. "The Effects of Dietary Silybin Supplementation on the Growth Performance and Regulation of Intestinal Oxidative Injury and Microflora Dysbiosis in Weaned Piglets" Antioxidants 12, no. 11: 1975. https://doi.org/10.3390/antiox12111975
APA StyleCai, L., Gao, G., Yin, C., Bai, R., Li, Y., Sun, W., Pi, Y., Jiang, X., & Li, X. (2023). The Effects of Dietary Silybin Supplementation on the Growth Performance and Regulation of Intestinal Oxidative Injury and Microflora Dysbiosis in Weaned Piglets. Antioxidants, 12(11), 1975. https://doi.org/10.3390/antiox12111975







