Unleashing the Potential of Nrf2: A Novel Therapeutic Target for Pulmonary Vascular Remodeling
Abstract
1. Introduction
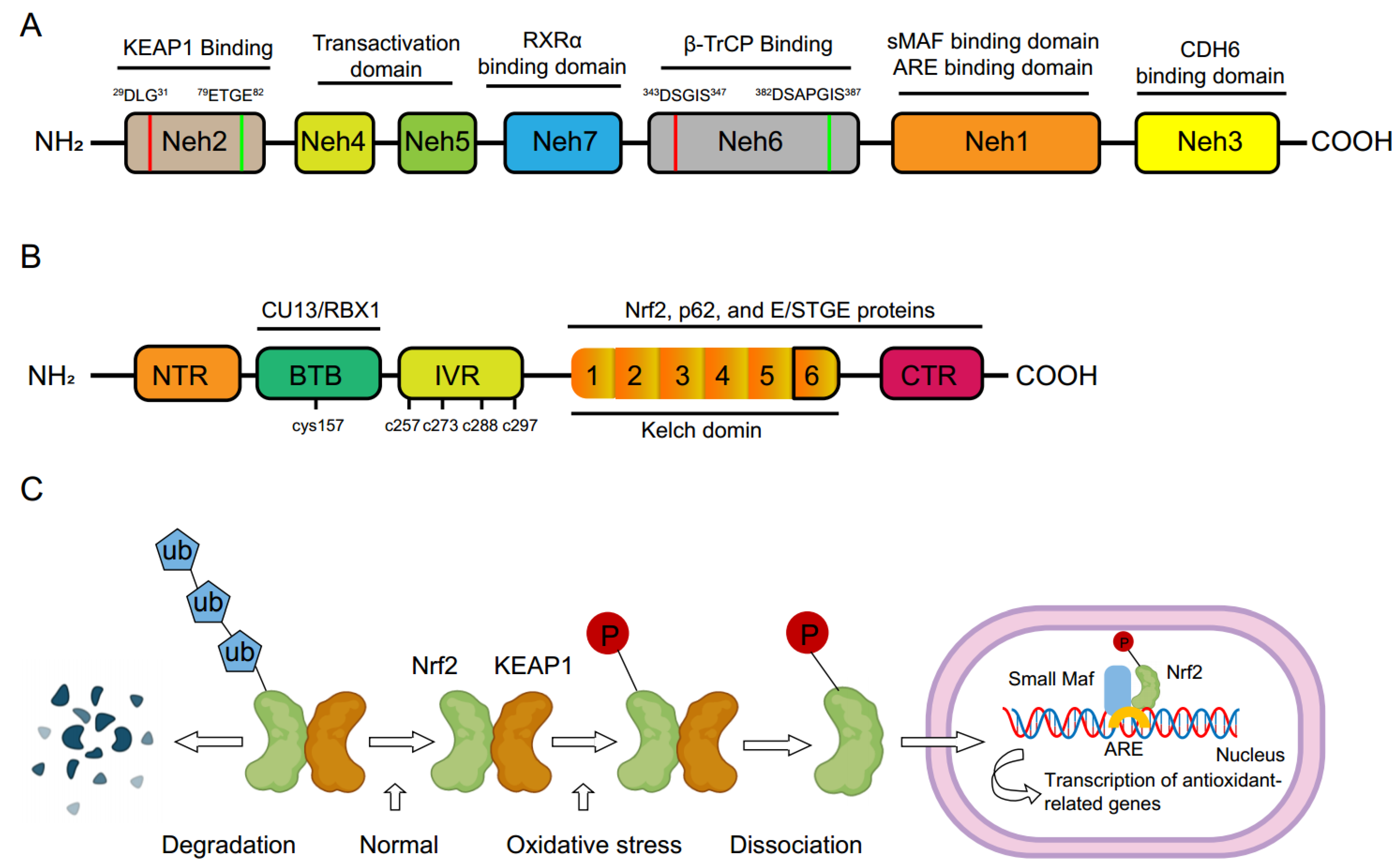
2. Molecular Basis of Nrf2
3. Signaling Transduction of Nrf2
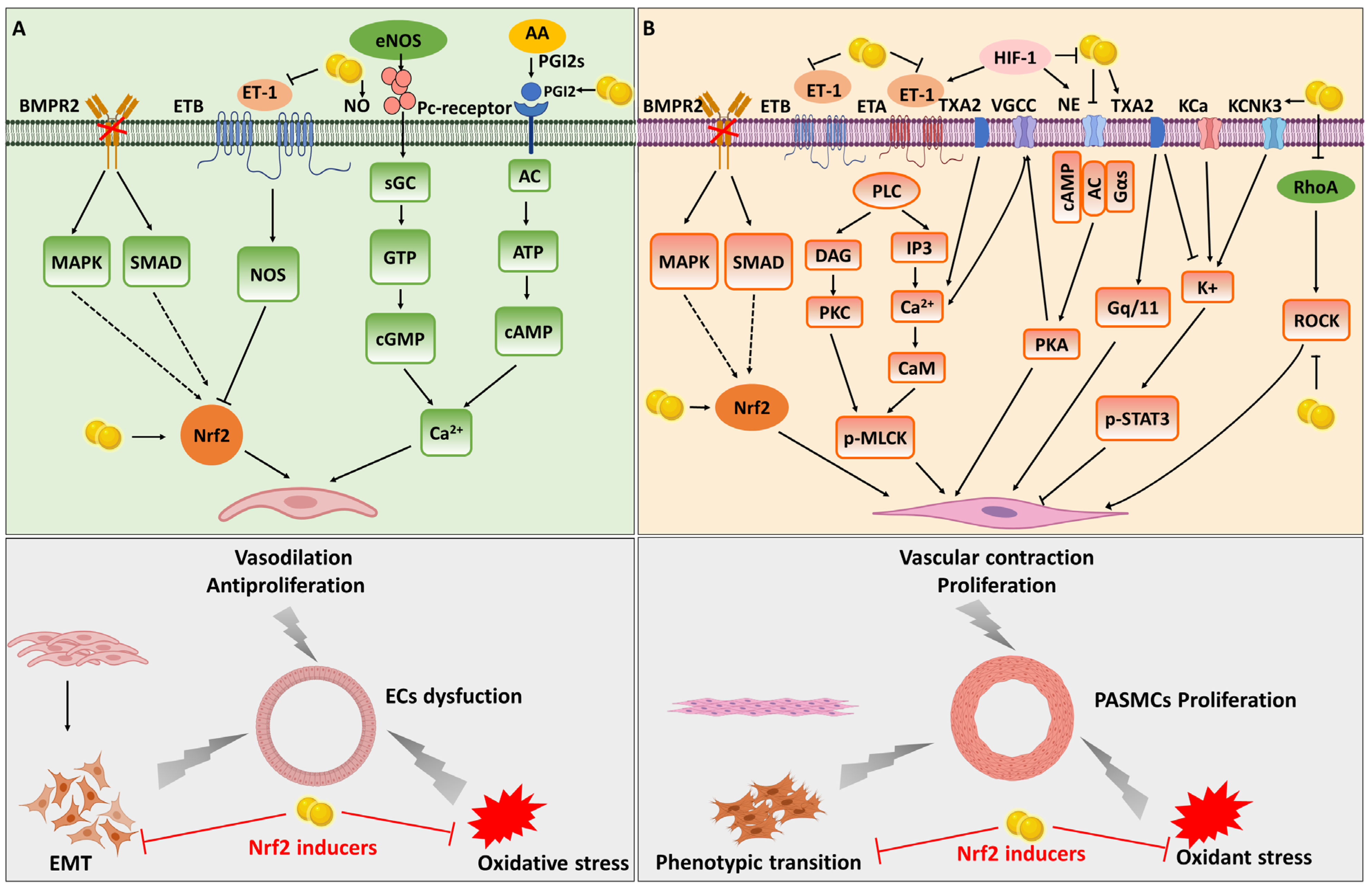
4. Role of Nrf2 in Pulmonary Vascular Remodeling
4.1. Nrf2 and Endothelial Dysfunction in the Intima Remodeling
4.2. Nrf2 and Smooth Muscle Cell Phenotypic Switching in Media Remodeling
5. The Therapeutic Potential of Nrf2 Inducers in PH
5.1. SFN
5.2. Oltipraz
5.3. Resveratrol
5.4. Rosiglitazone
5.5. Dimethyl Fumarate
5.6. Rutin
5.7. Curcumin
5.8. Natural Products
6. Concluding Remarks and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PH | Pulmonary hypertension |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| VSMC | vascular smooth muscle cell |
| PAEC | pulmonary arterial endothelial cell |
| PASMC | pulmonary arterial smooth muscle cell |
| PAH | Pulmonary arterial hypertension |
| ROS | reactive oxygen species |
| NOS | nitric oxide synthase |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| SOD | superoxide dismutase |
| ET-1 | endothelin-1 |
| PGI2 | prostacyclin |
| EC | endothelial cell |
| SMC | smooth muscle cell |
| EMT | epithelial-mesenchymal transition |
| NO | nitric oxide |
| EDHF | endothelium-derived hyperpolarizing factor |
| VGCC | Voltagegated calcium channels |
| MCT | monocrotaline |
| NHE | Na+/H+ exchanger |
| ARE | antioxidant response element |
| EpRE | electrophile response element |
| GST | glutathione S-transferase |
| GCL | glutamate-cysteine ligase |
| GPx | glutathione peroxidase |
| TXNRD | thioredoxin reductase |
| TXN | thioredoxin |
| GSH | glutathione |
| sMAF | small musculoaponeurotic fibrosarcoma |
| cAMP | cyclic adenosine monophosphate |
| β-TrCP | β-transducin repeat-containing protein |
| RXR-α | retinoic X receptor alpha |
| Keap1 | Kelch-like ECH-associated protein 1 |
| BTB | Bric-a-Brac domain |
| IVR | intervening region |
| LncRNA | long non-coding RNA |
| RVH | right ventricular hypertrophy |
| SFN | sulforaphane |
| KCNK3 | potassium channel subfamily K member 3 |
| Ipt | Iptakalim |
| PPARγ | peroxisome proliferator gamma |
| DMF | Dimethyl Fumarate |
| RVSP | right ventricular systolic pressure |
| SuHx | SU5416 and 10% hypoxia |
| GCL | glutamate-cysteine ligase |
| RV/(LV+S) | right ventricular hypertrophy index |
References
- Lambert, M.; Capuano, V.; Boet, A.; Tesson, L.; Bertero, T.; Nakhleh, M.K.; Remy, S.; Anegon, I.; Pechoux, C.; Hautefort, A.; et al. Characterization of Kcnk3-Mutated Rat, a Novel Model of Pulmonary Hypertension. Circ. Res. 2019, 125, 678–695. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, S.; Jiang, W.; Wang, J.; Xin, Q.; Sun, C.; Li, K.; Qi, T.; Luan, Y. Protective effect of baicalin against pulmonary arterial hypertension vascular remodeling through regulation of TNF-alpha signaling pathway. Pharmacol. Res. Perspect. 2021, 9, e00703. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Condon, D.F.; Nickel, N.P.; Anderson, R.; Mirza, S.; de Jesus Perez, V.A. The 6th World Symposium on Pulmonary Hypertension: What’s old is new. F1000Res 2019, 8, 888. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Channick, R.N.; Frantz, R.P.; Grunig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E.; Cordier, J.F.; Chabot, F.; et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010, 122, 156–163. [Google Scholar] [CrossRef]
- Benza, R.L.; Miller, D.P.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.S.H.; Massam, B.D.; Kulkarni, S.S.; Lang, C.C. Pulmonary Arterial Hypertension: Pathophysiology and Treatment. Diseases 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, A.; Pan, Y.; Wang, X.; Xu, Y.; Desai, A.A.; Tang, H.; Han, Y. Research Progress on Pulmonary Arterial Hypertension and the Role of the Angiotensin Converting Enzyme 2-Angiotensin-(1–7)-Mas Axis in Pulmonary Arterial Hypertension. Cardiovasc. Drugs Ther. 2022, 36, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Lau, E.M.; Montani, D.; Jais, X.; Sitbon, O.; Simonneau, G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014, 130, 2189–2208. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Cober, N.D.; Dai, Z.; Stewart, D.J.; Zhao, Y.Y. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur. Respir. J. 2021, 58, 2003957. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Chan, S.Y.; Weir, E.K. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1322–H1331. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Satoh, T.; Kikuchi, N.; Omura, J.; Kurosawa, R.; Suzuki, K.; Sugimura, K.; Aoki, T.; Nochioka, K.; Tatebe, S.; et al. Basigin mediates pulmonary hypertension by promoting inflammation and vascular smooth muscle cell proliferation. Circ. Res. 2014, 115, 738–750. [Google Scholar] [CrossRef]
- Tuder, R.M. Pathology of pulmonary arterial hypertension. Semin. Respir. Crit. Care Med. 2009, 30, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef] [PubMed]
- Smukowska-Gorynia, A.; Rzymski, P.; Marcinkowska, J.; Poniedzialek, B.; Komosa, A.; Cieslewicz, A.; Slawek-Szmyt, S.; Janus, M.; Araszkiewicz, A.; Jankiewicz, S.; et al. Prognostic Value of Oxidative Stress Markers in Patients with Pulmonary Arterial or Chronic Thromboembolic Pulmonary Hypertension. Oxidative Med. Cell. Longev. 2019, 2019, 3795320. [Google Scholar] [CrossRef]
- Pena, E.; Brito, J.; El Alam, S.; Siques, P. Oxidative Stress, Kinase Activity and Inflammatory Implications in Right Ventricular Hypertrophy and Heart Failure under Hypobaric Hypoxia. Int. J. Mol. Sci. 2020, 21, 6421. [Google Scholar] [CrossRef]
- Turck, P.; Fraga, S.; Salvador, I.; Campos-Carraro, C.; Lacerda, D.; Bahr, A.; Ortiz, V.; Hickmann, A.; Koetz, M.; Bello-Klein, A.; et al. Blueberry extract decreases oxidative stress and improves functional parameters in lungs from rats with pulmonary arterial hypertension. Nutrition 2020, 70, 110579. [Google Scholar] [CrossRef]
- Rudyk, O.; Aaronson, P.I. Redox Regulation, Oxidative Stress, and Inflammation in Group 3 Pulmonary Hypertension. Adv. Exp. Med. Biol. 2021, 1303, 209–241. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, M.; Guignabert, C.; Humbert, M.; Nicolls, M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Frid, M.G.; Thurman, J.M.; Hansen, K.C.; Maron, B.A.; Stenmark, K.R. Inflammation, immunity, and vascular remodeling in pulmonary hypertension; Evidence for complement involvement? Glob. Cardiol. Sci. Pract. 2020, 2020, e202001. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L.; et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H928–H939. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Design and development of Nrf2 modulators for cancer chemoprevention and therapy: A review. Drug Des. Dev. Ther. 2018, 12, 3181–3197. [Google Scholar] [CrossRef]
- Cores, A.; Piquero, M.; Villacampa, M.; Leon, R.; Menendez, J.C. NRF2 Regulation Processes as a Source of Potential Drug Targets against Neurodegenerative Diseases. Biomolecules 2020, 10, 904. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Nyul-Toth, A.; Kiss, T.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Benyo, Z.; Csiszar, A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 2019, 41, 727–738. [Google Scholar] [CrossRef]
- Kim, S.L.; Choi, H.S.; Ko, Y.C.; Yun, B.S.; Lee, D.S. 5-Hydroxymaltol Derived from Beetroot Juice through Lactobacillus Fermentation Suppresses Inflammatory Effect and Oxidant Stress via Regulating NF-kB, MAPKs Pathway and NRF2/HO-1 Expression. Antioxidants 2021, 10, 1324. [Google Scholar] [CrossRef]
- Gallorini, M.; Berardi, A.C.; Gissi, C.; Cataldi, A.; Osti, L. Nrf2-mediated cytoprotective effect of four different hyaluronic acids by molecular weight in human tenocytes. J. Drug Target. 2020, 28, 212–224. [Google Scholar] [CrossRef]
- Itoh, K.; Igarashi, K.; Hayashi, N.; Nishizawa, M.; Yamamoto, M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell Biol. 1995, 15, 4184–4193. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Kwong, M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta 2000, 1517, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Chanas, S.A.; Henderson, C.J.; McMahon, M.; Sun, C.; Moffat, G.J.; Wolf, C.R.; Yamamoto, M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000, 28, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Telkoparan-Akillilar, P.; Suzen, S.; Saso, L. The NRF2/KEAP1 Axis in the Regulation of Tumor Metabolism: Mechanisms and Therapeutic Perspectives. Biomolecules 2020, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Goncalves, F.M.; Abiko, Y.; Li, H.; Kumagai, Y.; Aschner, M. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 2020, 34, 101475. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Lee, D.Y.; Chun, K.S.; Kim, E.H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.B.; Gordan, J.D.; Jin, J.; Harper, J.W.; Diehl, J.A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: Oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell Biol. 2004, 24, 8477–8486. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Itoh, K.; Mimura, J.; Yamamoto, M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxid. Redox Signal. 2010, 13, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dutta, N.; Banerjee, P.; Gajbhiye, R.L.; Sareng, H.R.; Kapse, P.; Pal, S.; Burdelya, L.; Mandal, N.C.; Ravichandiran, V.; et al. Induction of monoamine oxidase A-mediated oxidative stress and impairment of NRF2-antioxidant defence response by polyphenol-rich fraction of Bergenia ligulata sensitizes prostate cancer cells in vitro and in vivo. Free Radic. Biol. Med. 2021, 172, 136–151. [Google Scholar] [CrossRef]
- Price, L.C.; Wort, S.J.; Perros, F.; Dorfmuller, P.; Huertas, A.; Montani, D.; Cohen-Kaminsky, S.; Humbert, M. Inflammation in pulmonary arterial hypertension. Chest 2012, 141, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Quarck, R.; Nawrot, T.; Meyns, B.; Delcroix, M. C-reactive protein: A new predictor of adverse outcome in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 53, 1211–1218. [Google Scholar] [CrossRef]
- Gong, J.; Feng, Z.; Peterson, A.L.; Carr, J.F.; Vang, A.; Braza, J.; Choudhary, G.; Dennery, P.A.; Yao, H. Endothelial to mesenchymal transition during neonatal hyperoxia-induced pulmonary hypertension. J. Pathol. 2020, 252, 411–422. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Y.; Wang, W.; Li, H.; Yang, M.; Ding, H.; Lv, X.; Lian, N.; Zhao, J.; Deng, C. The role of inflammation in a rat model of chronic thromboembolic pulmonary hypertension induced by carrageenan. Ann. Transl. Med. 2020, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Ruffenach, G.; O’Connor, E.; Vaillancourt, M.; Hong, J.; Cao, N.; Sarji, S.; Moazeni, S.; Papesh, J.; Grijalva, V.; Cunningham, C.M.; et al. Oral 15-Hydroxyeicosatetraenoic Acid Induces Pulmonary Hypertension in Mice by Triggering T Cell-Dependent Endothelial Cell Apoptosis. Hypertension 2020, 76, 985–996. [Google Scholar] [CrossRef]
- Frump, A.; Prewitt, A.; de Caestecker, M.P. BMPR2 mutations and endothelial dysfunction in pulmonary arterial hypertension (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045894018765840. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Stewart, S.; Upton, P.D.; Machado, R.; Thomson, J.R.; Trembath, R.C.; Morrell, N.W. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002, 105, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, C.; Liu, S.; Lu, W.; Li, Y.; Luo, X.; Ma, R.; Zhang, C.; Chen, H.; Chen, Y.; et al. Dysregulation of BMP9/BMPR2/SMAD signalling pathway contributes to pulmonary fibrosis and pulmonary hypertension induced by bleomycin in rats. Br. J. Pharmacol. 2021, 178, 203–216. [Google Scholar] [CrossRef]
- Tu, L.; Desroches-Castan, A.; Mallet, C.; Guyon, L.; Cumont, A.; Phan, C.; Robert, F.; Thuillet, R.; Bordenave, J.; Sekine, A.; et al. Selective BMP-9 Inhibition Partially Protects Against Experimental Pulmonary Hypertension. Circ. Res. 2019, 124, 846–855. [Google Scholar] [CrossRef]
- Sanchez-Duffhues, G.; Garcia de Vinuesa, A.; Ten Dijke, P. Endothelial-to-mesenchymal transition in cardiovascular diseases: Developmental signaling pathways gone awry. Dev. Dyn. 2018, 247, 492–508. [Google Scholar] [CrossRef]
- Medici, D.; Kalluri, R. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin. Cancer Biol. 2012, 22, 379–384. [Google Scholar] [CrossRef]
- Delaney, C.; Sherlock, L.; Fisher, S.; Maltzahn, J.; Wright, C.; Nozik-Grayck, E. Serotonin 2A receptor inhibition protects against the development of pulmonary hypertension and pulmonary vascular remodeling in neonatal mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L871–L881. [Google Scholar] [CrossRef]
- Gredic, M.; Wu, C.Y.; Hadzic, S.; Pak, O.; Savai, R.; Kojonazarov, B.; Doswada, S.; Weiss, A.; Weigert, A.; Guenther, A.; et al. Myeloid-cell-specific deletion of inducible nitric oxide synthase protects against smoke-induced pulmonary hypertension in mice. Eur. Respir. J. 2022, 59, 2101153. [Google Scholar] [CrossRef]
- Wang, M.; Luo, P.; Shi, W.; Guo, J.; Huo, S.; Yan, D.; Peng, L.; Zhang, C.; Lv, J.; Lin, L.; et al. S-Nitroso-L-Cysteine Ameliorated Pulmonary Hypertension in the MCT-Induced Rats through Anti-ROS and Anti-Inflammatory Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 6621232. [Google Scholar] [CrossRef]
- Budhiraja, R.; Tuder, R.M.; Hassoun, P.M. Endothelial dysfunction in pulmonary hypertension. Circulation 2004, 109, 159–165. [Google Scholar] [CrossRef]
- Geraci, M.W.; Gao, B.; Shepherd, D.C.; Moore, M.D.; Westcott, J.Y.; Fagan, K.A.; Alger, L.A.; Tuder, R.M.; Voelkel, N.F. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J. Clin. Investig. 1999, 103, 1509–1515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chester, A.H.; Yacoub, M.H. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pract. 2014, 2014, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Cinà, C.S.; Devereaux, P. Vascular viewpoint. Vasc. Med. 2006, 11, 61–63. [Google Scholar] [CrossRef] [PubMed]
- McFalls, E.O.; Ward, H.B.; Moritz, T.E.; Goldman, S.; Krupski, W.C.; Littooy, F.; Pierpont, G.; Santilli, S.; Rapp, J.; Hattler, B.; et al. Coronary-artery revascularization before elective major vascular surgery. N. Engl. J. Med. 2004, 351, 2795–2804. [Google Scholar] [CrossRef]
- Christman, B.W.; McPherson, C.D.; Newman, J.H.; King, G.A.; Bernard, G.R.; Groves, B.M.; Loyd, J.E. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N. Engl. J. Med. 1992, 327, 70–75. [Google Scholar] [CrossRef]
- Tuder, R.M.; Archer, S.L.; Dorfmuller, P.; Erzurum, S.C.; Guignabert, C.; Michelakis, E.; Rabinovitch, M.; Schermuly, R.; Stenmark, K.R.; Morrell, N.W. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D4–D12. [Google Scholar] [CrossRef]
- Tarantini, S.; Valcarcel-Ares, M.N.; Yabluchanskiy, A.; Tucsek, Z.; Hertelendy, P.; Kiss, T.; Gautam, T.; Zhang, X.A.; Sonntag, W.E.; de Cabo, R.; et al. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 853–863. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bailey-Downs, L.; Sosnowska, D.; Gautam, T.; Koncz, P.; Losonczy, G.; Ballabh, P.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Vascular oxidative stress in aging: A homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H363–H372. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Su, S.L.; Lu, C.Y.; Lin, A.H.; Lin, W.C.; Liu, C.S.; Yang, Y.C.; Wang, H.M.; Lii, C.K.; Chen, H.W. Andrographolide inhibits hypoxia-induced HIF-1alpha-driven endothelin 1 secretion by activating Nrf2/HO-1 and promoting the expression of prolyl hydroxylases 2/3 in human endothelial cells. Env. Toxicol. 2017, 32, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zheng, Y.; Hou, X. Lipoxin A4 restores oxidative stress-induced vascular endothelial cell injury and thrombosis-related factor expression by its receptor-mediated activation of Nrf2-HO-1 axis. Cell. Signal. 2019, 60, 146–153. [Google Scholar] [CrossRef]
- Ji, X.; Wang, H.; Zhu, J.; Zhu, L.; Pan, H.; Li, W.; Zhou, Y.; Cong, Z.; Yan, F.; Chen, S. Knockdown of Nrf2 suppresses glioblastoma angiogenesis by inhibiting hypoxia-induced activation of HIF-1α. Int. J. Cancer 2014, 135, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Diebold, I.; Hennigs, J.K.; Miyagawa, K.; Li, C.G.; Nickel, N.P.; Kaschwich, M.; Cao, A.; Wang, L.; Reddy, S.; Chen, P.I.; et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015, 21, 596–608. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, T.; Zhang, H.; Yan, Y.; Wang, D.; Fang, L.; Lu, Y.; Du, G. Activation of Nrf2 Attenuates Pulmonary Vascular Remodeling via Inhibiting Endothelial-to-Mesenchymal Transition: An Insight from a Plant Polyphenol. Int. J. Biol. Sci. 2017, 13, 1067–1081. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Huang, X.; Xie, Y.; Qu, Y.; Long, H.; Gu, N.; Jiang, W. Z-Ligustilide protects vascular endothelial cells from oxidative stress and rescues high fat diet-induced atherosclerosis by activating multiple NRF2 downstream genes. Atherosclerosis 2019, 284, 110–120. [Google Scholar] [CrossRef]
- Li, W.W.; Cao, A.H.; Sun, F.Y. LncRNA MIAT stimulates oxidative stress in the hypoxic pulmonary hypertension model by sponging miR-29a-5p and inhibiting Nrf2 pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9022–9029. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, H.J.; Sir, J.J.; Kim, B.K.; Hur, J.; Youn, S.W.; Yang, H.M.; Jun, S.I.; Park, K.W.; Hwang, S.J.; et al. Sulfasalazine induces haem oxygenase-1 via ROS-dependent Nrf2 signalling, leading to control of neointimal hyperplasia. Cardiovasc. Res. 2009, 82, 550–560. [Google Scholar] [CrossRef]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxidative Med. Cell. Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Frid, M.; Perros, F. Endothelial-to-Mesenchymal Transition: An Evolving Paradigm and a Promising Therapeutic Target in PAH. Circulation 2016, 133, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Yet, S.F.; Perrella, M.A.; Layne, M.D.; Hsieh, C.M.; Maemura, K.; Kobzik, L.; Wiesel, P.; Christou, H.; Kourembanas, S.; Lee, M.E. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J. Clin. Investig. 1999, 103, R23–R29. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Christou, H.; Hsieh, C.M.; Liu, Y.; Dhawan, V.; Abraham, N.G.; Perrella, M.A.; Mitsialis, S.A.; Kourembanas, S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc. Natl. Acad. Sci. USA 2001, 98, 8798–8803. [Google Scholar] [CrossRef]
- Goto, J.; Ishikawa, K.; Kawamura, K.; Watanabe, Y.; Matumoto, H.; Sugawara, D.; Maruyama, Y. Heme oxygenase-1 reduces murine monocrotaline-induced pulmonary inflammatory responses and resultant right ventricular overload. Antioxid. Redox Signal. 2002, 4, 563–568. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, Y.J.; Liu, C.P.; Yu, B.X.; Lu, W.X. Simvastatin protects against the development of monocrotaline-induced pulmonary hypertension in rats via a heme oxygenase-1-dependent pathway. Exp. Lung Res. 2011, 37, 492–499. [Google Scholar] [CrossRef]
- Liang, O.D.; Mitsialis, S.A.; Chang, M.S.; Vergadi, E.; Lee, C.; Aslam, M.; Fernandez-Gonzalez, A.; Liu, X.; Baveja, R.; Kourembanas, S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells 2011, 29, 99–107. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Bogaard, H.J.; Al Husseini, A.; Farkas, L.; Gomez-Arroyo, J.; Natarajan, R. Antioxidants for the treatment of patients with severe angioproliferative pulmonary hypertension? Antioxid. Redox Signal. 2013, 18, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Hartney, T.; Birari, R.; Venkataraman, S.; Villegas, L.; Martinez, M.; Black, S.M.; Stenmark, K.R.; Nozik-Grayck, E. Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PLoS ONE 2011, 6, e27531. [Google Scholar] [CrossRef]
- Coates, D.E.; Zafar, S.; Milne, T.J. Quantitative Real-Time Gene Profiling of Human Alveolar Osteoblasts. Methods Mol. Biol. 2017, 1537, 447–459. [Google Scholar] [CrossRef]
- Farrow, K.N.; Lakshminrusimha, S.; Reda, W.J.; Wedgwood, S.; Czech, L.; Gugino, S.F.; Davis, J.M.; Russell, J.A.; Steinhorn, R.H. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L979–L987. [Google Scholar] [CrossRef]
- Van Rheen, Z.; Fattman, C.; Domarski, S.; Majka, S.; Klemm, D.; Stenmark, K.R.; Nozik-Grayck, E. Lung extracellular superoxide dismutase overexpression lessens bleomycin-induced pulmonary hypertension and vascular remodeling. Am. J. Respir. Cell Mol. Biol. 2011, 44, 500–508. [Google Scholar] [CrossRef]
- Sentman, M.L.; Granstrom, M.; Jakobson, H.; Reaume, A.; Basu, S.; Marklund, S.L. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J. Biol. Chem. 2006, 281, 6904–6909. [Google Scholar] [CrossRef]
- Nozik-Grayck, E.; Suliman, H.B.; Majka, S.; Albietz, J.; Van Rheen, Z.; Roush, K.; Stenmark, K.R. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L422–L430. [Google Scholar] [CrossRef]
- Kamezaki, F.; Tasaki, H.; Yamashita, K.; Tsutsui, M.; Koide, S.; Nakata, S.; Tanimoto, A.; Okazaki, M.; Sasaguri, Y.; Adachi, T.; et al. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2008, 177, 219–226. [Google Scholar] [CrossRef]
- Xu, D.; Guo, H.; Xu, X.; Lu, Z.; Fassett, J.; Hu, X.; Xu, Y.; Tang, Q.; Hu, D.; Somani, A.; et al. Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension 2011, 58, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, N.Y.; Moonen, J.R.; Zhao, Z.; Shi, M.; Otsuki, S.; Wang, L.; Nguyen, T.; Yan, E.; Marciano, D.P.; et al. ALDH1A3 Coordinates Metabolism With Gene Regulation in Pulmonary Arterial Hypertension. Circulation 2021, 143, 2074–2090. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, L.A.; Sham, J.S.; Liu, Q.; Sylvester, J.T. Acute and chronic hypoxic pulmonary vasoconstriction: A central role for endothelin-1? Respir. Physiol. Neurobiol. 2002, 132, 93–106. [Google Scholar] [CrossRef]
- Undem, C.; Rios, E.J.; Maylor, J.; Shimoda, L.A. Endothelin-1 augments Na+/H+ exchange activity in murine pulmonary arterial smooth muscle cells via Rho kinase. PLoS ONE 2012, 7, e46303. [Google Scholar] [CrossRef] [PubMed]
- Whitman, E.M.; Pisarcik, S.; Luke, T.; Fallon, M.; Wang, J.; Sylvester, J.T.; Semenza, G.L.; Shimoda, L.A. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L309–L318. [Google Scholar] [CrossRef]
- Davie, N.J.; Schermuly, R.T.; Weissmann, N.; Grimminger, F.; Ghofrani, H.A. The science of endothelin-1 and endothelin receptor antagonists in the management of pulmonary arterial hypertension: Current understanding and future studies. Eur. J. Clin. Invest. 2009, 39 (Suppl. S2), 38–49. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Rich, S. Primary pulmonary hypertension: A vascular biology and translational research “Work in progress”. Circulation 2000, 102, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, S.; Nakahata, N.; Ohizumi, Y. Thromboxane A2-mediated shape change: Independent of Gq-phospholipase C-Ca2+ pathway in rabbit platelets. Br. J. Pharmacol. 1996, 117, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Cogolludo, A.; Moreno, L.; Bosca, L.; Tamargo, J.; Perez-Vizcaino, F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: Role of protein kinase Czeta. Circ. Res. 2003, 93, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.X.; Aldinger, A.M.; Juhaszova, M.; Wang, J.; Conte, J.V., Jr.; Gaine, S.P.; Orens, J.B.; Rubin, L.J. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation 1998, 98, 1400–1406. [Google Scholar] [CrossRef]
- Shimoda, L.A.; Polak, J. Hypoxia. 4. Hypoxia and ion channel function. Am. J. Physiol. Cell Physiol. 2011, 300, C951–C967. [Google Scholar] [CrossRef]
- Burg, E.D.; Remillard, C.V.; Yuan, J.X. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: Pharmacotherapeutic implications. Br. J. Pharmacol. 2008, 153 (Suppl. S1), S99–S111. [Google Scholar] [CrossRef]
- Michelakis, E.D.; McMurtry, M.S.; Wu, X.C.; Dyck, J.R.; Moudgil, R.; Hopkins, T.A.; Lopaschuk, G.D.; Puttagunta, L.; Waite, R.; Archer, S.L. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: Role of increased expression and activity of voltage-gated potassium channels. Circulation 2002, 105, 244–250. [Google Scholar] [CrossRef]
- Bonnet, S.; Rochefort, G.; Sutendra, G.; Archer, S.L.; Haromy, A.; Webster, L.; Hashimoto, K.; Bonnet, S.N.; Michelakis, E.D. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc. Natl. Acad. Sci. USA 2007, 104, 11418–11423. [Google Scholar] [CrossRef]
- Pozeg, Z.I.; Michelakis, E.D.; McMurtry, M.S.; Thebaud, B.; Wu, X.C.; Dyck, J.R.; Hashimoto, K.; Wang, S.; Moudgil, R.; Harry, G.; et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 2003, 107, 2037–2044. [Google Scholar] [CrossRef]
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994, 368, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Fisslthaler, B.; Popp, R.; Kiss, L.; Potente, M.; Harder, D.R.; Fleming, I.; Busse, R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 1999, 401, 493–497. [Google Scholar] [CrossRef]
- Luke, T.; Maylor, J.; Undem, C.; Sylvester, J.T.; Shimoda, L.A. Kinase-dependent activation of voltage-gated Ca2+ channels by ET-1 in pulmonary arterial myocytes during chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1128–L1139. [Google Scholar] [CrossRef] [PubMed]
- Sacks, R.S.; Firth, A.L.; Remillard, C.V.; Agange, N.; Yau, J.; Ko, E.A.; Yuan, J.X. Thrombin-mediated increases in cytosolic [Ca2+] involve different mechanisms in human pulmonary artery smooth muscle and endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L1048–L1055. [Google Scholar] [CrossRef]
- Quayle, J.M.; McCarron, J.G.; Brayden, J.E.; Nelson, M.T. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am. J. Physiol. 1993, 265, C1363–C1370. [Google Scholar] [CrossRef]
- Zuo, X.; Zong, F.; Wang, H.; Wang, Q.; Xie, W.; Wang, H. Iptakalim, a novel ATP-sensitive potassium channel opener, inhibits pulmonary arterial smooth muscle cell proliferation by downregulation of PKC-alpha. J. Biomed. Res. 2011, 25, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.E.; Brayden, J.E. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J. Physiol. 1995, 486 Pt 1, 47–58. [Google Scholar] [CrossRef]
- Kim, D.; Gnatenco, C. TASK-5, a new member of the tandem-pore K+ channel family. Biochem. Biophys. Res. Commun. 2001, 284, 923–930. [Google Scholar] [CrossRef]
- Olschewski, A.; Li, Y.; Tang, B.; Hanze, J.; Eul, B.; Bohle, R.M.; Wilhelm, J.; Morty, R.E.; Brau, M.E.; Weir, E.K.; et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ. Res. 2006, 98, 1072–1080. [Google Scholar] [CrossRef]
- Huetsch, J.C.; Walker, J.; Undem, C.; Lade, J.; Yun, X.; Baksh, S.; Jiang, H.; Lai, N.; Shimoda, L.A. Rho kinase and Na+/H+ exchanger mediate endothelin-1-induced pulmonary arterial smooth muscle cell proliferation and migration. Physiol. Rep. 2018, 6, e13698. [Google Scholar] [CrossRef]
- Quinn, D.A.; Du, H.K.; Thompson, B.T.; Hales, C.A. Amiloride analogs inhibit chronic hypoxic pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1998, 157, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Antoniu, S.A. PDE5 inhibitors for cystic fibrosis: Can they also enhance chloride transport? Evaluation of: Lubamba B, Lecourt H, Lebacq J; et al. Preclinical evidence that sildenafil and vardenafil activate chloride transport in cystic fibrosis. Am J Respir Crit Care Med 2008;177(5):506–15. Expert Opin. Investig. Drugs 2008, 17, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hales, C.A. Silencing of sodium-hydrogen exchanger 1 attenuates the proliferation, hypertrophy, and migration of pulmonary artery smooth muscle cells via E2F1. Am. J. Respir. Cell Mol. Biol. 2011, 45, 923–930. [Google Scholar] [CrossRef]
- Yu, A.Y.; Shimoda, L.A.; Iyer, N.V.; Huso, D.L.; Sun, X.; McWilliams, R.; Beaty, T.; Sham, J.S.; Wiener, C.M.; Sylvester, J.T.; et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J. Clin. Investig. 1999, 103, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, L.A.; Semenza, G.L. HIF and the lung: Role of hypoxia-inducible factors in pulmonary development and disease. Am. J. Respir. Crit. Care Med. 2011, 183, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, L.A. 55th Bowditch Lecture: Effects of chronic hypoxia on the pulmonary circulation: Role of HIF-1. J. Appl. Physiol. 2012, 113, 1343–1352. [Google Scholar] [CrossRef]
- Lyle, M.A.; Davis, J.P.; Brozovich, F.V. Regulation of Pulmonary Vascular Smooth Muscle Contractility in Pulmonary Arterial Hypertension: Implications for Therapy. Front. Physiol. 2017, 8, 614. [Google Scholar] [CrossRef]
- He, H.; Chai, X.; Zhou, Y.; Pan, X.; Yang, G. Association of Lactate Dehydrogenase with In-Hospital Mortality in Patients with Acute Aortic Dissection: A Retrospective Observational Study. Int. J. Hypertens. 2020, 2020, 1347165. [Google Scholar] [CrossRef]
- Shi, Y.; Li, S.; Song, Y.; Liu, P.; Yang, Z.; Liu, Y.; Quan, K.; Yu, G.; Fan, Z.; Zhu, W. Nrf-2 signaling inhibits intracranial aneurysm formation and progression by modulating vascular smooth muscle cell phenotype and function. J. Neuroinflam. 2019, 16, 185. [Google Scholar] [CrossRef]
- Zhang, B.; Niu, W.; Xu, D.; Li, Y.; Liu, M.; Wang, Y.; Luo, Y.; Zhao, P.; Liu, Y.; Dong, M.; et al. Oxymatrine prevents hypoxia- and monocrotaline-induced pulmonary hypertension in rats. Free Radic. Biol. Med. 2014, 69, 198–207. [Google Scholar] [CrossRef]
- Cartaya, A.E.; Lutz, H.; Maiocchi, S.; Nalesnik, M.; Bahnson, E.M. Delivery of Cinnamic Aldehyde Antioxidant Response Activating nanoParticles (ARAPas) for Vascular Applications. Antioxidants 2021, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Levonen, A.L.; Inkala, M.; Heikura, T.; Jauhiainen, S.; Jyrkkanen, H.K.; Kansanen, E.; Maatta, K.; Romppanen, E.; Turunen, P.; Rutanen, J.; et al. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Shawky, N.M.; Pichavaram, P.; Shehatou, G.S.; Suddek, G.M.; Gameil, N.M.; Jun, J.Y.; Segar, L. Sulforaphane improves dysregulated metabolic profile and inhibits leptin-induced VSMC proliferation: Implications toward suppression of neointima formation after arterial injury in western diet-fed obese mice. J. Nutr. Biochem. 2016, 32, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.L.; Liu, J.T.; Kuo, H.F.; Chong, I.W.; Hsieh, C.C. Epigallocatechin gallate attenuates proliferation and oxidative stress in human vascular smooth muscle cells induced by interleukin-1beta via heme oxygenase-1. Mediat. Inflamm. 2014, 2014, 523684. [Google Scholar] [CrossRef]
- Buglak, N.E.; Jiang, W.; Bahnson, E.S.M. Cinnamic aldehyde inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in Zucker Diabetic Fatty rats. Redox Biol. 2018, 19, 166–178. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, M.; Zhao, L.; Li, A.; Qin, X. Activation of Nrf2 contributes to the protective effect of Exendin-4 against angiotensin II-induced vascular smooth muscle cell senescence. Am. J. Physiol. Cell Physiol. 2016, 311, C572–C582. [Google Scholar] [CrossRef]
- Qin, X.; Qiu, C.; Zhao, L. Maslinic acid protects vascular smooth muscle cells from oxidative stress through Akt/Nrf2/HO-1 pathway. Mol. Cell. Biochem. 2014, 390, 61–67. [Google Scholar] [CrossRef]
- He, X.; Deng, J.; Yu, X.J.; Yang, S.; Yang, Y.; Zang, W.J. Activation of M3AChR (Type 3 Muscarinic Acetylcholine Receptor) and Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) Signaling by Choline Alleviates Vascular Smooth Muscle Cell Phenotypic Switching and Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2649–2664. [Google Scholar] [CrossRef]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A., Jr. Naringenin Inhibits Superoxide Anion-Induced Inflammatory Pain: Role of Oxidative Stress, Cytokines, Nrf-2 and the NO-cGMP-PKG-KATP Channel Signaling Pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- Ko, E.; Kim, D.; Min, D.W.; Kwon, S.H.; Lee, J.Y. Nrf2 regulates cell motility through RhoA-ROCK1 signalling in non-small-cell lung cancer cells. Sci. Rep. 2021, 11, 1247. [Google Scholar] [CrossRef]
- Guan, P.; Liang, Y.; Wang, N. Fasudil alleviates pressure overload-induced heart failure by activating Nrf2-mediated antioxidant responses. J. Cell. Biochem. 2018, 119, 6452–6460. [Google Scholar] [CrossRef]
- Lambert, M.; Capuano, V.; Olschewski, A.; Sabourin, J.; Nagaraj, C.; Girerd, B.; Weatherald, J.; Humbert, M.; Antigny, F. Ion Channels in Pulmonary Hypertension: A Therapeutic Interest? Int. J. Mol. Sci. 2018, 19, 3162. [Google Scholar] [CrossRef] [PubMed]
- Konduri, G.G.; Bakhutashvili, I.; Eis, A.; Gauthier, K.M. Impaired voltage gated potassium channel responses in a fetal lamb model of persistent pulmonary hypertension of the newborn. Pediatr. Res. 2009, 66, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L. Potassium channel diversity in the pulmonary arteries and pulmonary veins: Implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol. Ther. 2007, 115, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Morecroft, I.; Murray, A.; Nilsen, M.; Gurney, A.M.; MacLean, M.R. Treatment with the Kv7 potassium channel activator flupirtine is beneficial in two independent mouse models of pulmonary hypertension. Br. J. Pharmacol. 2009, 157, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Pang, Y.S.; Zeng, M. Change of voltage-gate potassium channel in pulmonary arterial smooth muscle cells of pulmonary hypertension induced by left-to-right shunt in rats. Zhonghua Er Ke Za Zhi = Chin. J. Pediatr. 2011, 49, 901–904. [Google Scholar]
- Ishii, T.; Warabi, E.; Siow, R.C.M.; Mann, G.E. Sequestosome1/p62: A regulator of redox-sensitive voltage-activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth. Free Radic. Biol. Med. 2013, 65, 102–116. [Google Scholar] [CrossRef]
- Cornfield, D.N.; Resnik, E.R.; Herron, J.M.; Abman, S.H. Chronic intrauterine pulmonary hypertension decreases calcium-sensitive potassium channel mRNA expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L857–L862. [Google Scholar] [CrossRef]
- Sun, X.; Qian, L.L.; Li, Y.; Pfiefer, T.M.; Wang, X.L.; Lee, H.C.; Lu, T. Regulation of KCNMA1 transcription by Nrf2 in coronary arterial smooth muscle cells. J. Mol. Cell. Cardiol. 2020, 140, 68–76. [Google Scholar] [CrossRef]
- Antigny, F.; Hautefort, A.; Meloche, J.; Belacel-Ouari, M.; Manoury, B.; Rucker-Martin, C.; Pechoux, C.; Potus, F.; Nadeau, V.; Tremblay, E.; et al. Potassium Channel Subfamily K Member 3 (KCNK3) Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 2016, 133, 1371–1385. [Google Scholar] [CrossRef]
- Le Ribeuz, H.; Dumont, F.; Ruellou, G.; Lambert, M.; Balliau, T.; Quatredeniers, M.; Girerd, B.; Cohen-Kaminsky, S.; Mercier, O.; Yen-Nicolay, S.; et al. Proteomic Analysis of KCNK3 Loss of Expression Identified Dysregulated Pathways in Pulmonary Vascular Cells. Int. J. Mol. Sci. 2020, 21, 7400. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xie, W.P.; Wang, H. Hypoxic pulmonary hypertension and novel ATP-sensitive potassium channel opener: The new hope on the horizon. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chin. J. Appl. Physiol. 2012, 28, 510–523. [Google Scholar]
- Zhao, X.J.; Zhao, Z.; Yang, D.D.; Cao, L.L.; Zhang, L.; Ji, J.; Gu, J.; Huang, J.Y.; Sun, X.L. Activation of ATP-sensitive potassium channel by iptakalim normalizes stress-induced HPA axis disorder and depressive behaviour by alleviating inflammation and oxidative stress in mouse hypothalamus. Brain Res. Bull. 2017, 130, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, G.; Huang, E.C.; Huang, J.; Cai, J.; Cai, L.; Wang, S.; Keller, B.B. Sulforaphane prevents right ventricular injury and reduces pulmonary vascular remodeling in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H853–H866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Kang, Y.; Cathey, D.; LeBlanc, A.J.; Cai, J.; Cai, L.; Wang, S.; Huang, J.; Keller, B.B. Sulforaphane Does Not Protect Right Ventricular Systolic and Diastolic Functions in Nrf2 Knockout Pulmonary Artery Hypertension Mice. Cardiovasc. Drugs Ther. 2022, 36, 425–436. [Google Scholar] [CrossRef]
- Eba, S.; Hoshikawa, Y.; Moriguchi, T.; Mitsuishi, Y.; Satoh, H.; Ishida, K.; Watanabe, T.; Shimizu, T.; Shimokawa, H.; Okada, Y.; et al. The nuclear factor erythroid 2-related factor 2 activator oltipraz attenuates chronic hypoxia-induced cardiopulmonary alterations in mice. Am. J. Respir. Cell Mol. Biol. 2013, 49, 324–333. [Google Scholar] [CrossRef]
- Paffett, M.L.; Lucas, S.N.; Campen, M.J. Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: A potential role for atrogin-1 in smooth muscle. Vasc. Pharmacol. 2012, 56, 64–73. [Google Scholar] [CrossRef]
- Yang, D.L.; Zhang, H.G.; Xu, Y.L.; Gao, Y.H.; Yang, X.J.; Hao, X.Q.; Li, X.H. Resveratrol inhibits right ventricular hypertrophy induced by monocrotaline in rats. Clin. Exp. Pharmacol. Physiol. 2010, 37, 150–155. [Google Scholar] [CrossRef]
- Wilson, D.N.; Schacht, S.E.; Al-Nakkash, L.; Babu, J.R.; Broderick, T.L. Resveratrol prevents pulmonary trunk remodeling but not right ventricular hypertrophy in monocrotaline-induced pulmonary hypertension. Pathophysiology 2016, 23, 243–250. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Olson, S.; Pinto, J.T.; Gupte, S.; Wu, J.M.; Hu, F.; Ballabh, P.; Podlutsky, A.; Losonczy, G.; et al. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 2009, 54, 668–675. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Zhang, B.; Wang, Y.; Liu, Y.; Luo, Y.; Niu, W.; Dong, M.; Liu, M.; Dong, H.; et al. Resveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in rats. Int. J. Med. Sci. 2016, 13, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, G.; Long, J.; Xiao, P.; Zeng, X.; Yang, H. Protective effects of resveratrol and SR1001 on hypoxia-induced pulmonary hypertension in rats. Clin. Exp. Hypertens. 2020, 42, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xue, J.; Meng, X.; Slutzky, J.L.; Calvert, A.E.; Chicoine, L.G. Resveratrol prevents hypoxia-induced arginase II expression and proliferation of human pulmonary artery smooth muscle cells via Akt-dependent signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L317–L325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Zhang, W.Y.; Wang, C.G.; Huang, J.A.; Jiang, J.H.; Zeng, D.X. Resveratrol prevented experimental pulmonary vascular remodeling via miR-638 regulating NR4A3/cyclin D1 pathway. Microvasc. Res. 2020, 130, 103988. [Google Scholar] [CrossRef]
- Shi, W.; Zhai, C.; Feng, W.; Wang, J.; Zhu, Y.; Li, S.; Wang, Q.; Zhang, Q.; Yan, X.; Chai, L.; et al. Resveratrol inhibits monocrotaline-induced pulmonary arterial remodeling by suppression of SphK1-mediated NF-kappaB activation. Life Sci. 2018, 210, 140–149. [Google Scholar] [CrossRef]
- Gosemann, J.H.; Friedmacher, F.; Hofmann, A.; Zimmer, J.; Kuebler, J.F.; Rittinghausen, S.; Suttkus, A.; Lacher, M.; Alvarez, L.; Corcionivoschi, N.; et al. Prenatal treatment with rosiglitazone attenuates vascular remodeling and pulmonary monocyte influx in experimental congenital diaphragmatic hernia. PLoS ONE 2018, 13, e0206975. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.Y.; Huang, Y.; Wang, N. Rosiglitazone Attenuated Endothelin-1-Induced Vasoconstriction of Pulmonary Arteries in the Rat Model of Pulmonary Arterial Hypertension via Differential Regulation of ET-1 Receptors. PPAR Res. 2014, 2014, 374075. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Lee, J.H.; Oh, Y.M.; Lee, Y.S.; Lee, S.D. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology 2010, 15, 659–668. [Google Scholar] [CrossRef]
- Nisbet, R.E.; Bland, J.M.; Kleinhenz, D.J.; Mitchell, P.O.; Walp, E.R.; Sutliff, R.L.; Hart, C.M. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am. J. Respir. Cell Mol. Biol. 2010, 42, 482–490. [Google Scholar] [CrossRef]
- Wang, X.F.; Lu, W.X.; Guo, J.; Li, G.; Zhang, Y.J. Protective effects of rosiglitazone intervention on monocrotaline-induced pulmonary arterial hypertension in rats and related inflammatory mechanisms. Zhonghua Yi Xue Za Zhi 2012, 92, 2144–2147. [Google Scholar]
- Crossno, J.T., Jr.; Garat, C.V.; Reusch, J.E.; Morris, K.G.; Dempsey, E.C.; McMurtry, I.F.; Stenmark, K.R.; Klemm, D.J. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L885–L897. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewska, A.P.; Seta, F.; Han, R.; Czajka, C.A.; Makino, K.; Stawski, L.; Isenberg, J.S.; Browning, J.L.; Trojanowska, M. Dimethyl Fumarate ameliorates pulmonary arterial hypertension and lung fibrosis by targeting multiple pathways. Sci. Rep. 2017, 7, 41605. [Google Scholar] [CrossRef] [PubMed]
- Shellenberger, N.W.; Collinsworth, K.K.; Subbiah, S.; Klein, D.; Neary, J.M. Hypoxia induces an increase in intestinal permeability and pulmonary arterial pressures in neonatal Holstein calves despite feeding the flavonoid rutin. J. Dairy Sci. 2020, 103, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, W.; Zhu, F.; Wang, Q.; Yang, H.; Wu, J. Curcumin Improves Pulmonary Hypertension Rats by Regulating Mitochondrial Function. Biomed. Res. Int. 2021, 2021, 1078019. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, L.X.; Chen, S.X.; Zhou, X.F.; Huang, X.Y.; Fan, X.F. Effect of curcumin on pulmonary hypertension and wall collagen of pulmonary arterioles of chronic hypoxic hypercapnic rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2006, 22, 257–261. [Google Scholar]
- Li, J.W.; Chen, P.; Guan, X.Q.; Gong, Y.S.; Yang, P.L. Inhibition of puerarin on pulmonary hypertension in rats with hypoxia and hypercapnia. Zhongguo Zhong Yao Za Zhi 2008, 33, 544–549. [Google Scholar] [PubMed]
- Zhang, X.D.; Du, W.; Zhang, C.; Wang, S.J.; Sheng, J.J.; Li, Y.; Si, G.L.; Zhu, D.L. Effect of puerarin on hypoxic pulmonary hypertension and accompanying pulmonary fibrosi. Zhongguo Zhong Yao Za Zhi 2018, 43, 2618–2623. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Zhang, C.; Sheng, J.; Li, S.; Li, W.; Yang, X.; Wang, X.; He, S.; Bai, J.; et al. Puerarin prevents progression of experimental hypoxia-induced pulmonary hypertension via inhibition of autophagy. J. Pharmacol. Sci. 2019, 141, 97–105. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.F.; Yuan, T.Y.; Sun, S.C.; Wang, R.R.; Wang, S.B.; Fang, L.H.; Lyu, Y.; Du, G.H. Puerarin-V prevents the progression of hypoxia- and monocrotaline-induced pulmonary hypertension in rodent models. Acta Pharmacol. Sin. 2022, 43, 2325–2339. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Obaid, A.A.; Zaki, H.F.; Agha, A.M. Naringenin adds to the protective effect of L-arginine in monocrotaline-induced pulmonary hypertension in rats: Favorable modulation of oxidative stress, inflammation and nitric oxide. Eur. J. Pharm. Sci. 2014, 62, 161–170. [Google Scholar] [CrossRef]
- Wande, Y.; Jie, L.; Aikai, Z.; Yaguo, Z.; Linlin, Z.; Yue, G.; Hang, Z. Berberine alleviates pulmonary hypertension through Trx1 and beta-catenin signaling pathways in pulmonary artery smooth muscle cells. Exp. Cell Res. 2020, 390, 111910. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Kan, J.; Zhang, J.; Ye, P.; Wang, D.; Jiang, X.; Li, M.; Zhu, L.; Gu, Y. Bioactive Compounds From Coptidis Rhizoma Alleviate Pulmonary Arterial Hypertension by Inhibiting Pulmonary Artery Smooth Muscle Cells’ Proliferation and Migration. J. Cardiovasc. Pharmacol. 2021, 78, 253–262. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Sun, Y.; Li, G. Tanshinone IIA alleviates monocrotaline-induced pulmonary hypertension in rats through the PI3K/Akt-eNOS signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, Y.; Chen, X.; Zhang, J.; Lu, W.; Wang, J. Tanshinone IIA sulfonate upregulated pulmonary artery smooth muscle peroxisome proliferator-activated receptor γ expression in monocrotaline induced pulmonary hypertension rat. Zhonghua Jie He He Hu Xi Za Zhi 2014, 37, 360–364. [Google Scholar] [PubMed]
- Zhang, N.; Dong, M.; Luo, Y.; Zhao, F.; Li, Y. Danshensu prevents hypoxic pulmonary hypertension in rats by inhibiting the proliferation of pulmonary artery smooth muscle cells via TGF-β-smad3-associated pathway. Eur. J. Pharmacol. 2018, 820, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Lu, W.; Luo, X.; Lin, Y.; Liu, S.; Qian, J.; Zhang, C.; Chen, H.; Li, Y.; et al. Sodium tanshinone IIA sulfonate enhances the BMP9-BMPR2-Smad1/5/9 signaling pathway in rat pulmonary microvascular endothelial cells and human embryonic stem cell-derived endothelial cells. Biochem. Pharmacol. 2022, 199, 114986. [Google Scholar] [CrossRef]
- Jiang, Q.; Lu, W.; Yang, K.; Hadadi, C.; Fu, X.; Chen, Y.; Yun, X.; Zhang, J.; Li, M.; Xu, L.; et al. Sodium tanshinone IIA sulfonate inhibits hypoxia-induced enhancement of SOCE in pulmonary arterial smooth muscle cells via the PKG-PPAR-gamma signaling axis. Am. J. Physiol. Cell Physiol. 2016, 311, C136–C149. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Q.; Wan, L.; Yang, K.; Zhang, Y.; Chen, Y.; Wang, E.; Lai, N.; Zhao, L.; Jiang, H.; et al. Sodium tanshinone IIA sulfonate inhibits canonical transient receptor potential expression in pulmonary arterial smooth muscle from pulmonary hypertensive rats. Am. J. Respir. Cell Mol. Biol. 2013, 48, 125–134. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, M.; Wei, M.; Liu, Y.; Dong, M.; Luo, Y.; Zhao, P.; Dong, H.; Niu, W.; Yan, Z.; et al. Tanshinone IIA attenuates hypoxic pulmonary hypertension via modulating KV currents. Respir. Physiol. Neurobiol. 2015, 205, 120–128. [Google Scholar] [CrossRef]
- Jabbarzadeh Kaboli, P.; Afzalipour Khoshkbejari, M.; Mohammadi, M.; Abiri, A.; Mokhtarian, R.; Vazifemand, R.; Amanollahi, S.; Yazdi Sani, S.; Li, M.; Zhao, Y.; et al. Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer-contradictory effects and future perspectives. Biomed. Pharmacother. = Biomed. Pharmacother. 2020, 121, 109635. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxidative Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef]
- Cho, H.Y.; Miller-DeGraff, L.; Blankenship-Paris, T.; Wang, X.; Bell, D.A.; Lih, F.; Deterding, L.; Panduri, V.; Morgan, D.L.; Yamamoto, M.; et al. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol. Appl. Pharmacol. 2019, 364, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tao, S.; Lian, F.; Chau, B.T.; Chen, J.; Sun, G.; Fang, D.; Lantz, R.C.; Zhang, D.D. Sulforaphane prevents pulmonary damage in response to inhaled arsenic by activating the Nrf2-defense response. Toxicol. Appl. Pharmacol. 2012, 265, 292–299. [Google Scholar] [CrossRef]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef]
- Jiang, Z.; Bian, M.; Wu, J.; Li, D.; Ding, L.; Zeng, Q. Oltipraz Prevents High Glucose-Induced Oxidative Stress and Apoptosis in RSC96 Cells through the Nrf2/NQO1 Signalling Pathway. BioMed Res. Int. 2020, 2020, 5939815. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.M.; Lee, J.M.; Kim, S.G. Antioxidant and mitochondrial protective effects of oxidized metabolites of oltipraz. Expert. Opin. Drug Metab. Toxicol. 2010, 6, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Javkhedkar, A.A.; Quiroz, Y.; Rodriguez-Iturbe, B.; Vaziri, N.D.; Lokhandwala, M.F.; Banday, A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R840–R846. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Roufogalis, B.D.; Banach, M.; Barati, M.; Sahebkar, A. Resveratrol: Mechanistic and therapeutic perspectives in pulmonary arterial hypertension. Pharmacol. Res. 2021, 163, 105287. [Google Scholar] [CrossRef] [PubMed]
- Mingfeng, D.; Xiaodong, M.; Yue, L.; Taikui, P.; Lei, X.; Ming, L. Effects of PPAR-gamma agonist treatment on LPS-induced mastitis in rats. Inflammation 2014, 37, 1919–1924. [Google Scholar] [CrossRef]
- Xin, G.L.L.; Khee, Y.P.; Ying, T.Y.; Chellian, J.; Gupta, G.; Kunnath, A.P.; Nammi, S.; Collet, T.; Hansbro, P.M.; Dua, K.; et al. Current Status on Immunological Therapies for Type 1 Diabetes Mellitus. Curr. Diabetes Rep. 2019, 19, 22. [Google Scholar] [CrossRef]
- Rashid, J.; Alobaida, A.; Al-Hilal, T.A.; Hammouda, S.; McMurtry, I.F.; Nozik-Grayck, E.; Stenmark, K.R.; Ahsan, F. Repurposing rosiglitazone, a PPAR-gamma agonist and oral antidiabetic, as an inhaled formulation, for the treatment of PAH. J. Control Release 2018, 280, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.; Nozik-Grayck, E.; McMurtry, I.F.; Stenmark, K.R.; Ahsan, F. Inhaled combination of sildenafil and rosiglitazone improves pulmonary hemodynamics, cardiac function, and arterial remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L119–L130. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Trujillo, Y.; Rodriguez-Esparragon, F.; Macias-Reyes, A.; Caballero-Hidalgo, A.; Rodriguez-Perez, J.C. Rosiglitazone but not losartan prevents Nrf-2 dependent CD36 gene expression up-regulation in an in vivo atherosclerosis model. Cardiovasc. Diabetol. 2008, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.L.; Yang, C.C.; Tseng, H.C.; Hsiao, L.D.; Lin, C.C.; Yang, C.M. Haem oxygenase-1 up-regulation by rosiglitazone via ROS-dependent Nrf2-antioxidant response elements axis or PPARgamma attenuates LPS-mediated lung inflammation. Br. J. Pharmacol. 2018, 175, 3928–3946. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, L.; Qu, Y.; Wang, D.; Zhu, Y.; Zhu, Y. Rosiglitazone Prevents Autophagy by Regulating Nrf2-Antioxidant Response Element in a Rat Model of Lithium-pilocarpine-induced Status Epilepticus. Neuroscience 2021, 455, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Kugler, S.; Lastres-Becker, I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018, 14, 522–534. [Google Scholar] [CrossRef]
- Pascale, C.L.; Martinez, A.N.; Carr, C.; Sawyer, D.M.; Ribeiro-Alves, M.; Chen, M.; O’Donnell, D.B.; Guidry, J.J.; Amenta, P.S.; Dumont, A.S. Treatment with dimethyl fumarate reduces the formation and rupture of intracranial aneurysms: Role of Nrf2 activation. J. Cereb. Blood Flow. Metab. 2020, 40, 1077–1089. [Google Scholar] [CrossRef]
- Muralidharan, P.; Hayes, D., Jr.; Black, S.M.; Mansour, H.M. Microparticulate/Nanoparticulate Powders of a Novel Nrf2 Activator and an Aerosol Performance Enhancer for Pulmonary Delivery Targeting the Lung Nrf2/Keap-1 Pathway. Mol. Syst. Des. Eng. 2016, 1, 48–65. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; El-Harbi, K.M.; Khoshhal, S.; Ahmed, N.; Elkablawy, M.A.; Shaaban, A.A.; Abo-Haded, H.M. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways. Cancer Manag. Res. 2019, 11, 47–61. [Google Scholar] [CrossRef]
- Verma, S.; Kalita, B.; Bajaj, S.; Prakash, H.; Singh, A.K.; Gupta, M.L. A Combination of Podophyllotoxin and Rutin Alleviates Radiation-Induced Pneumonitis and Fibrosis through Modulation of Lung Inflammation in Mice. Front. Immunol. 2017, 8, 658. [Google Scholar] [CrossRef]
- Bai, L.; Li, A.; Gong, C.; Ning, X.; Wang, Z. Protective effect of rutin against bleomycin induced lung fibrosis: Involvement of TGF-beta1/alpha-SMA/Col I and III pathway. Biofactors 2020, 46, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Tosun, M.; Olmez, H.; Unver, E.; Arslan, Y.K.; Cimen, F.K.; Ozcicek, A.; Aktas, M.; Suleyman, H. Oxidative and pro-inflammatory lung injury induced by desflurane inhalation in rats and the protective effect of rutin. Adv. Clin. Exp. Med. 2021, 30, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Niu, S.; Wang, R.; Li, Y.; Zhang, R.; Zhu, D. Mechanisms that underlie the induction of vasodilation in pulmonary artery by rutin. Int. Angiol. 2012, 31, 557–564. [Google Scholar] [PubMed]
- Li, Q.; Qiu, Y.; Mao, M.; Lv, J.; Zhang, L.; Li, S.; Li, X.; Zheng, X. Antioxidant mechanism of Rutin on hypoxia-induced pulmonary arterial cell proliferation. Molecules 2014, 19, 19036–19049. [Google Scholar] [CrossRef] [PubMed]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, T.; Long, M.; Chen, J.; Ren, D.M.; Wong, P.K.; Chapman, E.; Zhou, B.; Zhang, D.D. A Curcumin Derivative That Inhibits Vinyl Carbamate-Induced Lung Carcinogenesis via Activation of the Nrf2 Protective Response. Antioxid. Redox Signal. 2015, 23, 651–664. [Google Scholar] [CrossRef]
- Devadasu, V.R.; Wadsworth, R.M.; Ravi Kumar, M.N. Tissue localization of nanoparticles is altered due to hypoxia resulting in poor efficacy of curcumin nanoparticles in pulmonary hypertension. Eur. J. Pharm. Biopharm. 2012, 80, 578–584. [Google Scholar] [CrossRef]
- Kruangtip, O.; Chootip, K.; Temkitthawon, P.; Changwichit, K.; Chuprajob, T.; Changtam, C.; Suksamrarn, A.; Khorana, N.; Scholfield, C.N.; Ingkaninan, K. Curcumin analogues inhibit phosphodiesterase-5 and dilate rat pulmonary arteries. J. Pharm. Pharmacol. 2015, 67, 87–95. [Google Scholar] [CrossRef]
- Wei, S.Y.; Chen, Y.; Xu, X.Y. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar] [CrossRef]
- Chen, X.; Huang, C.; Sun, H.; Hong, H.; Jin, J.; Bei, C.; Lu, Z.; Zhang, X. Puerarin suppresses inflammation and ECM degradation through Nrf2/HO-1 axis in chondrocytes and alleviates pain symptom in osteoarthritic mice. Food Funct. 2021, 12, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Aimaier, S.; Tao, Y.; Lei, F.; Yupeng, Z.; Wenhui, S.; Aikemu, A.; Maimaitiyiming, D. Protective effects of the Terminalia bellirica tannin-induced Nrf2/HO-1 signaling pathway in rats with high-altitude pulmonary hypertension. BMC Complement. Med. Ther. 2023, 23, 150. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Zhang, Q.; Hu, Q.; Liu, Y.; Zhang, L.; Liu, R. Tannins in Terminalia bellirica inhibits hepatocellular carcinoma growth via re-educating tumor-associated macrophages and restoring CD8+T cell function. Biomed. Pharmacother. 2022, 154, 113543. [Google Scholar] [CrossRef]
- Feng, J.; Luo, J.; Deng, L.; Zhong, Y.; Wen, X.; Cai, Y.; Li, J. Naringenin-induced HO-1 ameliorates high glucose or free fatty acids-associated apoptosis via PI3K and JNK/Nrf2 pathways in human umbilical vein endothelial cells. Int. Immunopharmacol. 2019, 75, 105769. [Google Scholar] [CrossRef]
- Yang, X.J.; Liu, F.; Feng, N.; Ding, X.S.; Chen, Y.; Zhu, S.X.; Yang, L.C.; Feng, X.F. Berberine Attenuates Cholesterol Accumulation in Macrophage Foam Cells by Suppressing AP-1 Activity and Activation of the Nrf2/HO-1 Pathway. J. Cardiovasc. Pharmacol. 2020, 75, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Fekri, H.S.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Therapeutic and biological activities of berberine: The involvement of Nrf2 signaling pathway. J. Cell. Biochem. 2020, 121, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Wang, S.Q. Nrf2 is involved in the effect of tanshinone IIA on intracellular redox status in human aortic smooth muscle cells. Biochem. Pharmacol. 2007, 73, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, M.Q.; Liu, M.L.; Xu, D.Q.; Luo, Y.; Zhang, B.; Liu, L.L.; Xu, M.; Zhao, P.T.; Gao, Y.Q.; et al. Tanshinone IIA modulates pulmonary vascular response to agonist and hypoxia primarily via inhibiting Ca2+ influx and release in normal and hypoxic pulmonary hypertension rats. Eur. J. Pharmacol. 2010, 640, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Clay, A.; Hearle, P.; Schadt, K.; Lynch, D.R. New developments in pharmacotherapy for Friedreich ataxia. Expert Opin. Pharmacother. 2019, 20, 1855–1867. [Google Scholar] [CrossRef]
| Function | Gene | Extended Name |
|---|---|---|
| CAT | catalase | |
| GCLC | glutamate-cysteine ligase catalytic subunit | |
| GCLM | glutamate-cysteine ligase modifier subunit | |
| GGT1 | gamma-glutamyltransferase 1 | |
| GPX1 | glutathione peroxidase 1 | |
| GPX2 | glutathione peroxidase 2 | |
| GPX4 | glutathione peroxidase 4 | |
| GSR1 | glutathione reductase | |
| Antioxidants | HO-1 | heme oxygenase 1 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 | |
| PRDX1 | peroxiredoxin 1 | |
| PRDX6 | peroxiredoxin 6 | |
| SLC7A11 | solute carrier family 7 member 11 | |
| SOD | superoxide dismutase | |
| SRXN1 | sulfiredoxin 1 | |
| TXN1 | thioredoxin 1 | |
| TXNRD1 | thioredoxin reductase 1 |
| Compound | Chemical Structure | Model | Treatment Strategy | Effects | References |
|---|---|---|---|---|---|
| SFN | 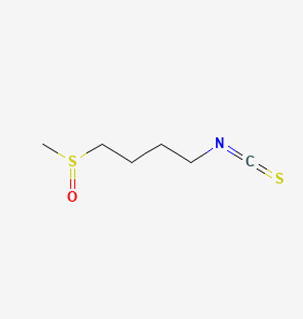 | SuHx in mice | SFN (0.5 mg/kg 5 days per week) for 28 days | Prevented SuHx-induced RV dysfunction and remodeling, reduced RV inflammation and fibrosis, reduced SuHx-induced pulmonary vascular remodeling, inflammation, and fibrosis. | [154] |
| global Nrf2-knockout mice, SuHx in mice | SFN (0.5 mg/kg 5 days per week) for 28 days | Partially or completely reversed SuHx-induced RV diastolic/systolic dysfunction and increased RV systolic pressure. | [155] | ||
| Oltipraz |  | Hypoxia mice | Oltipraz (5, 50, or 500 mg/kg/day) for 3 days | Decreased RVH and pulmonary vascular remodeling. | [156] |
| Resveratrol | 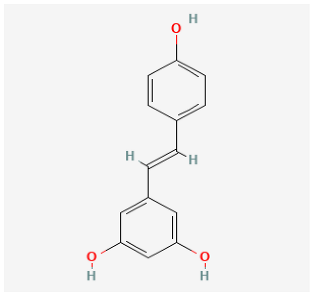 | MCT rat | resveratrol (3 mg/kg/day) for 14 days | Attenuated established MCT-induced PH indices, including right ventricular systolic pressure, right ventricular hypertrophy, and medial thickening of intrapulmonary. | [157] |
| MCT rat | resveratrol (10 and 30 mg/kg) twice daily for 21 days. | Attenuated RV hypertrophy, swollen mitochrondria and cardiomyocyte apoptosis. | [158] | ||
| MCT rat | resveratrol (25 mg/kg/day) for 21 days. | Reduced the thickness of the pulmonary trunk tunica media. | [159] | ||
| MCT rat | resveratrol (25 mg/kg/day) for 21 days | Exerted anti-inflammatory, antioxidant, and antiproliferative effects. | [160] | ||
| Hypoxia rat | resveratrol (40 mg/kg/day) for 28 days | Prevented pulmonary hypertension through its antiproliferation, anti-inflammation and antioxidant effects. | [161] | ||
| Hypoxia rat | resveratrol (40 mg/kg/day) for 21 days | Prevented pulmonary hypertension and RVH. | [162] | ||
| Hypoxia rat | resveratrol (100 mg/kg/day) for 14 days | Prevented proliferation of human pulmonary artery smooth muscle cells and RVH. | [163] | ||
| MCT rat | resveratrol (25 mg/kg/day) for 28 days | Prevented pulmonary vascular remodeling. | [164] | ||
| MCT rat | resveratrol (25 mg/kg/day) for 28 days | Inhibited pulmonary arterial remodeling. | [165] | ||
| Rosiglitazone |  | Pregnant rats were treated with nitrofen | Rosiglitazone (3 mg/kg/day) for 3 days | Reduced pulmonary vascular remodeling. | [166] |
| Hypoxia rat | rosiglitazone (20 mg/kg per day) with oral gavage for 3 days | Inhibited pulmonary artery vasoconstrictive. | [167] | ||
| Hypoxia rat | rosiglitazone (8 mg/kg orally, 5 days/week) for 28 days | Reduced chronic hypoxic pulmonary hypertension. | [168] | ||
| Hypoxia mice | rosiglitazone (10 mg/kg/d, gavage) for 35 days | Reduced pulmonary vascular remodeling and hypertension. | [169] | ||
| MCT rat | rosiglitazone (5, 2.5 mg/kg/day) for 21 days | Reduced perivascular inflammation. | [170] | ||
| Hypoxia rat | rosiglitazone (5 mg/kg/day) for 21 days | Attenuated hypoxia-induced pulmonary arterial remodeling. | [171] | ||
| DMF |  | Hypoxia mice | DMF (90 mg/kg/day) for 21 days | Reversed hemodynamic changes, reducing inflammation, oxidative damage, and fibrosis. | [172] |
| Rutin | 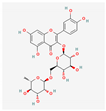 | Hypoxia calves | glucorhamnoside rutin orally administered for 14 days | Led to pulmonary arteriolar medial hypertrophy and adventitial hyperplasia. | [173] |
| Curcumin | 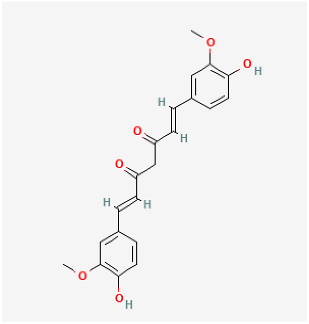 | MCT rat | curcumin (30 mg/kg/day) for 18 days | Improved pulmonary vascular remodeling, promote PASMC apoptosis, and protect mitochondrial function. | [174] |
| Hypoxia rat | curcumin (50 mg/kg/day) administrated for 28 days | Decreased pulmonary arterial pressure, improve pulmonary vessel remodeling and inhibit the deposition of collagen I in pulmonary arterioles. | [175] | ||
| Puerarin | 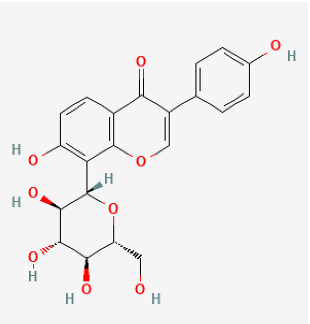 | Hypoxia rat | puerarin (20 mg/kg/day) for 21 days | Improved pulmonary vascular remodeling. | [176] |
| Hypoxia rat | puerarin intraperitoneal injection, 20 mg/kg/d for 21 days | Inhibition of vascular wall thickening pulmonary fibrosis. | [177] | ||
| Hypoxia rat | puerarin (80 mg/kg/day, orally) for 21 days | Reduced autophagy and suppressing cell proliferation. | [178] | ||
| MCT rat or Hypoxia mice | puerarin (10, 30, 100 mg/kg/d, i.g.) for 28 days or puerarin (60 mg/kg/d, i.g.) for 7 days | Reduced RVSP and lung injury, improved pulmonary artery blood flow, inhibit inflammatory responses, improved resistance to apoptosis and abnormal proliferation, attenuate right ventricular injury and remodeling, and maintained normal function of the right ventricle. | [179] | ||
| Naringenin |  | MCT rat | naringenin (50 mg/kg) were orally administered daily for 21 days | Alleviated oxidative stress, inflammatory and apoptotic markers. | [180] |
| Berberine |  | SuHx in rats | berberine (100 mg per kg day) for 28 days | Reversed right ventricular systolic pressure and right ventricular hypertrophy and decrease pulmonary vascular remodeling. | [181] |
| MCT rat | berberine (50 mg/kg/d) for 28 days | Inhibited pulmonary artery smooth muscle cells’ proliferation and migration. | [182] | ||
| Tanshinone IIA | 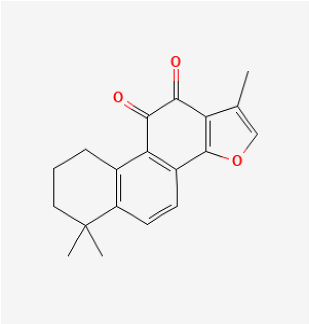 | MCT rat | tanshinone IIA (10 mg/kg) for 14 days | Inhibited pulmonary artery intimamedia thickening and muscularization of the pulmonary arterioles. | [183] |
| MCT rat | tanshinone IIA (30 mg/kg/d) for 21 days | Decreased RVSP, MRVP, RV/(LV+S) and pulmonary vascular remodeling. | [184] | ||
| Hypoxia rat | tanshinone IIA (160 mg/kg/d) for 28 days | Inhibited proliferation of pulmonary artery smooth muscle cells. | [185] | ||
| Hypoxia rat | tanshinone IIA (30 mg/kg/d) for 21 days | Normalized the RVSP and RVH, improved the cardiac output. | [186] | ||
| Hypoxia rat | tanshinone IIA (30 mg/kg/d) for 21 days | Prevented hypoxia-mediated increases in intracellular calcium homeostasis and cell proliferation. | [187] | ||
| MCT rat or Hypoxia mice | tanshinone IIA (10 mg/kg/d) for 21 days | Relieved RVSP and RVH. | [188] | ||
| Hypoxia rat | tanshinone IIA (10 mg/kg/d) for 28 days | Restrained pulmonary artery wall remodeling. | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Q.; Bai, Y.; Hu, S.; Ding, J.; Liu, L.; Dai, M.; Qiu, J.; Wu, L.; Rao, X.; Wang, Y. Unleashing the Potential of Nrf2: A Novel Therapeutic Target for Pulmonary Vascular Remodeling. Antioxidants 2023, 12, 1978. https://doi.org/10.3390/antiox12111978
Fang Q, Bai Y, Hu S, Ding J, Liu L, Dai M, Qiu J, Wu L, Rao X, Wang Y. Unleashing the Potential of Nrf2: A Novel Therapeutic Target for Pulmonary Vascular Remodeling. Antioxidants. 2023; 12(11):1978. https://doi.org/10.3390/antiox12111978
Chicago/Turabian StyleFang, Qin, Yang Bai, Shuiqing Hu, Jie Ding, Lei Liu, Meiyan Dai, Jie Qiu, Lujin Wu, Xiaoquan Rao, and Yan Wang. 2023. "Unleashing the Potential of Nrf2: A Novel Therapeutic Target for Pulmonary Vascular Remodeling" Antioxidants 12, no. 11: 1978. https://doi.org/10.3390/antiox12111978
APA StyleFang, Q., Bai, Y., Hu, S., Ding, J., Liu, L., Dai, M., Qiu, J., Wu, L., Rao, X., & Wang, Y. (2023). Unleashing the Potential of Nrf2: A Novel Therapeutic Target for Pulmonary Vascular Remodeling. Antioxidants, 12(11), 1978. https://doi.org/10.3390/antiox12111978







