Chemical Composition of Salvia fruticosa Mill. Essential Oil and Its Protective Effects on Both Photosynthetic Damage and Oxidative Stress in Conocephalum conicum L. Induced by Environmental Heavy Metal Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil Extraction
2.2. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis of Essential Oil
2.3. Conocephalum Conicum Material
2.4. In Vitro Growth
2.5. Treatment with the Total Extract of EO and the Individual Compounds
2.6. Detection of ROS and Antioxidant Enzymes’ Activity
2.7. Measurements of Chlorophyll Fluorescence
3. Results and Discussion
3.1. Chemical Profiling of Salvia Fruticosa EO
3.2. ROS Quantificaztion and Antioxidant Enzymes
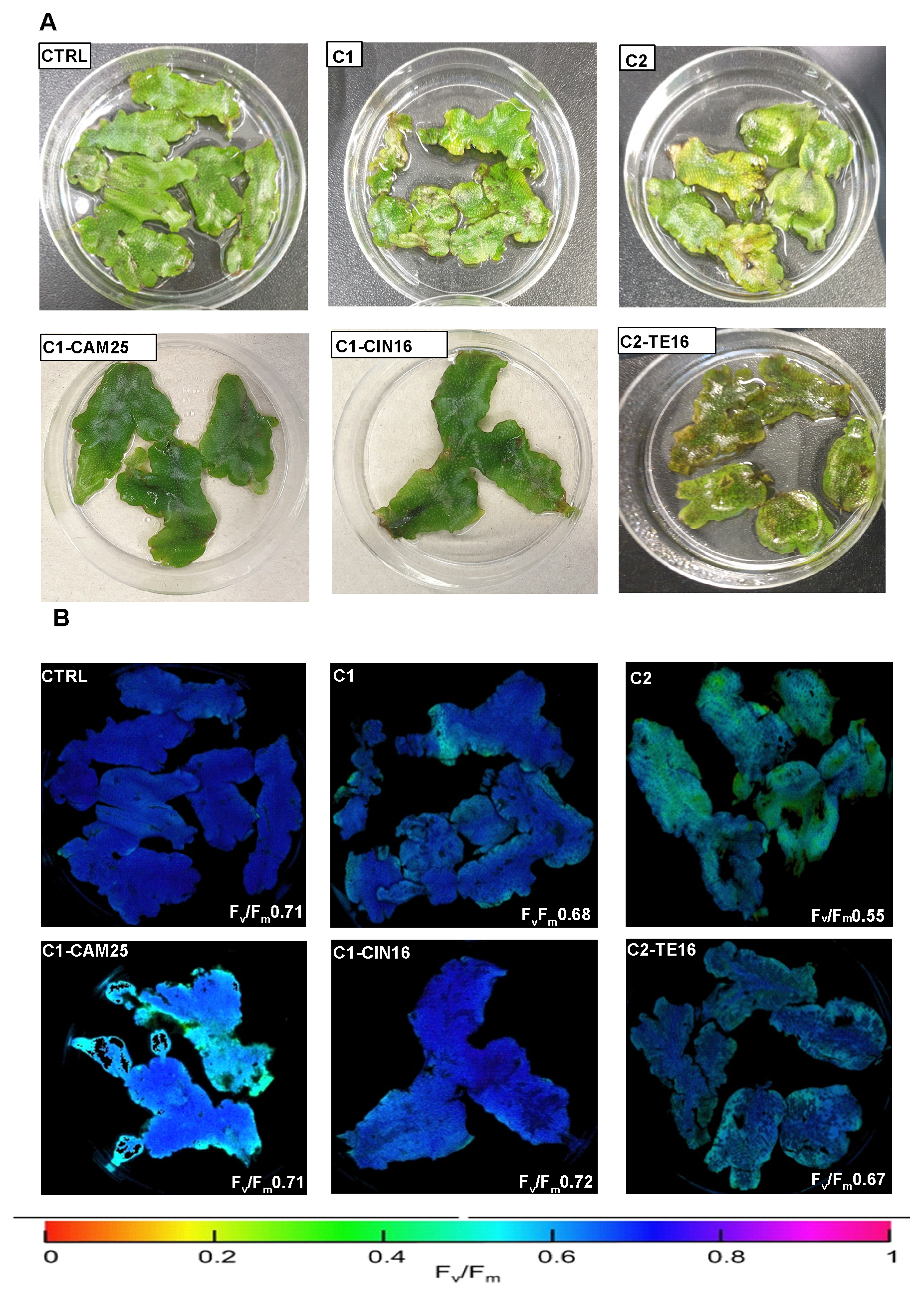
3.3. Measurements of Chlorophyll Fluorescence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.-B.; Ni, Z.-Y.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Constituents from Salvia Species and Their Biological Activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Mulas, M. Traditional Uses of Labiatae in The Mediterranean Area. Acta Hortic. 2006, 723, 25–32. [Google Scholar] [CrossRef]
- Ulubelen, A.; Birman, H.; Öksüz, S.; Topçu, G.; Kolak, U.; Barla, A.; Voelter, W. Cardioactive Diterpenes from the Roots of Salvia eriophora. Planta Med. 2002, 68, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Pazoki, A.; Akhlaghi, H. Chemical Composition of the Essential Oils from Flowers, Stems, and Roots of Salvia Multicaulis Growing Wild in Iran. Chem. Nat. Compd. 2008, 44, 127–128. [Google Scholar] [CrossRef]
- Guajardo Touche, E.M.; Loprz, E.G.; Reyes, A.P.; Sánchez, H.; Honecker, F.; Achenbach, H. Parryin, a Diterpene with a Tricyclic 6-7-5-Ring System from Salvia Parryi. Phytochemistry 1997, 45, 387–390. [Google Scholar] [CrossRef]
- Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/ (accessed on 13 September 2023).
- Rivera, D.; Obon, C.; Cano, F. The Botany, History and Traditional Uses of Three-Lobed Sage (Salvia fruticosa Miller) (Labiatae). Econ. Bot. 1994, 48, 190–195. [Google Scholar] [CrossRef]
- Yaniv, Z.; Dafni, A.; Palevitch, D. Labiatae as Medicinal Plants in Israel. In Aromatic Plants: Basic and Applied Aspects; Margaris, N., Koedam, A., Vokou, D., Eds.; World Crops: Production, Utilization, and Description ; Springer: Dordrecht, The Netherlands, 1982; pp. 265–269. ISBN 978-94-009-7642-9. [Google Scholar]
- Paleviteh, D.; Yaniv, Z.; Dafni, A.; Friedman, J. Medicinal Plants of Israel: An Ethnobotanical Survey. In Herbs, Spices, and Medicinal Plants; Craker, L., Simon, J., Eds.; Oryx Press: Phoenix, AZ, USA, 1986; pp. 281–345. [Google Scholar]
- Boulos, L. Medicinal Plants of North Africa; Reference Publications, Incorporated: Algonac, MI, USA, 1983; ISBN 978-0-917256-16-5. [Google Scholar]
- Karim, F.; Quraan, S. Medicinal Plants of Jordan; Centre for Jordanian Studies, Yarmouk University: Irbid, Jordan, 1986. [Google Scholar]
- Başer, K.H.C.; Honda, G.; Miki, W. Herb Drugs and Herbalists in Turkey; Institute for the Study of Languages and Cultures of Asia and Africa: Tokyo, Japan, 1986. [Google Scholar]
- Huxley, A.J.; Huxley, A.; Taylor, A.W. Flowers of Greece and the Aegean; Chatto & Windus: London, UK, 1977; ISBN 978-0-7011-2190-7. [Google Scholar]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H. Anticancer and Medicinal Properties of Essential Oil and Extracts of East Mediterranean Sage (Salvia triloba). In Advances in Phytomedicine; Khan, M.T.H., Ather, A., Eds.; Lead Molecules from Natural Products; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 169–180. [Google Scholar]
- Nikolova, M.; Aneva, I. European Species of Genus Salvia: Distribution, Chemodiversity and Biological Activity. In Salvia Biotechnology; Georgiev, V., Pavlov, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–30. ISBN 978-3-319-73900-7. [Google Scholar]
- Abdelhalim, A.; Chebib, M.; Aburjai, T.; Johnston, G.A.R.; Hanrahan, J.R. GABAA Receptor Modulation by Compounds Isolated from Salvia Triloba. ABC 2014, 04, 148–159. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A Review. Phytochem. 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Cvetkovikj, I.; Stefkov, G.; Acevska, J.; Stanoeva, J.P.; Karapandzova, M.; Stefova, M.; Dimitrovska, A.; Kulevanova, S. Polyphenolic Characterization and Chromatographic Methods for Fast Assessment of Culinary Salvia Species from South East Europe. J. Chromatogr. A 2013, 1282, 38–45. [Google Scholar] [CrossRef] [PubMed]
- De Feo, V.; Bruno, M.; Tahiri, B.; Napolitano, F.; Senatore, F. Chemical Composition and Antibacterial Activity of Essential Oils from Thymus spinulosus Ten. (Lamiaceae). J. Agric. Food Chem. 2003, 51, 3849–3853. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M.; Maggi, F.; Leporini, M.; Falco, T.; Loizzo, M.R.; Tundis, R. Ferulago nodosa Subsp. Geniculata (Guss.) Troia & Raimondo from Sicily (Italy): Isolation of Essential Oil and Evaluation of Its Bioactivity. Molecules 2020, 25, 3249. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Gagliano Candela, R.; Maggi, F. Chemical Composition of the Essential Oil of Elaeoselinum asclepium (L.) Bertol subsp. meoides (Desf.) Fiori (Umbelliferae) Collected Wild in Central Sicily and Its Antimicrobial Activity. Nat. Prod. Res. 2022, 36, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Badalamenti, N.; Ilardi, V.; Bruno, M.; Bontempo, P.; Basile, A. Chemical Composition of Thymus leucotrichus Var. Creticus Essential Oil and Its Protective Effects on Both Damage and Oxidative Stress in Leptodictyum riparium Hedw. Induced by Cadmium. Plants 2022, 11, 3529. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Maresca, V.; Varcamonti, M.; Bruno, M.; Badalamenti, N.; Basile, A.; Zanfardino, A. (+)-(E)-Chrysanthenyl Acetate: A Molecule with Interesting Biological Properties Contained in the Anthemis secundiramea (Asteraceae) Flowers. Appl. Sci. 2020, 10, 6808. [Google Scholar] [CrossRef]
- Lauricella, M.; Maggio, A.; Badalamenti, N.; Bruno, M.; D’Angelo, G.D.; D’Anneo, A. Essential Oil of Foeniculum vulgare Subsp. Piperitum Fruits Exerts an Anti-tumor Effect in Triple-negative Breast Cancer Cells. Mol. Med. Rep. 2022, 26, 243. [Google Scholar] [CrossRef]
- Cvetkoviky, I.; Stefkov, G.; Karapandzova, M.; Kulevanova, S. Essential Oil Composition of Salvia Fruticosa Mill. Populations from Balkan Peninsula. Macedon. Pharm. Bull. 2015, 61, 19–26. [Google Scholar] [CrossRef]
- Schmiderer, C.; Torres-Londoño, P.; Novak, J. Proof of Geographical Origin of Albanian Sage by Essential Oil Analysis. Biochem. Syst. Ecol. 2013, 51, 70–77. [Google Scholar] [CrossRef]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial Activity of the Essential Oils of Salvia officinalis L. and Salvia triloba L. Cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and Acetyl-Cholinesterase Inhibitory Activities in Essential Oils of Salvia Species and Their Constituents. Phytother. Res. 2004, 18, 315–324. [Google Scholar] [CrossRef]
- Bellomaria, B.; Arnold, N.; Valentini, G.; Arnold, H.J. Contribution to the Study of the Essential Oils from Three Species of Salvia Growing Wild in the Eastern Mediterranean Region. J. Essent. Oil Res. 1992, 4, 607–614. [Google Scholar] [CrossRef]
- Pitarokili, D.; Tzakou, O.; Loukis, A.; Harvala, C. Volatile Metabolites from Salvia Fruticosa as Antifungal Agents in Soilborne Pathogens. J. Agric. Food Chem. 2003, 51, 3294–3301. [Google Scholar] [CrossRef]
- Katsiotis, S.; Iconomou, N.J. Qualitative and Quantitative Gasliquid Chromatographic Analysis of the Essential Oil of Salvia triloba Grown in Greece. Pharm. Acta Helv. 1984, 59, 29–32. [Google Scholar]
- Koliopoulos, G.; Pitarokili, D.; Kioulos, E.; Michaelakis, A.; Tzakou, O. Chemical Composition and Larvicidal Evaluation of Mentha, Salvia, and Melissa Essential Oils against the West Nile Virus Mosquito Culex Pipiens. Parasitol. Res. 2010, 107, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Tzakou, O.; Pitarokili, D.; Couladis, M. Antifungal Activities of Selected Aromatic Plants Growing Wild in Greece. Nahrung 2002, 46, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Karousou, R.; Vokou, D.; Kokkini, S. Variation of Salvia Fruticosa Essential Oils on the Island of Crete (Greece). Bot. Acta 1998, 111, 250–254. [Google Scholar] [CrossRef]
- Skoula, M.; Hilali, I.E.; Makris, A.M. Evaluation of the Genetic Diversity of Salvia Fruticosa Mill. Clones Using RAPD Markers and Comparison with the Essential Oil Profiles. Biochem. Syst. Ecol. 1999, 27, 559–568. [Google Scholar] [CrossRef]
- Karioti, A.; Skaltsa, H.; Demetzos, C.; Perdetzoglou, D.; Economakis, C.D.; Salem, A.B. Effect of Nitrogen Concentration of the Nutrient Solution on the Volatile Constituents of Leaves of Salvia fruticosa Mill. in Solution Culture. J. Agric. Food Chem. 2003, 51, 6505–6508. [Google Scholar] [CrossRef]
- Skoula, M.; Abbes, J.E.; Johnson, C.B. Genetic Variation of Volatiles and Rosmarinic Acid in Populations of Salvia Fruticosa Mill Growing in Crete. Biochem. Syst. Ecol. 2000, 28, 551–561. [Google Scholar] [CrossRef]
- Kanias, G.D.; Souleles, C.; Loukis, A.; Philotheou-Panou, E. Statistical Study of Essential Oil Composition in Three Cultivated Sage Species. J. Essent. Oil Res. 1998, 10, 395–403. [Google Scholar] [CrossRef]
- Leontaritou, P.; Lamari, F.N.; Papasotiropoulos, V.; Iatrou, G. Morphological, Genetic and Essential Oil Variation of Greek Sage (Salvia fruticosa Mill.) Populations from Greece. Ind. Crops Prod. 2020, 150, 112346. [Google Scholar] [CrossRef]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite Profiling and Antioxidative Activity of Sage (Salvia fruticosa Mill.) under the Influence of Genotype and Harvesting Period. Ind. Crops Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Antonopoulou, V.; Vlassi, A.; Antonatos, S.; Michaelakis, A.; Papachristos, D.P.; Tzakou, O. Chemical Composition and Fumigant Activity of Essential Oils from Six Plant Families against Sitophilus oryzae (Col: Curculionidae). J. Pest. Sci. 2018, 91, 873–886. [Google Scholar] [CrossRef]
- Adam, K.; Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antifungal Activities of Origanum Vulgare Subsp. Hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa Essential Oils against Human Pathogenic Fungi. J. Agric. Food Chem. 1998, 46, 1739–1745. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Gardeli, C.; Mallouchos, A.; Papaioannou, M.; Komaitis, M. Variation of the Chemical Profile and Antioxidant Behavior of Rosmarinus officinalis L. and Salvia fruticosa Miller Grown in Greece. J. Agric. Food Chem. 2008, 56, 7254–7264. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahab, M.A.; Toaima, W.I.M.; Hamed, E.S. Effect of Different Planting Locations in Egypt on Salvia fruticosa Mill. Plants. Egypt. J. Desert Res. 2015, 65, 291–307. [Google Scholar] [CrossRef]
- Máthé, I.; Máthé, Á.; Hohmann, J.; Janicsák, G. Volatile and Some Non-Volatile Chemical Constituents of Mediterranean Salvia Species beyond Their Native Area. Isr. J. Plant Sci. 2010, 58, 273–277. [Google Scholar] [CrossRef]
- Elmann, A.; Mordechay, S.; Rindner, M.; Larkov, O.; Elkabetz, M.; Ravid, U. Protective Effects of the Essential Oil of Salvia Fruticosa and Its Constituents on Astrocytic Susceptibility to Hydrogen Peroxide-Induced Cell Death. J. Agric. Food. Chem. 2009, 57, 6636–6641. [Google Scholar] [CrossRef]
- Putievsky, E.; Ravid, U.; Dudai, N. The Essential Oil and Yield Components from Various Plant Parts of Salvia fruticosa. J. Nat. Prod. 1986, 49, 1015–1017. [Google Scholar] [CrossRef]
- Vergine, M.; Nicoli, F.; Negro, C.; Luvisi, A.; Nutricati, E.; Accogli, R.A.; Sabella, E.; Miceli, A. Phytochemical Profiles and Antioxidant Activity of Salvia Species from Southern Italy. Rec. Nat. Prod. 2019, 13, 205–215. [Google Scholar] [CrossRef]
- Al-Kalaldeh, J.Z.; Abu-Dahab, R.; Afifi, F.U. Volatile Oil Composition and Antiproliferative Activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against Human Breast Adenocarcinoma Cells. Nutr. Res. 2010, 30, 271–278. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, R.; Michael Jubeli, R.; El Beyrouthy, M.; Baillet Guffroy, A.; Rizk, T.; Tfayli, A.; Lteif, R. Phytochemical Screening and Antityrosinase Activity of Carvacrol, Thymoquinone, and Four Essential Oils of Lebanese Plants. J. Cosmet. Dermatol. 2019, 18, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid. Based Complement Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.; Nasser, H.; Baydoun, S. Chemical Composition of the Essential Oils of Five Salvia Species Growing Wild or Cultivated from Lebanon. Food Sci. Nutr. Res. 2018, 1, 1–5. [Google Scholar]
- Iriti, M.; Vitalini, S.; Arnold Apostolides, N.; El Beyrouthy, M. Chemical Composition and Antiradical Capacity of Essential Oils from Lebanese Medicinal Plants. J. Essent. Oil Res. 2014, 26, 466–472. [Google Scholar] [CrossRef]
- Giweli, A.A.; Džamić, A.M.; Soković, M.; Ristić, M.S.; Janaćković, P.; Marin, P.D. The Chemical Composition, Antimicrobial and Antioxidant Activities of the Essential Oil of Salvia Fruticosa Growing Wild in Libya. Arch. Biol. Sci. 2013, 65, 321–329. [Google Scholar] [CrossRef]
- Kocabas, I.; Kaplan, M.; Kurkcuoglu, M.; Baser, K.H.C. Effects of Different Organic Manure Applications on the Essential Oil Components of Turkish Sage (Salvia fruticosa Mill.). Asian J. Chem 2010, 22, 1599–1605. [Google Scholar]
- Arslan, I.; Çelik, A. Free Radical Scavenging Activities and Essential Oil Analysis of Salvia cedronella Boiss. and S. fruticosa Mill. J. Essent. Oil-Bear. Plants 2010, 13, 545–550. [Google Scholar] [CrossRef]
- Karadağ, A.; İpekçi, E.; Yağcılar, A.; Demirbolat, I.; Kartal, M.; Siafaka, P.; Okur, N. Antibacterial Evaluation of Elettaria cardamomum (L.) Maton, Lavandula angustifolia Mill. and Salvia fruticosa Mill. Essential Oil Combinations in Mouthwash Preparations. Nat. Volatiles Essent. Oils 2020, 7, 685474. [Google Scholar] [CrossRef]
- Karik, Ü.; Çinar, O.; Tunçtürk, M.; Şekeroğlu, N.; Gezici, S. Essential Oil Composition of Some Sage (Salvia Spp.) Species Cultivated in İzmir (Turkey) Ecological Conditions. Indian J. Pharm. Educ. Res. 2018, 52, s102–s107. [Google Scholar] [CrossRef]
- Figueredo, G.; Ünver, A.; Chalchat, J.C.; Arslan, D.; Özcan, M.M. A Research on the Composition of Essential Oil Isolated from Some Aromatic Plants By Microwave And Hydrodistillation: The Composition Of Some Essential Oils. J. Food Biochem. 2012, 36, 334–343. [Google Scholar] [CrossRef]
- Senol, F.S.; Orhan, I.E.; Erdem, S.A.; Kartal, M.; Sener, B.; Kan, Y.; Celep, F.; Kahraman, A.; Dogan, M. Evaluation of Cholinesterase Inhibitory and Antioxidant Activities of Wild and Cultivated Samples of Sage (Salvia Fruticosa) by Activity-Guided Fractionation. J. Med. Food 2011, 14, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Aşkun, T.; Başer, K.; Tümen, G.; Kürkçüoğlu, M. Characterization of Essential Oils of Some Salvia Species and Their Antimycobacterial Activities. Turk. J. Biol. 2010, 34, 89–95. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Özcan, M.M.; Figueredo, G. The Composition of Essential Oils of Different Parts of Laurel, Mountain Tea, Sage and Ajowan. J. Food Biochem. 2011, 35, 484–499. [Google Scholar] [CrossRef]
- Topçu, G.; Öztürk, M.; Kuşman, T.; Demirkoz, A.; Kolak, U.; Ulubelen, A. Terpenoids, Essential Oil Composition, Fatty Acid Profile, and Biological Activities of Anatolian Salvia fruticosa Mill. Turk. J. Chem. 2013, 37, 619–632. [Google Scholar] [CrossRef]
- Bayrak, A.; Akgül, A. Composition of Essential Oils from Turkish Salvia Species. Phytochemistry 1987, 26, 846–847. [Google Scholar] [CrossRef]
- Özcan, M.M.; Figueredo, G.; Chalchat, J.C.; Chalard, P.; Al-Juhaimi, F.; Ghafoor, K.; El-Babiker, E.F. Chemical Constituents in Essential Oils of Salvia officinalis L. and Salvia fruticosa Mill. Z. Arznei Gewurzpfla. 2015, 20, 181–184. [Google Scholar]
- Gursoy, U.K.; Gursoy, M.; Gursoy, O.V.; Cakmakci, L.; Könönen, E.; Uitto, V.-J. Anti-Biofilm Properties of Satureja hortensis L. Essential Oil against Periodontal Pathogens. Anaerobe 2009, 15, 164–167. [Google Scholar] [CrossRef]
- Süzgeç Selçuk, S.; Ozek, T.; Özek, G.; Yur, S.; Göger, F.; Gürdal, B.; Gülsoy Toplan, G.; Meriçli, A.H.; Baser, K.H.C. The Leaf and the Gall Volatiles of Salvia Fruticosa Miller from Turkey: Chemical Composition and Biological Activities. Rec. Nat. Prod. 2020, 15, 10–24. [Google Scholar] [CrossRef]
- Kosar, M.; Tunalier, Z.; Ozek, T.; Kürcüglu, M.; Can Baser, K.H. A Simple Method to Obtain Essential Oils from Salvia Triloba L. and Laurus nobilis L. by Using Microwave-Assisted Hydrodistillation. Z. Für Naturforschung C 2005, 60, 501–504. [Google Scholar] [CrossRef] [PubMed]
- di Martino, D. Ecologie dell’inquinamento Progetto di Territorio Attraverso la Bonifica. Available online: http://www.fedoa.unina.it/10025/ (accessed on 18 November 2021).
- Grezzi, G.; Ayuso, R.A.; De Vivo, B.; Lima, A.; Albanese, S. Lead Isotopes in Soils and Groundwaters as Tracers of the Impact of Human Activities on the Surface Environment: The Domizio-Flegreo Littoral (Italy) Case Study. J. Geochem. Explor. 2011, 109, 51–58. [Google Scholar] [CrossRef]
- Bove, M.A.; Ayuso, R.A.; De Vivo, B.; Lima, A.; Albanese, S. Geochemical and Isotopic Study of Soils and Waters from an Italian Contaminated Site: Agro Aversano (Campania). J. Geochem. Explor. 2011, 109, 38–50. [Google Scholar] [CrossRef]
- Council of Europe. Determination of Essential Oils in Herbal Drugs. In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2008; pp. 251–252. [Google Scholar]
- Rigano, D.; Formisano, C.; Rosselli, S.; Badalamenti, N.; Bruno, M. GC and GC-–MS Analysis of Volatile Compounds from Ballota nigra Subsp. Uncinata Collected in Aeolian Islands, Sicily (Southern Italy). Nat. Prod. Commun. 2020, 15, 1934578X20920483. [Google Scholar] [CrossRef]
- Esposito, S.; Sorbo, S.; Conte, B.; Basile, A. Effects of Heavy Metals on Ultrastructure and HSP70S Induction in the Aquatic Moss Leptodictyum Riparium Hedw. Int. J. Phytoremediation 2012, 14, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and Structural Biomarkers to Monitor Heavy Metal Pollution of One of the Most Contaminated Freshwater Sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Experim. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- del Río, L.A. ROS and RNS in Plant Physiology: An Overview. J. Experim. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-Metal-Induced Reactive Oxygen Species: Phytotoxicity and Physicochemical Changes in Plants. In Reviews of Environmental Contamination and Toxicology Volume 232; Whitacre, D.M., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–44. ISBN 978-3-319-06746-9. [Google Scholar]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]

| Origin | P.p. | Compounds (%) | Ref. |
|---|---|---|---|
| Albania | l. | 1,8-cineole (38.9), camphor (8.4), α-pinene (5.9), α-thujone (5.4), α-terpineol (4.9), β-pinene (4.4), borneol (3.8), camphene (3.8), (E)-β-caryophyllene (3.5), β-thujone (3.4), myrcene (3.2) | [26] |
| Albania, 7 localities | l. | 1,8-cineole (51.2–17.2), camphor (18.6–2.9), (E)-β-caryophyllene (16.0–0.7), β-thujone (10.4–1.1), α-pinene (6.0–1.7), camphene (6.0–0.5), α-terpineol (5.7–0), globulol (5.0–0), β-pinene (4.5–2.2), manool (4.5–0.8), α-terpenyl acetate (4.2–0), α-thujone (4.1–0.9) | [27] |
| Albania, Vlora | a.p. | 1,8-cineole (37.5–30.1), camphor (21.5–13.9), camphene (9.0–6.4), (E)-β-caryophyllene (8.1–5.3), α-pinene (7.1–6.6), β-pinene (5.6–4.5), myrcene (5.5–4.0) | [28] |
| Brasil | a.p. | α-thujone (20.1), 1,8-cineole (15.7), camphor (12.6), (E)-β-caryophyllene (11.8), α-humulene (7.5), viridiflorol (6.3), β-thujone (4.8), β-pinene (3.9) | [29] |
| Cyprus, Troodos | l. | camphor (49.3–49.2), 1,8-cineole (21.5–17.6), (E)-β-caryophyllene (11.9–6.6), camphene (5.0–0), borneol (4.6–1.7), limonene (3.6–0) | [30] |
| Cyprus, 6 localities | l. | 1,8-cineole (67.5–19.3), camphor (44.5–5.7), camphene (7.3–1.4), β-pinene (6.9–2.2), limonene (5.3–1.1), α-pinene (4.3–3.3) | [31] |
| Cyprus, 4 localities | f. | 1,8-cineole (52.0–14.3), camphor (41.8–6.3), β-pinene (13.9–3.0), (E)-β-caryophyllene (8.7–4.4), camphene (6.5–2.1), α-pinene (5.7–1.8), borneol (5.2–3.5), limonene (3.1–1.6) | [32] |
| Cyprus, 5 localities | s. | 1,8-cineole (54.7–4.0), camphor (44.2–7.6), (E)-β-caryophyllene (23.0–3.4), caryophyllene oxide (12.2–1.9), β-pinene (9.7–0), borneol (7.6–1.5), camphene (6.5–0), α-pinene (4.2–0) | [31] |
| Greece, 3 localities | l. | 1,8-cineole (58.3–23.7), globulol (9.9–0), β-thujone (9.8–2.6), α-terpineol (6.4–3.2), manool (6.4–0), β-pinene (6.1–0.8), α-terpenyl acetate (5.2–0), α-pinene (4.2–0.4), (E)-β-caryophyllene (3.8–0.8), α-thujone (3.5–1.2), | [27] |
| Greece, 15 localities | l. | 1,8-cineole (54.4–16.9), (E)-β-caryophyllene (15.6–0), camphor (15.4–0.6), α-thujone (14.5–0), β-thujone (9.0–0.6), β-pinene (9.0–0), viridiflorol (8.4–0), borneol (8.0–0), α-pinene (7.4–1.5), camphene (7.0–0), bornyl acetate (6.8–0), α-terpineol (6.7–0), myrcene (5.2–1.6) | [32] |
| Greece, 8 localities | l. | 1,8-cineole (66.2–38.8), camphor (23.8–1,7), thujone (12.1–1.4), β-pinene (10.7–2.9), camphene (7.4–0.4), (E)-β-caryophyllene (7.3–1.2), α-pinene (6.7–3.7), myrcene (6.7–0) | [33] |
| Greece, Ikaria | a.p. | camphor (23.1), α-pinene (12.7), borneol (12.6), camphene (9.0), 1,8-cineole (6.9), β-pinene (5.8), (E)-β-caryophyllene (5.3), α-terpineol (4.6), caryophyllene oxide (3.8) | [34] |
| Greece, Kalymnos | a.p. | 1,8-cineole (31.4), camphor (22.6), α-pinene (8.7), camphene (8.5), α-thujone (7.5), β-pinene (4.5), β-thujone (4.1) | [34] |
| Greece, Krete | a.p. | 1,8-cineole (41.4), camphor (12.1), β-thujone (10.3), β-pinene (6.4), α-pinene (5.4), α-terpineol (5.0), camphene (3.1) | [35] |
| Greece, Krete | a.p. | 1,8-cineole (64.2–22.7), camphor (30.3–0.8), β-thujone (25.6–0.9), α-thujone (19.2–1.0), camphene (9.9–0.2), β-pinene (9.4–3.5), α-terpineol (7.5–1.2), (E)-β-caryophyllene (6.9–0.2), myrcene (5.3–1.6), α-pinene (5.2–1.8), | [36] |
| Greece, Krete | l. | 1,8-cineole (51.0–35.6), camphor (11.6–3.7), β-thujone (11.5–1.9), β-pinene (7.0–5.0), α-thujone (5.6–2.9), (E)-β-caryophyllene (4.7–1.3), α-pinene (4.5–3.6), camphene (3.2–0.7) | [37] |
| Greece, Krete, clt NO3-N, 100 mg/L | l. | 1,8-cineole (37.5–26.8), viridiflorol (15.7–7.2), (E)-β-caryophyllene (13.0–0.2), 13-epi-manool (11.4–4.6), myrcene (7.0–3.7), α-humulene (6.0–4.8), α-pinene (5.1–3.7), β-pinene (4.6–4.5), α-aromadendrene (4.5–3.1), α-terpineol (3.6–2.5) | [38] |
| Greece, Krete, clt NO3-N, 150 mg/L | l. | 1,8-cineole (28.6–22.5), 13-epi-manool (13.1–12.9), (E)-β-caryophyllene (12.2), viridiflorol (10.9–10.7), α-humulene (4.9–4.7), α-pinene (4.1–2.6), β-pinene (4.0–3.7), α-terpineol (3.8–3.0), myrcene (3.7–2.6), α-aromadendrene (3.6–3.0) | [38] |
| Greece, Krete, clt NO3-N, 200 mg/L | l. | viridiflorol (37.9–23.3), 13-epi-manool (25.7–14.3), (E)-β-caryophyllene (11.5–9.9), α-humulene (10.2–8.6), α-terpineol (6.6–6.2), caryophyllene oxide (3.9–3.3) | [38] |
| Greece, Krete, clt | l. | 1,8-cineole (59.3–48.1), β-pinene (11.9–10.3), α-pinene (10.0–9.3), myrcene (7.8–3.7), camphor (5.9–1.3), β-thujone (4.5–0.5) | [39] |
| Greece, Krete, clt | l. | 1,8-cineole (62.9–28.2), α-thujone (34.1–2.0), camphor (10.3–0.4), β-pinene (8.8–0.9), β-thujone (8.6–0.9), (E)-β-caryophyllene (5.6–1.4), myrcene (5.2–1.1), caryophyllene oxide (5.2–0.2), α-pinene (3.9–0.2) | [40] |
| Greece, Peloponnese | l. | 1,8-cineole (46.6–27.8), camphor (15.6–6.2), (E)-β-caryophyllene (9.7–4.0), camphene (7.4–2.5), α-pinene (7.1–4.1), β-pinene (5.4–3.3), myrcene (5.4–3.1), α-terpineol (4.0–2.0), β-thujone (3.0–0.6) | [41] |
| Greece, clt | a.p. | 1,8-cineole (55.7–44.7), camphor (14.9–1.3), β-pinene (14.1–5.8), (E)-β-caryophyllene (7.2–1.4), camphene (5.9–0.5), α-pinene (5.9–3.5), myrcene (5.6–2.7), α-terpineol (5.2–2.1), | [42] |
| Greece, clt | a.p. | camphor (18.6), 1,8-cineole (16.6), camphene (7.0), (E)-β-caryophyllene (5.4), β-pinene (5.3), α-pinene (5.2), bornyl acetate (4.4), α-terpineol (3.9), α-thujone (3.8), β-thujone (4.1), limonene (3.1) | [43] |
| Greece, Mt. Ochi, Eubea | a.p. | 1,8-cineole (56.3), β-pinene (7.8), (E)-β-caryophyllene (7.0), α-terpineol (5.6), β-thujone (4.1), α-pinene (4.0), myrcene (3.0) | [43] |
| Greece, Sithonia | l. | 1,8-cineole (43.1), camphor (18.3), β-pinene (8.2), α-pinene (6.8), sabinene (4.8), myrcene (3.2), | [44] |
| Greece, Zakynthos | a.p. | 1,8-cineole (58.9–46.0), viridiflorol (7.0–2.1), camphor (5.8–07), (E)-β-caryophyllene (5.1–1.0), β-pinene (5.0–2.0), myrcene (4.6–3.2), α-terpineol (4.3–2.8), α-pinene (4.0–3.2), α-thujone (3.1–1.1) | [45] |
| Egypt, 3 localities | a.p. | camphor (23.7–5.1), 1,8-cineole (45.7–31.9), (E)-β-caryophyllene (11.5–0.9), β-pinene (9.9–6.7), camphene (8.7–1.9), α-pinene (5.7–2.9), myrcene (4.0–1.6) | [46] |
| Hungary, clt | a.p. | camphor (26.0), α-thujone (21.4), 1,8-cineole (16.9), viridiflorol (5.6), myrcene (4.3) | [47] |
| Israel, clt | a.p. | 1,8-cineole (26.4), camphor (18.9), camphene (9.5), α-thujone (9.1), (E)-β-caryophyllene (5.0), α-humulene (3.9), β-pinene (4.7), α-pinene (4.4) | [48] |
| Israel, clt | l. | 1,8-cineole (44.0), α-pinene (18.6), (E)-β-caryophyllene (11.3), β-pinene (5.0), camphor (3.3) | [49] |
| Israel, clt | s. | α-pinene (37.3), 1,8-cineole (31.5), (E)-β-caryophyllene (7.6), β-pinene (7.0), camphor (6.8) | [49] |
| Israel, clt | f. | α-pinene (31.5), 1,8-cineole (30.8), (E)-β-caryophyllene (10.4), β-pinene (6.6), camphor (5.6), α-terpinil acetate (3.4), camphene (3.1) | [49] |
| Italy, Salento, clt | a.p. | 1,8-cineole (27.6), (E)-β-caryophyllene (18.3), limonene (8.8), humulene (7.6), myrcene (5.0), α-pinene (3.7), γ-gurjunene (3.7) | [50] |
| Jordan, Amman | l. | 1,8-cineole (45.2), camphor (11.5), β-pinene (9.0), γ-terpineol (4.4), α-pinene (3.3) | [51] |
| Lebanon, Ebrine | a.p. | 1,8-cineole (33.5), β-pinene (9.8), α-pinene (8.0), (E)-β-caryophyllene (7.6), α-thujone (7.1), α-terpineol (6.4), camphor (5.6), α-terpinyl acetate (3.7), myrcene (3.5) | [52] |
| Lebanon | a.p. | 1,8-cineole (57.3), (E)-β-caryophyllene (8.3), camphor (4.8), α-terpineol (4.2) | [53] |
| Lebanon | a.p. | 1,8-cineole (21.5), β-pinene (10.1), α-terpineol (9.2), (E)-β-caryophyllene (7.3), camphor (6.3), camphene (5.0), γ-gurjunene (4.4) | [54] |
| Lebanon, Nahr Ibrahim | a.p. | 1,8-cineole (48.7), (E)-β-caryophyllene (30.8), aromadendrene (3.3), β-pinene (3.2) | [55] |
| Lybia, Biadda | a.p. | 1,8-cineole (49.3), camphor (7.5), β-pinene (7.4), myrcene (7.4), α-pinene (5.1), (E)-β-caryophyllene (4.1), α-terpineol (3.2) | [56] |
| Turkey, cultivated | a.p. | 1,8-cineole (45.0), camphor (7.0), (E)-β-caryophyllene (5.7), β-pinene (5.3), β-thujone (5.1), α-pinene (5.0), camphene (3.0) | [57] |
| Turkey, ÇakIroluk | a.p. | 1,8-cineole (11.6), camphor (10.4), α-thujone (10.4), β-gurjunene (8.2), α-humulene (7.5), β-thujone (4.8), β-pinene (3.9) | [58] |
| Turkey, Iskilip, Çorum | a.p. | 1,8-cineole (40.0), camphor (11.3), α-pinene (7.3), myrcene (4.5), camphene (3.9) | [59] |
| Turkey, Izmir, cultivated | a.p. | 1,8-cineole (57.2), β-pinene (8.2), myrcene (5.7), (E)-β-caryophyllene (4.8), α-pinene (3.4), camphor (3.1), β-thujone (3.1) | [60] |
| Turkey, Konya market | l. | 1,8-cineole (51.2), α-thujone (5.8), α-pinene (4.4), β-pinene (3.1) | [61] |
| Turkey, Kalkan | l. | 1,8-cineole (456 mg/mL), thymol (39 mg/mL), camphor (36 mg/mL), α-pinene (27 mg/mL), β-pinene (20 mg/mL) | [61] |
| Turkey, Konya, clt | a.p. | 1,8-cineole (36.2), camphor (19.1), thujone (7.8), β-pinene (6.4), α-pinene (5.3), (E)-β-caryophyllene (4.8), α-terpineol (3.9) | [62] |
| Turkey, Marmara | a.p. | 1,8-cineole, (52.8), camphor (5.8), α-pinene (5.8), β-pinene (4.5), myrcene (3.8), camphene (3.1) | [63] |
| Turkey, Mersin | a.p. | α-pinene (31.0), isoborneol (27.2), borneol (7.6), 1,8-cineole, (6.9), camphene (6.1), β-pinene (3.9) | [64] |
| Turkey, Muğla | a.p. | 1,8-cineole (58.9), α-pinene (5.6), β-pinene (5.2), myrcene (5.2), camphor (4.5), (E)-β-caryophyllene (4.2), α-terpineol (3.0) | [65] |
| Turkey, Muğla | a.p. | 1,8-cineole (55.5), camphor (8.4), (E)-β-caryophyllene (5.2), borneol (4.6), β-pinene (4.3), α-pinene (3.2), myrcene (3.1) | [66] |
| Turkey, Muğla | a.p. | 1,8-cineole (40.1), camphor (26.8), borneol (8.9), camphene (5.3), α-pinene (3.6) | [67] |
| Turkey, West Mediteraean | a.p. | 1,8-cineole (49.5), camphor (13.3), β-pinene (7.2), α-pinene (5.8), camphene (5.0), β-thujone (3.6) | [68] |
| Turkey, 3 localities | l. | 1,8-cineole (47.1–27.2), camphor (19.8–9.3), camphene (10.7–3.8), α-pinene (7.1–5.7), β-pinene (5.8–5.7), borneol (4.4–1.5), α-thujone (3.4–1.9), (E)-β-caryophyllene (3.1–1,5) | [69] |
| Turkey, commercial | l. | 1,8-cineole (52.0), camphor (10.4), α-pinene (6.0), camphene (4.7), β-pinene (3.9), myrcene (3.3) | [70] |
| C1 | C2 | |
|---|---|---|
| Cu | 4743.46 ± 24.41 a | 10,812.52 ± 43.94 b |
| Zn | 4260.64 ± 11.02 a | 396,728.84 ± 1633.1 b |
| Cd | 1804.90 ± 9.38 a | 278,743.55 ± 685.84 b |
| Pb | 35.94 ± 4.50 a | 943.77 ± 22.53 b |
| Heavy Metals Exposure | EO Treatment | Code |
|---|---|---|
| No exposure (without DMSO) | No essential oil | CTRL |
| No exposure (with DMSO) | No essential oil | CTRL-D |
| C1 Heavy Metals mix | No essential oil | C1 |
| C2 Heavy Metals mix | No essential oil | C2 |
| Total EO extract treatments | ||
| No exposure (with DMSO) | Total EO extract 0.16% | CTRL-TE16 |
| C1 Heavy Metals mix | Total EO extract 0.16% | C1-TE16 |
| C2 Heavy Metals mix | Total EO extract 0.16% | C2-TE16 |
| No exposure (with DMSO) | Total EO extract 0.25% | CTRL-TE25 |
| C1 Heavy Metals mix | Total EO extract 0.25% | C1-TE25 |
| C2 Heavy Metals mix | Total EO extract 0.25% | C2-TE25 |
| Pure EOs treatments | ||
| No exposure (with DMSO) | Camphor 0.16% | CTRL-CAM16 |
| C1 Heavy Metals mix | Camphor 0.16% | C1-CAM16 |
| C2 Heavy Metals mix | Camphor 0.16% | C2-CAM16 |
| No exposure (with DMSO) | Camphor 0.25% | CTRL-CAM25 |
| C1 Heavy Metals mix | Camphor 0.25% | C1-CAM25 |
| C2 Heavy Metals mix | Camphor 0.25% | C2-CAM25 |
| No exposure (with DMSO) | β-myrcene 0.16% | CTRL-MYR16 |
| C1 Heavy Metals mix | β-myrcene 0.16% | C1-MYR16 |
| C2 Heavy Metals mix | β-myrcene 0.16% | C2-MYR16 |
| No exposure (with DMSO) | β-myrcene 0.25% | CTRL-MYR25 |
| C1 Heavy Metals mix | β-myrcene 0.25% | C1-MYR25 |
| C2 Heavy Metals mix | β-myrcene 0.25% | C2-MYR25 |
| No exposure (with DMSO) | 1,8-cineole 0.16% | CTRL-CIN16 |
| C1 Heavy Metals mix | 1,8-cineole 0.16% | C1-CIN16 |
| C2 Heavy Metals mix | 1,8-cineole 0.16% | C2-CIN16 |
| No exposure (with DMSO) | 1,8-cineole 0.25% | CTRL-CIN25 |
| C1 Heavy Metals mix | 1,8-cineole 0.25% | C1-CIN25 |
| C2 Heavy Metals mix | 1,8-cineole 0.25% | C2-CIN25 |
| LRI a | LRI b | Compound | % | Identification c |
|---|---|---|---|---|
| 855 | 1318 | 1-Hexanol | 0.03 ± 0.00 | 1, 2, 3 |
| 860 | 1344 | (Z)-4-Hexen-1-ol | 0.04 ± 0.00 | 1, 2 |
| 923 | 1007 | Tricyclene | 0.12 ± 0.00 | 1, 2 |
| 933 | 1025 | α-Pinene | 6.51 ± 0.27 | 1, 2, 3 |
| 950 | 1040 | Camphene | 8.69 ± 0.38 | 1, 2, 3 |
| 975 | 1080 | β-Pinene | 6.70 ± 0.21 | 1, 2, 3 |
| 980 | 1412 | Oct-1-en-3-ol | 0.13 ± 0.00 | 1, 2 |
| 989 | 1137 | β-Myrcene | 9.13 ± 0.38 | 1, 2, 3 |
| 1028 | 1172 | 1,8-Cineole (Eucalyptol) | 17.56 ± 0.74 | 1, 2, 3 |
| 1057 | 1240 | γ-Terpinene | 1.38 ± 0.04 | 1, 2 |
| 1060 | 1470 | (E)-Sabinene hydrate | 0.30 ± 0.01 | 1, 2 |
| 1074 | 1493 | (Z)-Sabinene hydrate | 0.06 ± 0.00 | 1, 2 |
| 1089 | 1250 | Terpinolene | 0.45 ± 0.02 | 1, 2 |
| 1097 | 1513 | β-Linalool | 0.19 ± 0.00 | 1, 2, 3 |
| 1100 | 1368 | α-Thujone | 1.26 ± 0.04 | 1, 2 |
| 1106 | 1386 | β-Thujone | 2.11 ± 0.08 | 1, 2 |
| 1128 | 1564 | (E)-p-2-Menthen-1-ol | 0.13 ± 0.00 | 1, 2 |
| 1130 | 1515 | Camphor | 13.63 ± 0.54 | 1, 2, 3 |
| 1142 | 1818 | p-Cymene-8-ol | 0.03 ± 0.00 | 1, 2 |
| 1151 | 1640 | Isoborneol | 0.04 ± 0.00 | 1, 2 |
| 1162 | 1690 | Borneol | 3.69 ± 0.13 | 1, 2 |
| 1168 | 1592 | Terpinene-4-ol | 1.90 ± 0.06 | 1, 2 |
| 1180 | 1705 | α-Terpineol | 6.56 ± 0.27 | 1, 2, 3 |
| 1185 | 1342 | (E)-1-Octenyl acetate | 0.07 ± 0.00 | 1, 2 |
| 1265 | 1546 | Bornyl acetate | 1.63 ± 0.06 | 1, 2 |
| 1300 | 2167 | Carvacrol | 0.02 ± 0.00 | 1, 2 |
| 1366 | 1674 | Isoledene | 0.04 ± 0.00 | 1, 2 |
| 1432 | 1583 | (E)-β-Caryophyllene | 2.12 ± 0.09 | 1, 2 |
| 1439 | 1833 | (E)-Geranylacetone | 0.04 ± 0.00 | 1, 2 |
| 1448 | 1690 | α-Humulene | 0.27 ± 0.01 | 1, 2 |
| 1518 | 1796 | (E)-Calamene | 0.16 ± 0.00 | 1, 2 |
| 1532 | 1716 | δ-Cadinene | 0.78 ± 0.02 | 1, 2 |
| 1552 | 2019 | Ledol | 0.03 ± 0.00 | 1, 2 |
| 1567 | 2119 | (Z)-3-Hexen-1-yl-benzoate | 0.12 ± 0.00 | 1, 2 |
| 1569 | 2129 | Spathulenol | 0.18 ± 0.00 | 1, 2 |
| 1578 | 1960 | Caryophyllene oxide | 1.21 ± 0.04 | 1, 2, |
| 1590 | 2027 | Globulol | 4.07 ± 0.15 | 1, 2 |
| 1592 | 2073 | Viridiflorol | 0.04 ± 0.00 | 1, 2 |
| 1648 | 2250 | α-Eudesmol | 0.05 ± 0.00 | 1, 2 |
| 2034 | 2603 | Manool | 3.01 ± 0.11 | 1, 2 |
| 2319 | 3203 | Ferruginol | 0.17 ± 0.00 | 1, 2 |
| Class of Compounds | ||||
| Aliphatic alcohols | 0.20 ± 0.00 | |||
| Aliphatic esters | 0.07 ± 0.00 | |||
| Aromatic esters | 0.12 ± 0.00 | |||
| Monoterpene hydrocarbons | 31.60 ± 1.30 | |||
| Oxygenated monoterpenes | 49.11 ± 1.93 | |||
| Sesquiterpene hydrocarbons | 3.41 ± 0.12 | |||
| Oxygenated sesquiterpenes | 5.58 ± 0.19 | |||
| Oxygenated diterpenes | 3.18 ± 0.11 | |||
| Total | 93.27 ± 3.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalamenti, N.; Salbitani, G.; Cianciullo, P.; Bossa, R.; De Ruberto, F.; Greco, V.; Basile, A.; Maresca, V.; Bruno, M.; Carfagna, S. Chemical Composition of Salvia fruticosa Mill. Essential Oil and Its Protective Effects on Both Photosynthetic Damage and Oxidative Stress in Conocephalum conicum L. Induced by Environmental Heavy Metal Concentrations. Antioxidants 2023, 12, 1990. https://doi.org/10.3390/antiox12111990
Badalamenti N, Salbitani G, Cianciullo P, Bossa R, De Ruberto F, Greco V, Basile A, Maresca V, Bruno M, Carfagna S. Chemical Composition of Salvia fruticosa Mill. Essential Oil and Its Protective Effects on Both Photosynthetic Damage and Oxidative Stress in Conocephalum conicum L. Induced by Environmental Heavy Metal Concentrations. Antioxidants. 2023; 12(11):1990. https://doi.org/10.3390/antiox12111990
Chicago/Turabian StyleBadalamenti, Natale, Giovanna Salbitani, Piergiorgio Cianciullo, Rosanna Bossa, Francesca De Ruberto, Valeria Greco, Adriana Basile, Viviana Maresca, Maurizio Bruno, and Simona Carfagna. 2023. "Chemical Composition of Salvia fruticosa Mill. Essential Oil and Its Protective Effects on Both Photosynthetic Damage and Oxidative Stress in Conocephalum conicum L. Induced by Environmental Heavy Metal Concentrations" Antioxidants 12, no. 11: 1990. https://doi.org/10.3390/antiox12111990
APA StyleBadalamenti, N., Salbitani, G., Cianciullo, P., Bossa, R., De Ruberto, F., Greco, V., Basile, A., Maresca, V., Bruno, M., & Carfagna, S. (2023). Chemical Composition of Salvia fruticosa Mill. Essential Oil and Its Protective Effects on Both Photosynthetic Damage and Oxidative Stress in Conocephalum conicum L. Induced by Environmental Heavy Metal Concentrations. Antioxidants, 12(11), 1990. https://doi.org/10.3390/antiox12111990














